Submitted:
21 June 2023
Posted:
22 June 2023
You are already at the latest version
Abstract
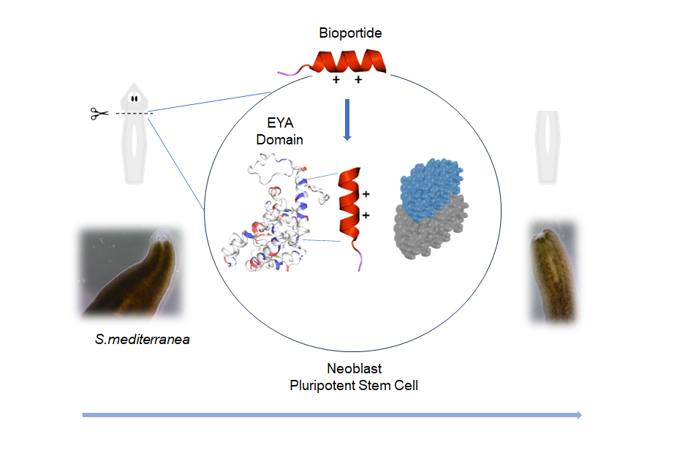
Keywords:
1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis and Secondary Structure Analysis
2.1.1. Microwave Enhanced Solid Phase Peptide Synthesis
2.1.2. Circular Dichroism
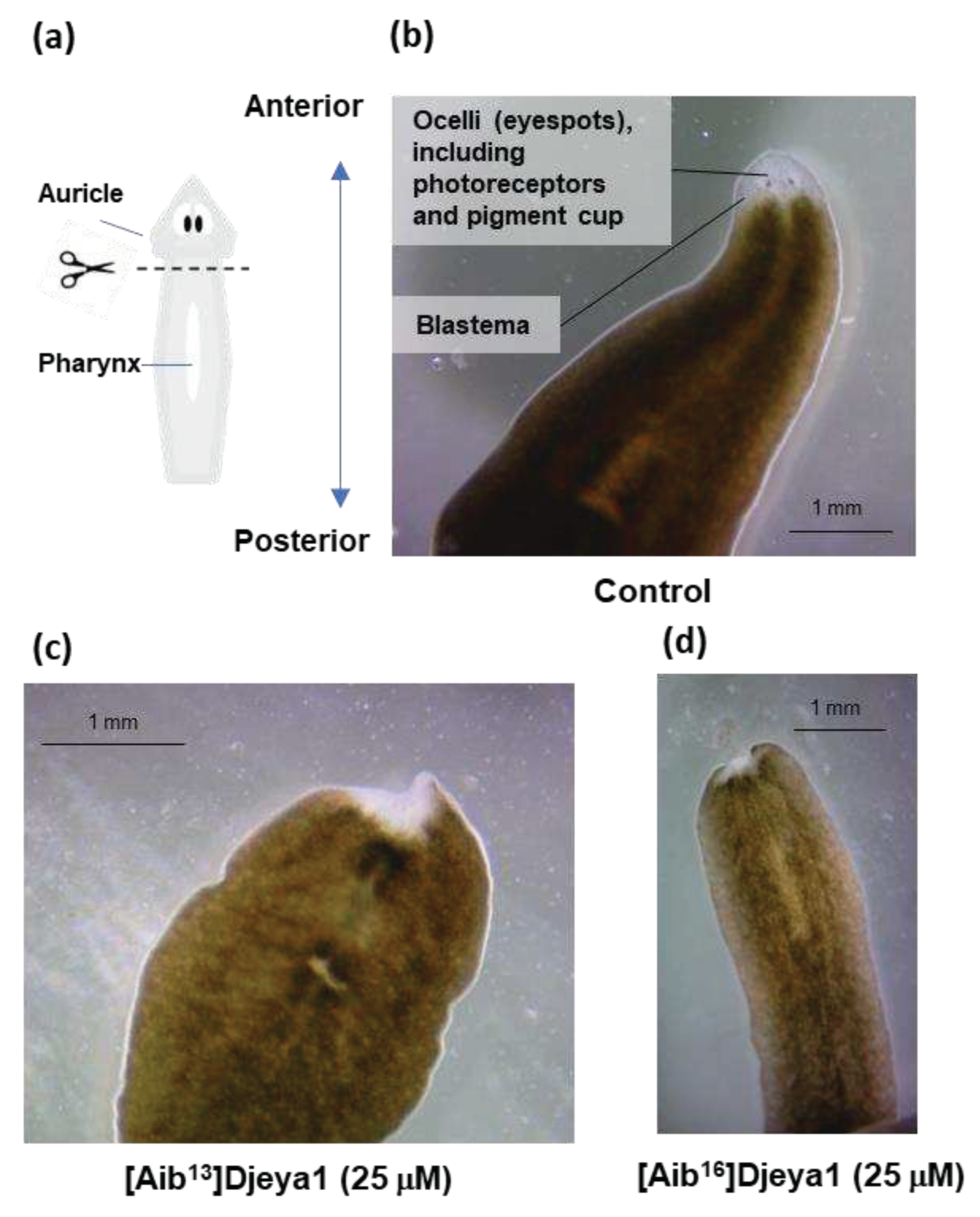
2.2. Schmidtea mediterranea Culture and Head Morphogenesis
2.2.1. Planarian Maintenance
2.2.3. Anterior Pole Regeneration and Eye Development Assay
2.3. Biological Characterisation of Aib-substituted Djeya1 Analogues
2.3.1. Cell Culture Maintenance
2.3.2. Qualitative Peptide Uptake Analyses
2.3.3. Quantitative Peptide Uptake Analyses
2.3.4. Cytotoxicity Assays
2.3.5. Cellular Proliferation
2.3.6. Cell Migration Assays
2.4. Graphical Representations and Statistical Analyses
3. Results
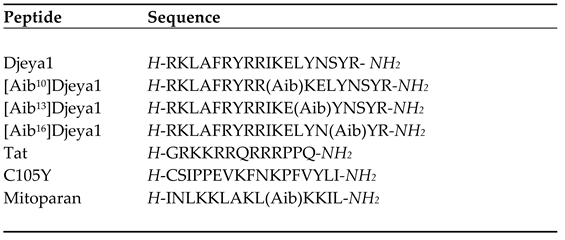
3.1. Site-directed Bioengineering of the Djeya1 CPP
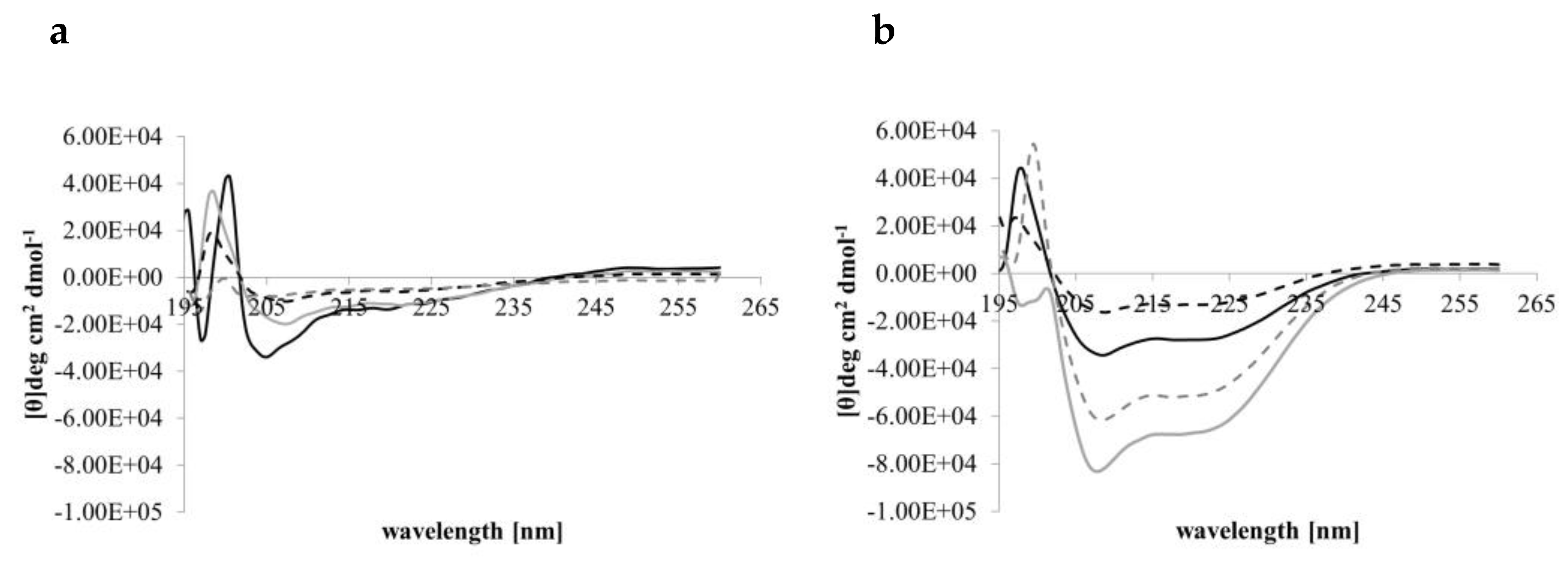
3.2. CD Spectral Analyses of Peptide Helicity
| Peptide | α-helix | ß-Strand | ß-Turns | Unordered |
|---|---|---|---|---|
| Djeya1 | 43.00 ± 1.73 | 30.33 ± 0.58 | 6.67 ± 0.58 | 20.00 ± 1.73 |
| [Aib10]Djeya1 | 41.33 ± 1.15 | 31.33 ± 0.58 | 7.00 ± 0.0 | 20.67 ± 0.58 |
| [Aib13]Djeya1 | 40.67 ± 1.52 | 30.00 ± 1.00 | 7.00 ± 0.0 | 22.33 ± 1.52 |
| [Aib16]Djeya1 | 39.67 ± 4.04 | 31.67 ± 2.87 | 7.33 ± 0.58 | 21.00 ± 1.00 |
| Peptide | α-helix | ß-Strand | ß-Turns | Unordered |
|---|---|---|---|---|
| Djeya1 | 45.50 ± 4.94 | 27.55 ± 3.53 | 7.00 ± 0.0 | 20.0 ± 1.41 |
| [Aib10]Djeya1 | 51.50 ± 0.07 | 21.00 ± 0.02 | 7.50 ± 0.71 | 20.0 ± 0.0 |
| [Aib13]Djeya1 | 40.00 ± 2.64 | 31.00 ± 2.08 | 7.33 ± 0.05 | 22.33 ± 1.5 |
| [Aib16]Djeya1 | 46.00 ± 1.41 | 27.50 ± 0.71 | 7.00 ± 0.0 | 19.50 ± 0.71 |
3.3. Exogenous Application of [Aib13]Djeya1 and [Aib16]Djeya1 Delays Anterior Pole Regeneration and Eye Development in S. mediterranea.
3.4. Aib-substituted Djeya1 Analogues are Efficient CPPs.
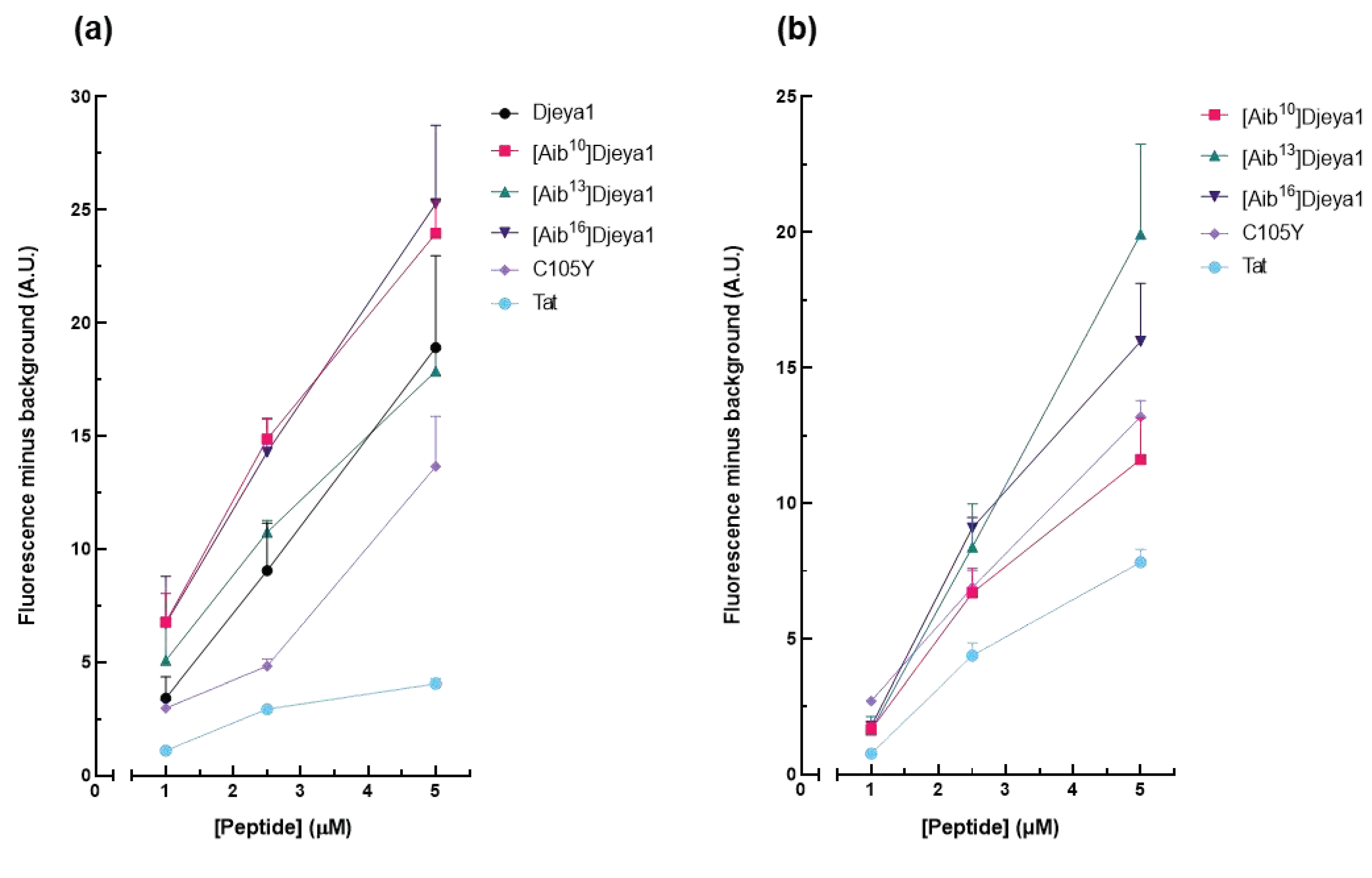
3.4.1. Qualitative Peptide Uptake Analyses.
3.4.2. Quantitative Peptide Uptake Analyses.
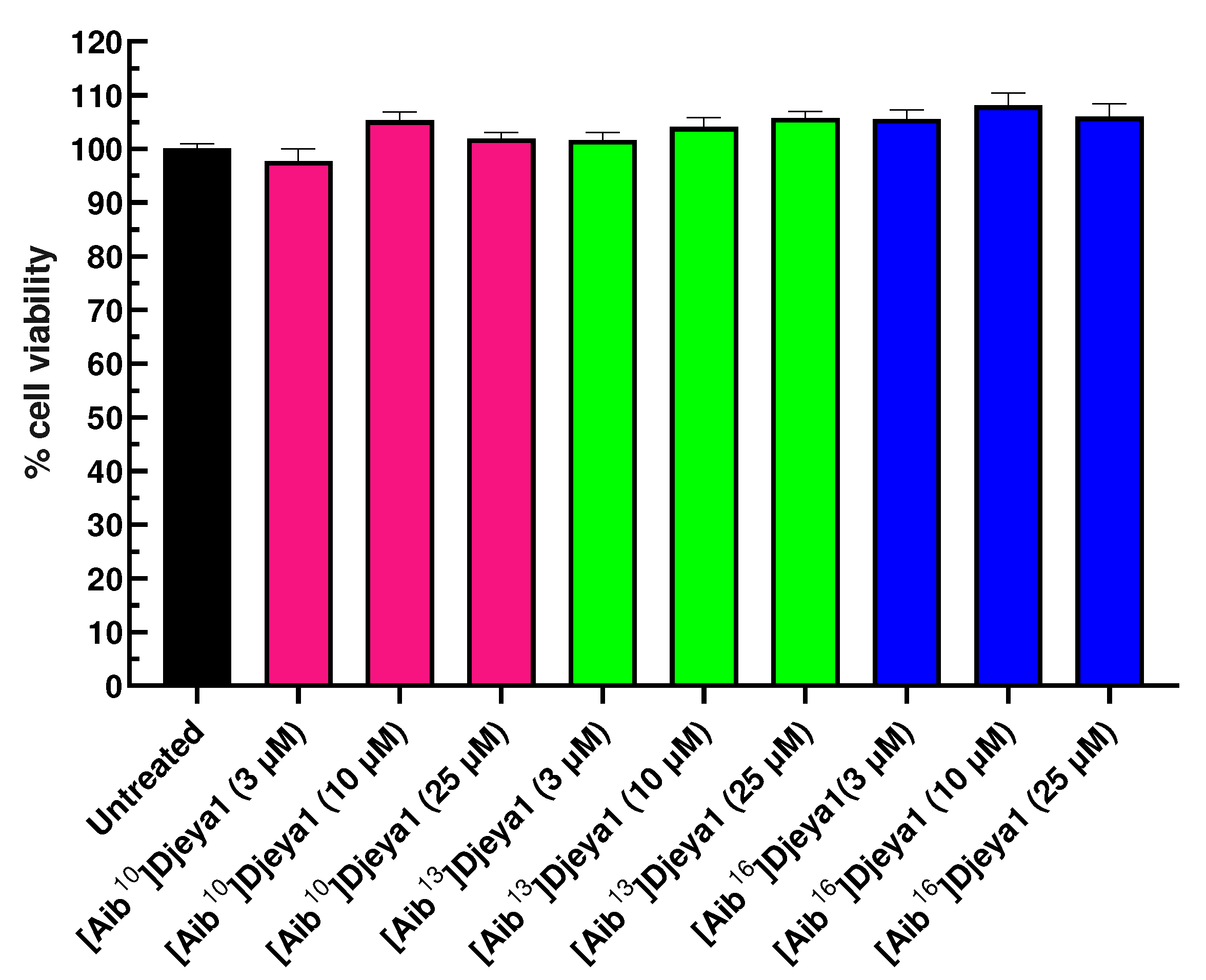
3.5. Cytotoxicity of Djeya1 Analogues.
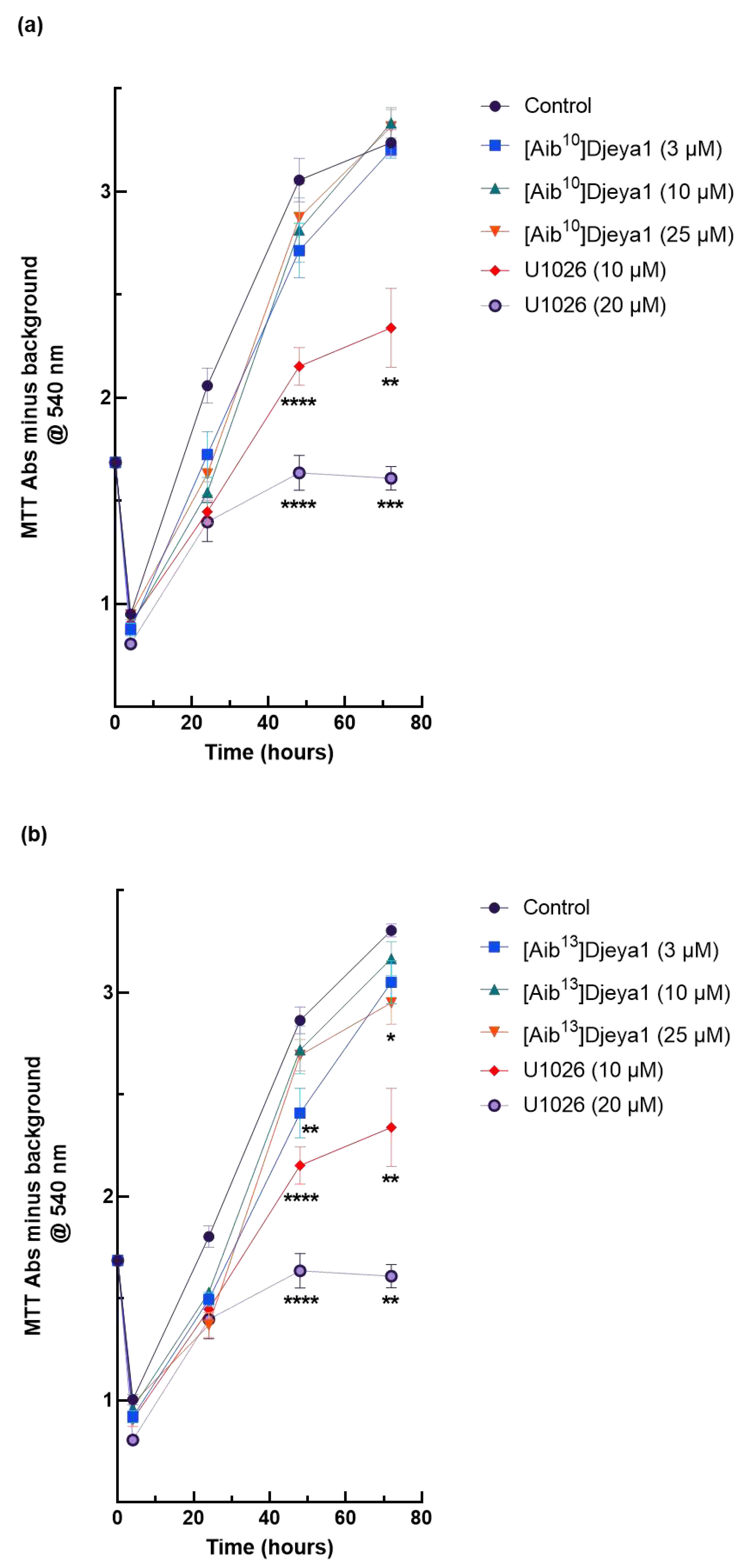
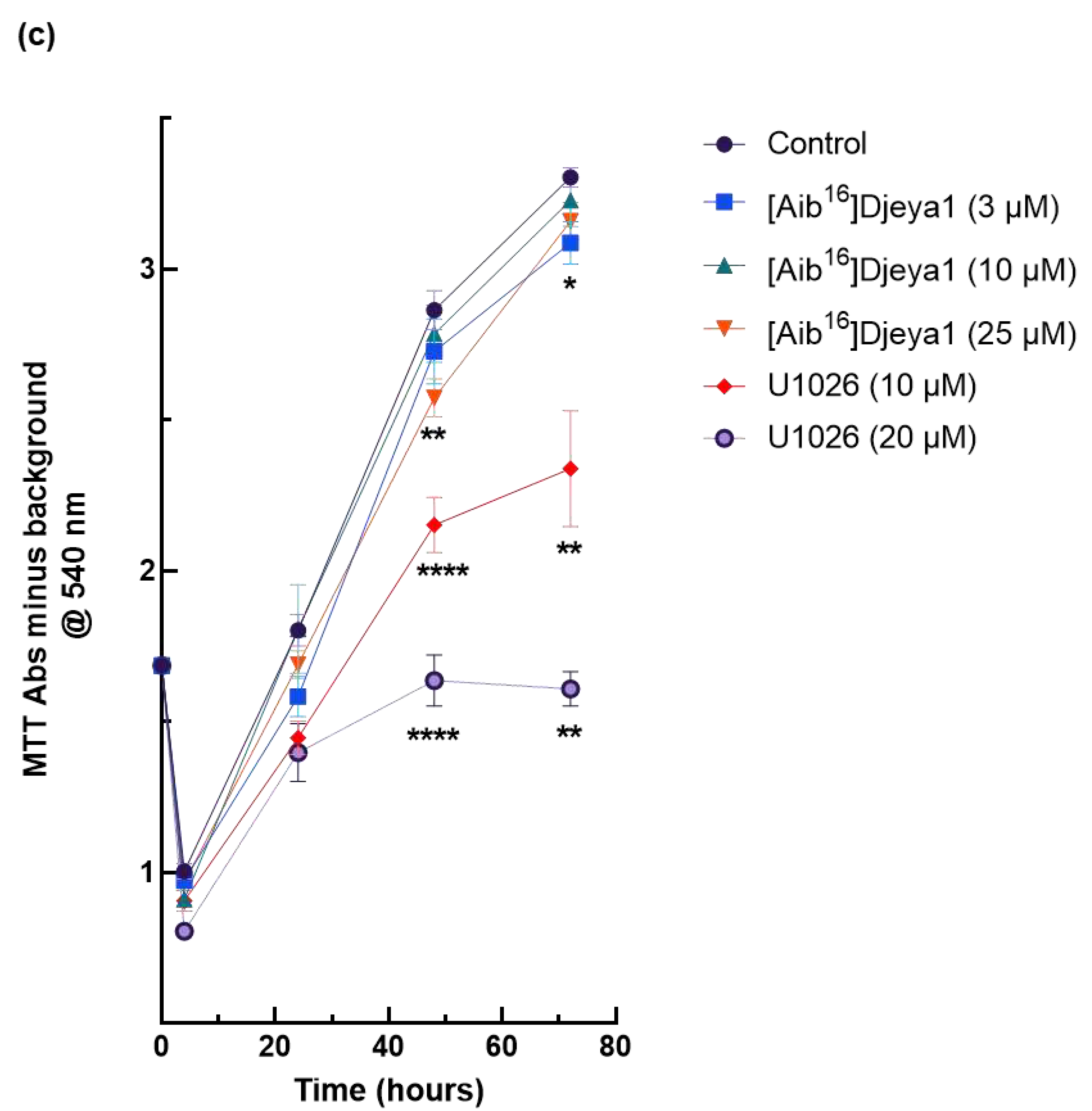
3.6. Impact of Djeya1 Analogues upon U251 Cellular Proliferation.
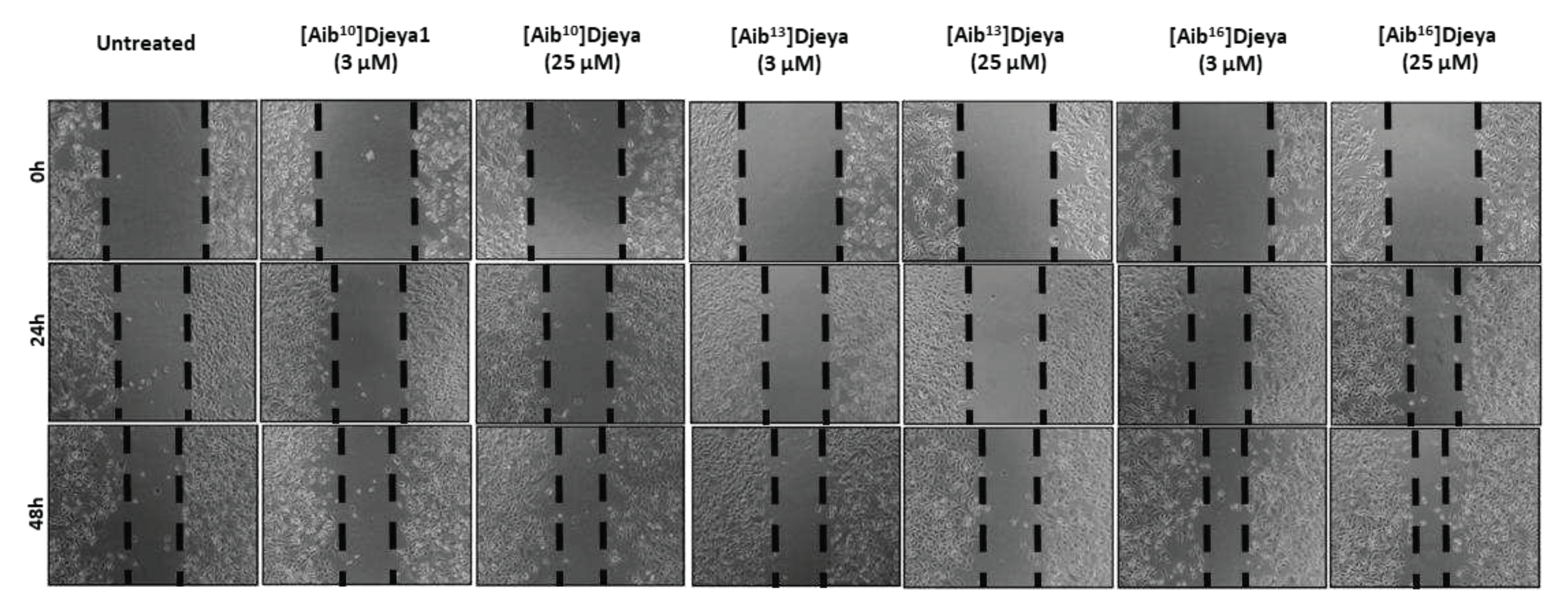
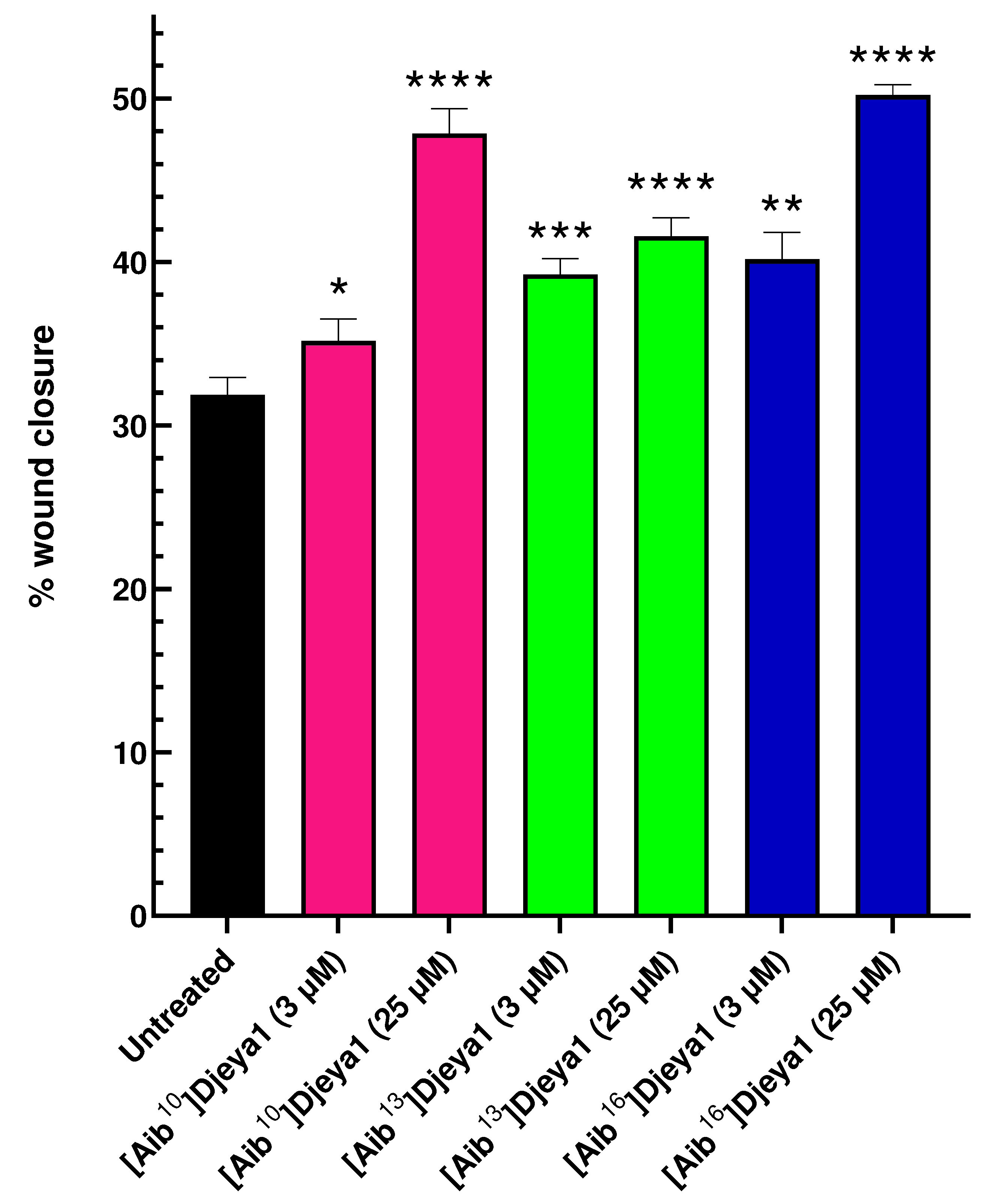
3.7. Aib-substituted Djeya1 Analogues Enhance Cell Migration.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Kurrikoff, K.; Vunk, B.; Langel, Ü. Status update in the use of cell-penetrating peptides for the delivery of macromolecular therapeutics. Expert Opin. Biol. Ther. 2021, 21, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-penetrating peptides in diagnosis and treatment of human diseases: From preclinical research to clinical application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Howl, J.; Matou-Nasri, S.; West, D.S.; Farquhar, M.; Slaninová, J.; Östenson, C.-G.; Zorko, M.; Östlund, P.; Kumar, S.; Langel, Ü.; McKeating, J.; Jones, S. Bioportide: An emergent concept of bioactive cell penetrating peptides. Cell. Mol. Life Sci. 2012, 69, 2951–2966. [Google Scholar] [CrossRef]
- Howl, J.; Jones, S. Insights into the molecular mechanisms of action of bioportides: a strategy to target protein-protein interactions. Expert Rev. Mol. Med. 2015, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.V.; Freitas, M.J.; Santiago, J.; Jones, S.; Guimarães, S.; Vijayaraghavan, S.; Publicover, S.; Colombo, G.; Howl, J.; Fardilha, M. Disruption of protein phosphatase 1 complexes with the use of bioportides as a novel approach to target sperm motility. Fertil. Steril. 2021, 115, 348–362. [Google Scholar] [CrossRef]
- Aboobaker, A.A. Planarian stem cells: a simple paradigm for regeneration. Trends Cell. Biol. 2011, 21, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Owlarn, S.; Bartscherer, K. Go ahead, grow a head! A planarian's guide to anterior regeneration. Regeneration, 2016, 24, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Robb, S.M.C.; Gotting, K.; Ross, E.; Sánchez Alvarado, A. SmedGD 2.0: the Schmidtea mediterranea genome database. Genesis 2015, 53, 535–546. [Google Scholar] [CrossRef]
- Sánchez Alvarado, A.; Newmark, P.A. , Robb, S.M.; Juste, R. The Schmidtea mediterranea database as a molecular resource for studying platyhelminthes, stem cells and regeneration. Development 2002, 129, 5659–5665. [Google Scholar] [CrossRef]
- Jones, S.; Osman, S.; Howl, J. The planarian Schmidtea mediterranea as a model system for the discovery and characterization of cell penetrating peptides and bioportides. Chem. Biol. Drug Des. 2019, 93, 1036–1049. [Google Scholar] [CrossRef]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef]
- Mannini, L.; Rossi, L.; Deri, P.; Gremigni, V.; Salvetti, A.; Saló, E.; Batistoni, R. Djeyes absent (Djeya) controls prototypic planarian eye regeneration by cooperating with the transcription factor Djsix-1. Dev. Biol. 2004, 269, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Rayapureddi, J.P.; Kattamuri, C.; Steinmetz, B.D.; Frankfort, B.J.; Ostrin, E.J.; Mardon, G.; Hedge, R.S. Eyes absent represents a class of protein tyrosine phosphatases. Nature, 2003, 426, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Hedge, R.S.; Roychoudhury, K.; Pandey, R.M. The multi-functional eyes absent proteins. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 372–385. [Google Scholar] [CrossRef]
- Eisenhoffer, G.T.; Kang,H. ; Sánchez Alvarado, A. Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell Stem Cell. 2008, 3, 327–339. [Google Scholar] [CrossRef]
- Baguñà, J. The planarian neoblast: The rambling history of its origin and some current black boxes. Int. J. Dev. Biol. 2012, 56, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Alvarado, A. Stem cells and the planarian Schmidtea mediterranea. C. R. Biol. 2007, 330, 498–503. [Google Scholar] [CrossRef]
- Xu, P.X.; Zheng, W.; Luclef, C.; Maire, P.; Maas, R.L.; Peters, H.; Xu, X. Eya1 is required for the morphogenesis of mammalian thymus. Development 2002, 129, 3033–3034. [Google Scholar] [CrossRef]
- Jones, S.; Osman, S.; Howl, J. (2018) A high-throughput synthetic platform enables the discovery of proteomimetic cell penetrating peptides and bioportides. Int. J. Pept. Res. Ther. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Subirs-Fumosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An efficient additive for peptide synthesis to replace the benzotrialzole-based HOBt and HOAt with a lower risk of explosion. Chemistry, 2009, 15, 9394–9403. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Uusna, J.; Langel, Ü.; Howl, J. Intracellular target-specific accretion of cell penetrating peptides and bioportides: Ultrastructural and biological correlates. Bioconjugate Chem. 2016, 27, 121–129. [Google Scholar] [CrossRef]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta, 2005, 1751, 119–39. [Google Scholar] [CrossRef]
- Tanaka, F.; Forster, L.S.; Pal, P.K.; Rupley, J.A. The circular dichroism of lysozyme. J. Biol. Chem. 1975, 250, 6977–6982. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P.; Ruban, A.V. Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes. Photosynth. Res. 2014, 119, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Cebriá, F.; Newmark, P.A. Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development, 2005, 132, 3691–3703. [Google Scholar] [CrossRef] [PubMed]
- Howl, J.; Howl, L.; Jones, S. The cationic tetradecapeptide mastoparan as a privileged structure for drug discovery: Enhanced antimicrobial properties of mitoparan analogues modified at position-14. Peptides 2018, 101, 95–105. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, L.; Song, Y. Eya2 is overexpressed in human prostate cancer and regulates docetaxel sensitivity and mitochondrial membrane potential through AKT/Bcl-2 signaling. BioMed. Res. Int. 2019.
- Wen, Z.; Liang, C.; Pan, Q.; Wang, Y. Eya2 overexpression promotes the invasion of human astrocytoma through the regulation of ERK/MMP9 signaling. Int. J. Mol. Med. 2017, 40, 1315–1322. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, R.; Qiu, X.; Tian, T. EYA4 promotes cell proliferation through downregulation of p27Kip1 in glioma. Cell. Physiol. Biochem. 2018, 49, 1856–1869. [Google Scholar] [CrossRef]
- Jones, S.; Martel, C.; Belzacq-Casagrande, A.S.; Brenner, C.; Howl, J. (2008). Mitoparan and target-selective chimeric analogues: membrane translocation and intracellular redistribution induces mitochondrial apoptosis. Biochim. Biophys. Acta, 2008; 1783, 849–863. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Tadjuidje, E.; Hedge, R.S. The eyes absent proteins in development and disease. Cell. Mol. Life Sci. 2013, 70, 1897–1913. [Google Scholar] [CrossRef]
- Jung, S.-K.; Jeong, D.G.; Chung, S.J.; Kim, J.H.; Park, B.C.; Tonks, N.K.; Ryu, S.E.; Kim, S.J. ; Crystal structure of ED-Eya2: insight into dual roles as a protein tyrosine phosphatase and a transcription factor. FASEB J. 2010, 24, 560–569. [Google Scholar] [CrossRef]
- Seelig, J. Thermodynamics of lipid–peptide interactions. Biochim. et Biophys. Acta 2004, 1666, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.Z.; Baldwin, R.L. Mechanism of helix induction by trifluoroethanol: A framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry 1997, 36, 8413–8421. [Google Scholar] [CrossRef] [PubMed]
- Cammers-Goodwin, A.; Allen, T.J.; Oslick, S.L.; McClure, K.F.; Lee, J.H.; Kemp, D.S. Mechanism of stabilization of helical conformations of polypeptides by water containing trifluoroethanol. J. Am. Chem. Soc. 1996, 118, 3082–3090. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Pelletieri, J.; Sánchez Alvarado, A. Cell turnover and adult tissue homeostasis: from humans to planarians. Ann. Rev. Genet. 2007, 41, 83–105. [Google Scholar] [CrossRef]
- Bohr, T.E.; Shiroor, D.A.; Adler, C.E. Planarian stem cells sense the identity of the missing pharynx to launch its targeted regeneration. eLife, 2021. [CrossRef]
- Wen, Z.; Liang, C.; Pan, Q.; Wang, Y. Eya2 overexpression promotes the invasion of human astrocytoma through the regulation of ERK/MMP9 signaling. Int. J. Mol. Med. 2017, 40, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Abnave, P.; Aboukhatwa, E.; Kosaka, N.; Thompson, J.; Hill, M.A.; Aboobaker, A.A. Epithelial-mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians. Development 2017, 144, 3440–3453. [Google Scholar] [CrossRef] [PubMed]
- Lukanowska, M.; Howl, J.; Jones, S. Bioportides: Bioactive cell-penetrating peptides that modulate cellular dynamics. Biotechnol. J., 2013, 8, 918–930. [Google Scholar] [CrossRef]
- Zorko, M.; Langel, Ü. Studies of cell-penetrating peptides by biophysical methods. Q. Rev. Biophys. 2022, 55, e3–1. [Google Scholar] [CrossRef] [PubMed]
- Infield, D.T.; Rasouli, A.; Galles, G.D.; Chipot, C.; Tajkhorshid, E.; Ahern, C.A. Cation-π interactions and their functional roles in membrane proteins. J. Mol. Biol. 2021, 433, 167035. [Google Scholar] [CrossRef] [PubMed]
- Buller, C.; Xu, X.; Marquis, V.; Schwanke, R.; Xu, P.-X. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum. Mol. Genet. 2001, 10, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Lapan, S.W.; Reddien, P.W. Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration. Cell Rep. 2012, 2, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, L.; Vartuli, R.L.; Ford, H.L.; Zhao, R. The Eya phosphatase: Its unique role in cancer. Int. J. Biochem. Cell Biol. 2018, 96, 165–170. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





