Submitted:
13 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Performance
2.2. Necrotic Enteritis Challenge and Lesion Scoring
2.3. Total RNA Extraction and Reverse Transcription
2.4. Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
3.1. Performance Parameters
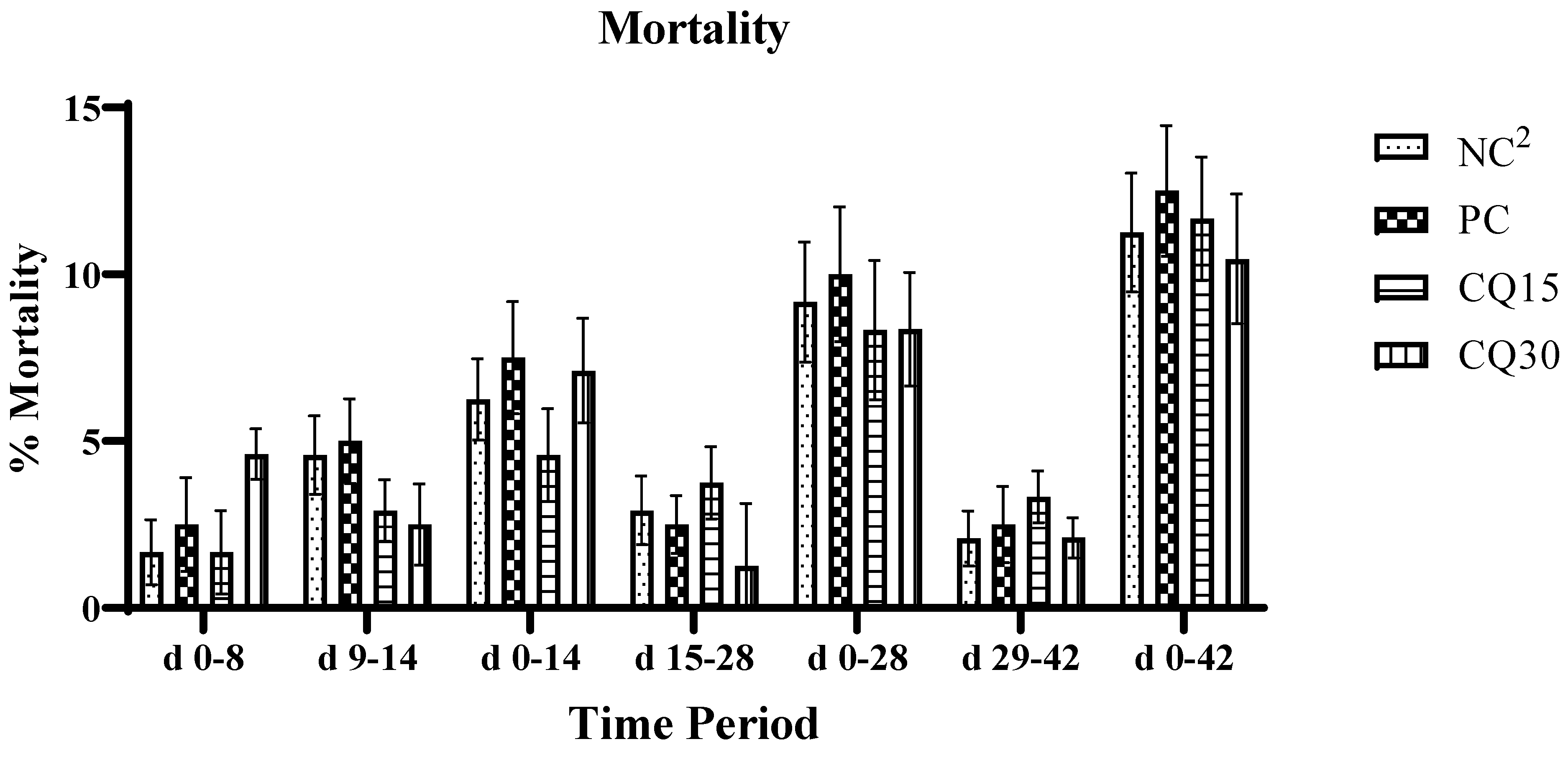
3.2. Mortality Rate
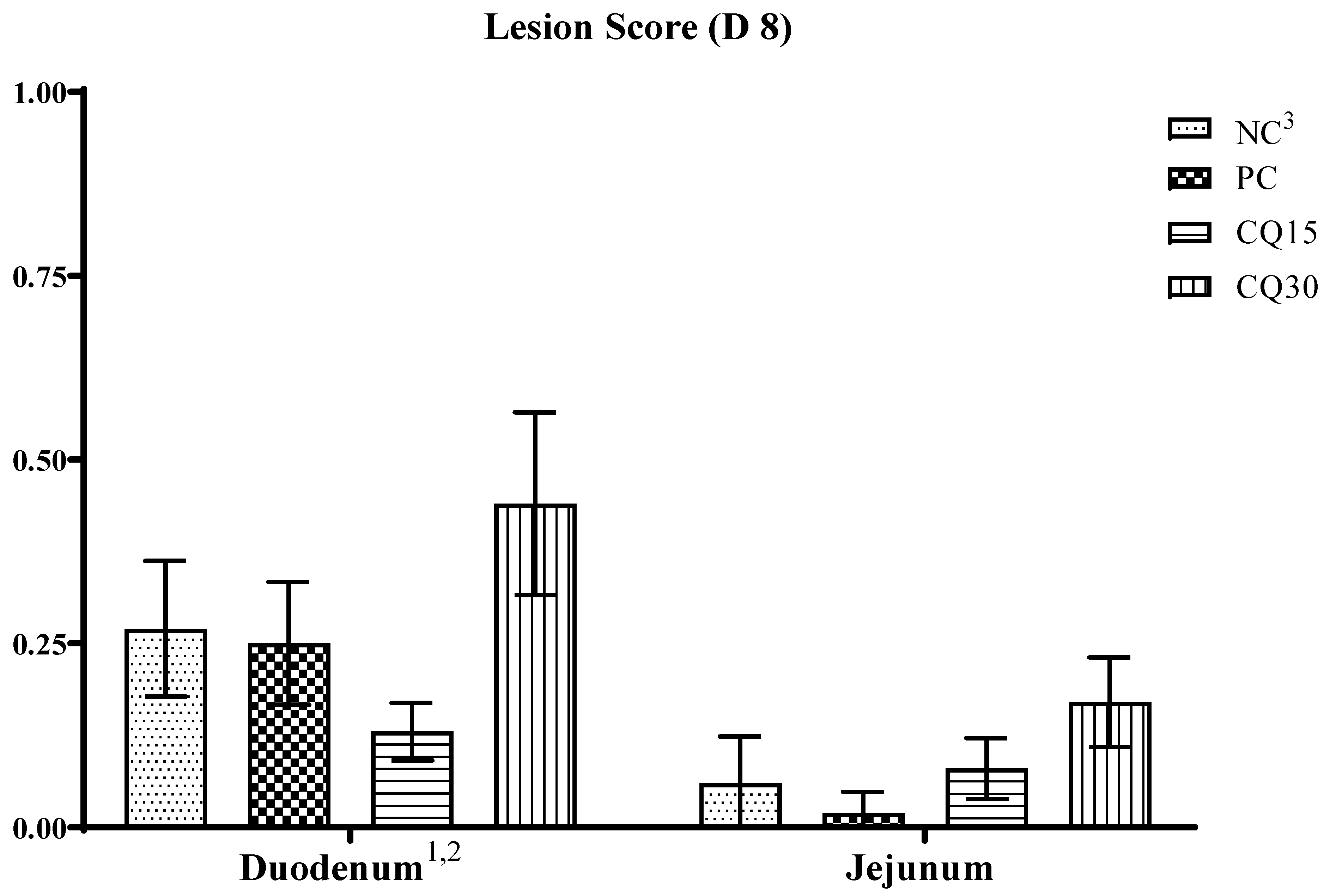
3.3. Necrotic Enteritis Lesion Scores
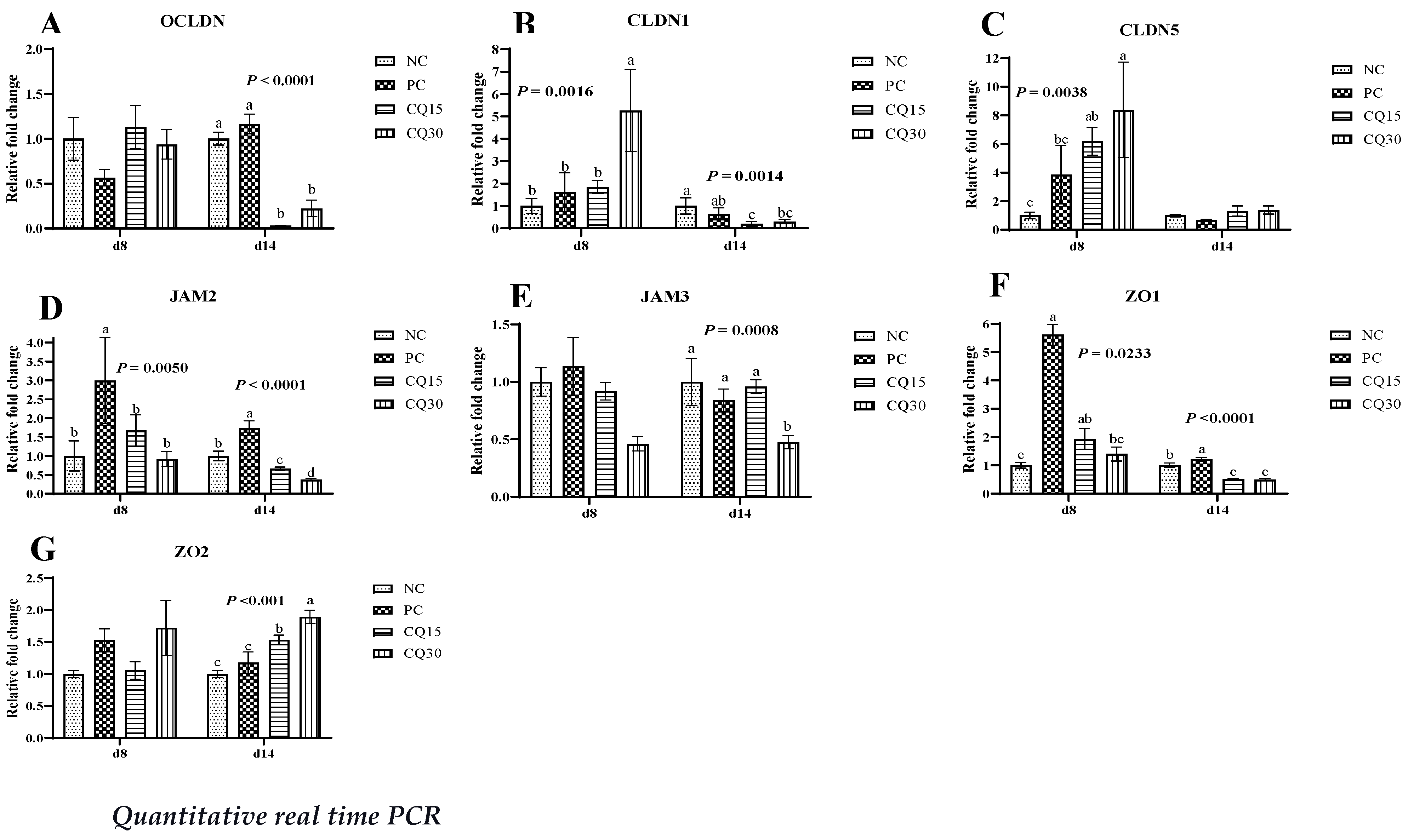
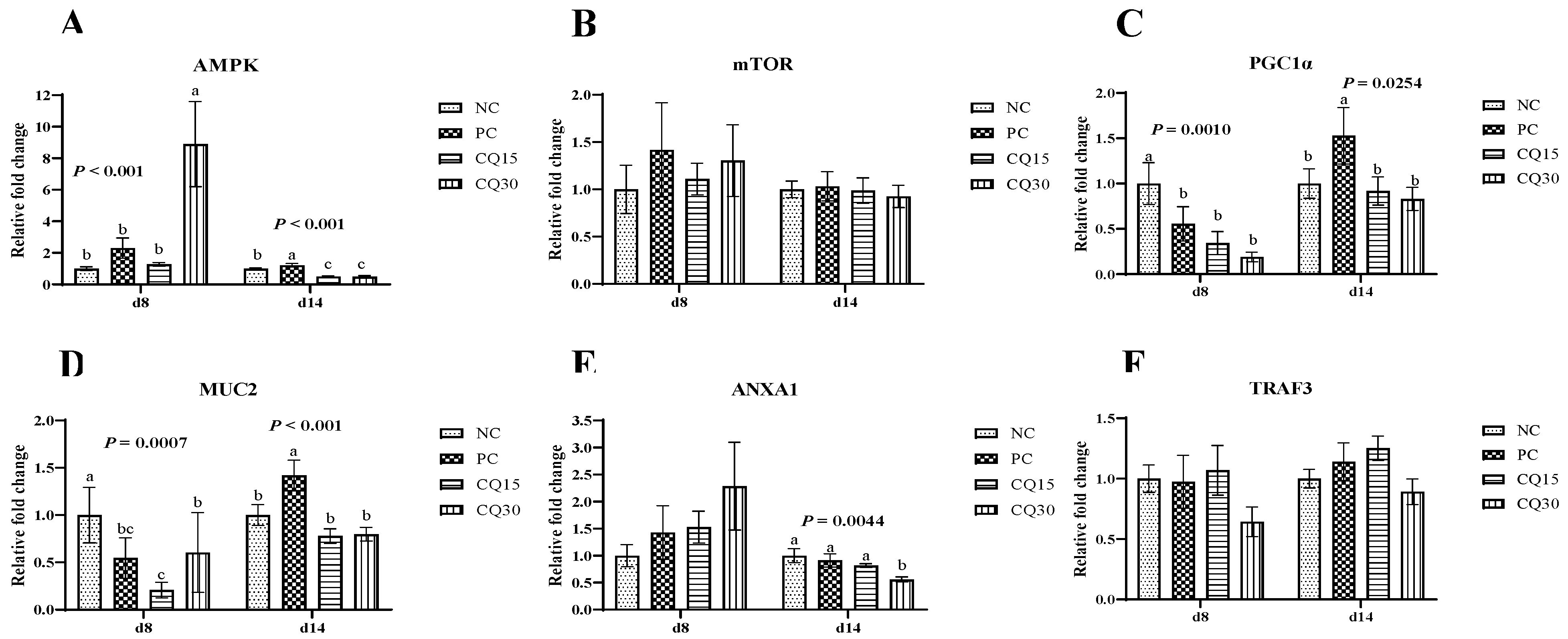
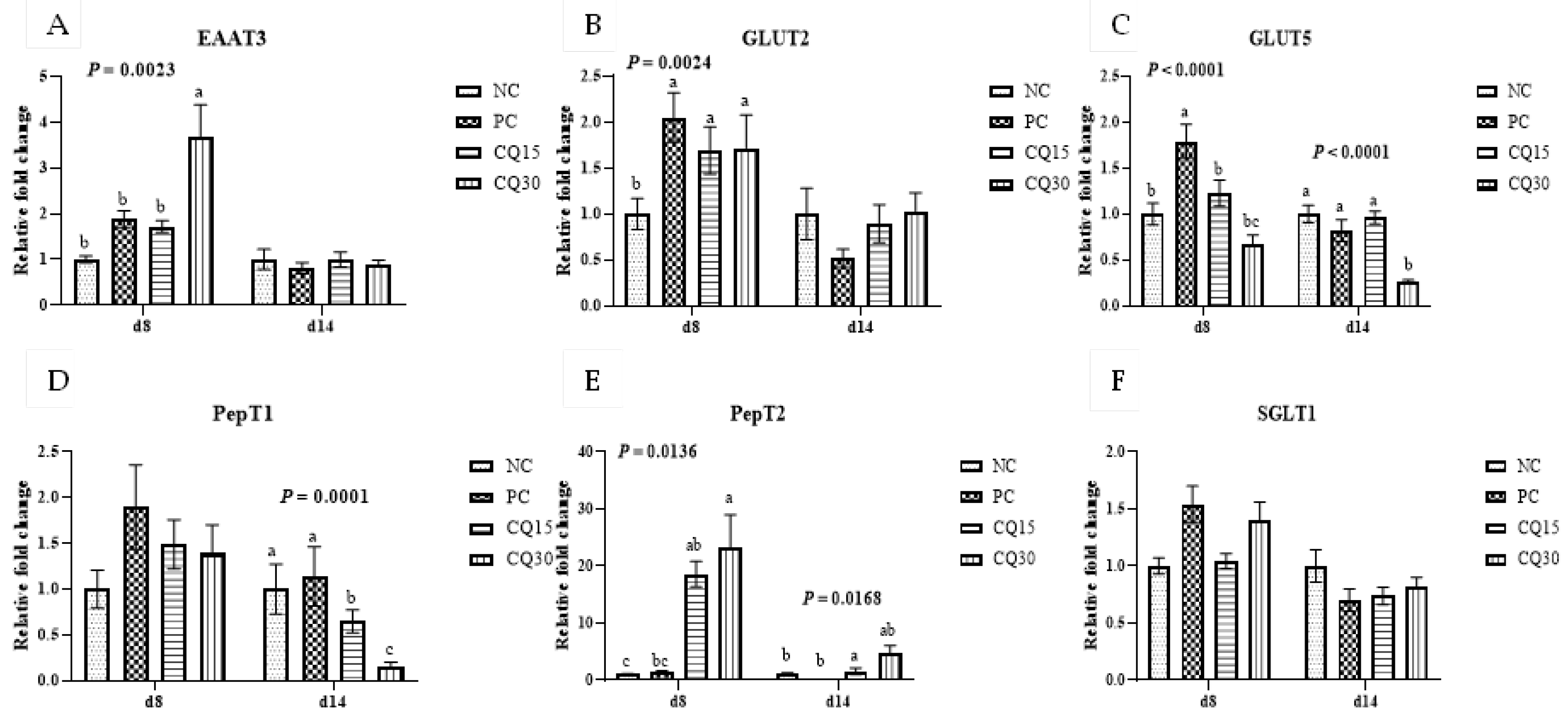
3.4. mRNA Abundance of Tight Junction Proteins, Cellular Energy Homeostasis Pathways, and Nutrient Transporters
4. Discussion
Declaration of Interest
References
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; Uzal, F.A.; Van Immerseel, F. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe. 2018, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Dalloul, R.A. Centennial Review: Recent developments in host-pathogen interactions during necrotic enteritis in poultry. Poult. Sci. 2021, 100, 101330. [Google Scholar] [CrossRef] [PubMed]
- Lepp, D.; Zhou, Y.; Ojha, S.; Mehdizadeh Gohari, I.; Carere, J.; Yang, C.; Prescott, J.F.; Gong, J. Clostridium perfringens produces an adhesive pilus required for the pathogenesis of necrotic enteritis in poultry. J. Bacteriol. 2021, 203. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wade, B.; Keyburn, A.L. The true cost of necrotic enteritis. World Poult. 2015, 31, 16–17. [Google Scholar]
- Bedford, M. Removal of antibiotic growth promoters from poultry diets: implications and strategies to minimize subsequent problems. World’s Poult. Sci. J. 2000, 56, 347–365. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J. D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Rood, J.I.; Moore, R.J.; Titball, R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009, 17, 32–36. [Google Scholar] [CrossRef]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]
- Castanon, J.I.R. History of the use of antibiotics as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef]
- Amad, A.; Männer, K.; Wendler, K.; Neumann, K; Zentek J. Effects of a phytogenic feed additive on growth performance and ileal nutrient digestibility in broiler chickens. Poult. Sci. 2011, 90, 2811–2816. [CrossRef] [PubMed]
- Paraskeuas, V.; Mountzouris, K.C. Modulation of broiler gut microbiota and gene expression of Toll-like receptors and tight junction proteins by diet type and inclusion of phytogenics. Poult. Sci. 2019, 98, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Bafundo, K.W.; Johnson, A.B.; Mathis, G.F. The effects of a combination of Quillaja saponaria and Yucca schidigera on Eimeria spp., in broiler chickens. Avian Dis. 2020, 64, 300–304. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J. F. Phytogenic feed Additives in poultry: Achievements, prospective and challenges. Animals (Basel). 2021, 11. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chowdhury, M.A.K.; Hou, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. 2015, 4, 137–156. [Google Scholar] [CrossRef]
- Greene, E.S.; Cauble, R.; Kadhim, H.; de Almeida Mallmann, B.; Gu, I.; Lee, S.O.; Orlowski, S.; Dridi, S. Protective effects of the phytogenic feed additive "comfort" on growth performance via modulation of hypothalamic feeding- and drinking-related neuropeptides in cyclic heat-stressed broilers. Domest. Anim. Endocrinol. 2021, 74, 106487. [Google Scholar] [CrossRef]
- Youssef, I.M.I.; Männer, K.; Zentek, J. Effect of essential oils or saponins alone or in combination on productive performance, intestinal morphology and digestive enzymes' activity of broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl). 2021, 105, 99–107. [Google Scholar] [CrossRef]
- Bafundo, K.W.; Männer, K.; Duerr, I. The combination of quillaja and yucca saponins in broilers: effects on performance, nutrient digestibility and ileal morphometrics. Br. Poult. Sci. 2021, 62, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Bafundo, K.W.; Duerr, I.; McNaughton, J.L.; Johnson, A.B. The effects of a quillaja and yucca combination on performance and carcass traits of coccidia-vaccinated broilers exposed to an enteric disease challenge. Poult. Sci. 2021, 100, 101391. [Google Scholar] [CrossRef]
- Bafundo, K.W.; Gomez, L; Lumpkins, B.; Mathis, G.F.; McNaughton, J.L.; Duerr, I. Concurrent use of saponins and live coccidiosis vaccines: the influence of a quillaja and yucca combination on anticoccidial effects and performance results of coccidia-vaccinated broilers. Poult. Sci 2021, 100, 100905.
- Cheeke, P. Actual and potential applications of Yucca schidigera and Quillaja saponaria saponins in human and animal nutrition. J. Anim. Sci. 2000, 77, 1–10. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Fleck, J.D.; Betti, A.H.; Pereira da Silva, F.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: particular chemical characteristics and biological activities. Molecules. 2019, 24. [Google Scholar] [CrossRef]
- Calik, A.; Omara, I.I.; White, M.B.; Evans, N.P.; Karnezos, T.P; Dalloul, R.A. Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms. 2019, 7. [Google Scholar] [CrossRef]
- Emami, N.K.; Calik, A.; White, M.B.; Kimminau, E.A.; Dalloul, R.A. Effect of probiotics and multi-component feed additives on microbiota, gut barrier, and immune responses in broiler chickens during subclinical necrotic enteritis. Front. Vet. Sci. 2020, 7, 572142. [Google Scholar] [CrossRef]
- Emami, N.K.; White, M.B.; Calik, A.; Kimminau, E.A.; Dalloul, R.A. Managing broilers gut health with antibiotic-free diets during subclinical necrotic enteritis. Poult. Sci. 2021, 100, 101055. [Google Scholar] [CrossRef]
- Prescott, J.F.; Sivendra, R.; Barnum, D.A. The use of bacitracin in the prevention and treatment of experimentally induced necrotic enteritis in the chicken. Can. Vet. J. 1978, 19, 181–183. [Google Scholar]
- Hofacre, C.L.; Smith, J.A.; Mathis, G.F. An optimist's view on limiting necrotic enteritis and maintaining broiler gut health and performance in today's marketing, food safety, and regulatory climate. Poult. Sci. 2018, 97, 1929–1933. [Google Scholar] [CrossRef]
- Amad, A.; Männer, K; Wendler, K; Neumann, K; Zentek J. Effects of a phytogenic feed additive on growth performance and ileal nutrient digestibility in broiler chickens. Poult. Sci. 2011, 90, 2811–2816. [CrossRef]
- Cho, J.; Kim, H; Kim, I. Effects of phytogenic feed additive on growth performance, digestibility, blood metabolites, intestinal microbiota, meat color and relative organ weight after oral challenge with Clostridium perfringens in broilers. Livest. Sci. 2014, 160, 82–88. [CrossRef]
- Du, E.; Wang, W; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19–25. [CrossRef]
- Pirgozliev, V.; Beccaccia, A.; Rose, S.P.; Bravo, D. Partitioning of dietary energy of chickens fed maize- or wheat-based diets with and without a commercial blend of phytogenic feed additives. J. Anim. Sci. 2015, 93, 1695–1702. [Google Scholar] [CrossRef]
- Pirgozliev, V.; Mansbridge, S.C.; Rose, S.P.; Lillehoj, H.S.; Bravo, D. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult. Sci. 2019, 98, 3443–3449. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Calik, A.; White, M.B.; Young, M.; Dalloul, R.A. Necrotic enteritis in broiler chickens: The role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms. 2019, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Suzuki, H.; Tani, K.; Nishikawa, K.; Irie, K.; Ogura, Y.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science. 2015, 347, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Golder, H. M.; Geier, M.S.; Forder, R.E.; Hynd, P.I.; Hughes, R.J. Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. Br. Poult. Sci. 2011, 52, 500–506. [Google Scholar] [CrossRef]
- Stefanello, C.; Rosa, D.P.; Dalmoro, Y.K.; Segatto, A. L.; Vieira, M.S.; Moraes, M.L.; Santin, E. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci. 2020, 6, 491. [Google Scholar] [CrossRef]
- Fathima, S.; Hakeem, W.G.A.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic enteritis in broiler chickens: A review on the pathogen, pathogenesis, and prevention. Microorganisms. 2022, 10, 1958. [Google Scholar] [CrossRef]
- Daneshmand, A.; Kermanshahi, H.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M.; Alizadeh.; Aldawoodi, A. Effects of cLFchimera peptide on intestinal morphology, integrity, microbiota, and immune cells in broiler chickens challenged with necrotic enteritis. Sci. Rep. 2020, 10, 17704. [CrossRef] [PubMed]
- Flees, J.J.; Ganguly, B.; Dridi, S. Phytogenic feed additives improve broiler feed efficiency via modulation of intermediary lipid and protein metabolism-related signaling pathways. Poult. Sci. 2020, 100, 100963. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. L.; Ga, C. Q.; Li, X. G.; Jin, C. L.; Wang, D.; Shu, G.; Wang, W. C.; Kong, X. F.; Yao, K.; Yan, H. C.; Wang, X. Q. EAAT3 promotes amino acid transport and proliferation of porcine intestinal epithelial cells. Oncotarget. 2016, 7, 38681–38692. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.R.; Romero, L.F.; Persia, M.E. Effects of protease, phytase and a Bacillus sp. direct-fed microbial on nutrient and energy digestibility, ileal brush border digestive enzyme activity and cecal short-chain fatty acid concentration in broiler chickens. PLoS One. 2014, 9, 101888. [Google Scholar] [CrossRef]
- Reyer, H.; Zentek, J.; Manner, K.; Youssef, I.M.I.; Aumiller, T.; Weghuber, J.; Mueller, A.S. Possible molecular mechanisms by which an essential oil blend from star anise, rosemary, thyme, and oregano and saponins increase the performance and ileal protein digestibility of growing broilers. J. Agric. Food Chem. 2017, 65, 6821–6830. [Google Scholar] [CrossRef] [PubMed]
- Zwarycz, B.; Wong, E.A. Expression of the peptide transporters PepT1, PepT2, and PHT1 in the embryonic and post hatch chick. Poult. Sci. 2013, 92, 1314–1321. [Google Scholar] [CrossRef]
- Chen, H.; Pan, Y.X.; Wong, E.A.; Webb, K.E., Jr. Dietary protein level and stage of development affect expression of an intestinal peptide transporter (cPepT1) in chickens. J. Nutr. 2005, 135, 193–198. [Google Scholar] [CrossRef]
- Farrokhifar, S.H.; Ali Jafari, R.; Erfani Majd, N.; Fatemi Tabatabaee, S.R.; Mayahi, M. Effects of dietary vitamin E on mucosal maltase and alkaline phosphatase enzyme activities and on the amount of mucosal malonyldialdehyde in broiler chickens. Vet. Res. Forum. 2013, 4, 221–225. [Google Scholar]
| Feeding Phase (days) | |||
|---|---|---|---|
|
Ingredients (%) |
Starter (1-14) |
Grower (14-28) |
Finisher (28-42) |
| Corn (7.81% CP) | 59.53 | 64.12 | 65.70 |
| Soybean meal (48% CP) | 33.5 | 28.80 | 26.86 |
| Soybean Oil (9000 kcal/kg) | 2.18 | 2.60 | 3.50 |
| Dicalcium Phosphate (18.5% P, 22% Ca) | 2.05 | 1.92 | 1.70 |
| Calcium Carbonate (37% Calcium) | 1.11 | 1.00 | 0.90 |
| Sodium Chloride | 0.3 | 0.3 | 0.3 |
| Sodium Bicarbonate | 0.07 | 0.07 | 0.05 |
| DL-Methionine (990 g/kg)b | 0.38 | 0.34 | 0.29 |
| L-Lysine Hydrochloride (788g L-Lysine/kg)c | 0.37 | 0.35 | 0.24 |
| L-Threonine (985g/kg)d | 0.15 | 0.14 | 0.10 |
| Vitamin/Trace Mineral Premixe | 0.36 | 0.36 | 0.36 |
| Calculated Analysis (% unless specified) | |||
| ME (kCal/kg) | 3007 | 3087 | 3168 |
| Crude protein | 21.81 | 19.90 | 18.94 |
| Total phosphorus | 0.76 | 0.71 | 0.66 |
| Available phosphorus | 0.45 | 0.42 | 0.38 |
| Calcium | 0.90 | 0.84 | 0.76 |
| Chlorine | 0.33 | 0.33 | 0.29 |
| Sodium | 0.16 | 0.16 | 0.15 |
| Potassium | 0.85 | 0.77 | 0.73 |
| Methionine | 0.67 | 0.61 | 0.55 |
| Methionine+Cysteine | 0.98 | 0.89 | 0.82 |
| Lysine | 1.32 | 1.19 | 1.05 |
| Threonine | 0.86 | 0.78 | 0.71 |
| Linoleic acid | 1.44 | 1.52 | 1.55 |
| Dietary cation-anion balance (mEq) | 194 | 174 | 170 |
| Target gene | Primer sequence | Accession # |
|---|---|---|
| Occludin (OCLN) |
F- CCGTAACCCCGAGTTGGAT R- ATTGAGGCGGTCGTTGATG |
NM_205128.1 |
| Claudin 1 (CLDN1) |
F- GTGTTCAGAGGCATCAGGTATC R- TCAGGTCAAACAGAGGTACAA |
NM_001013611.2 |
| Claudin 5 (CLDN5) |
F- AGGTGTCAGCCTTCATCGAC R- CCAGGATGGAATCGTACACC |
NM_204201.1 |
| Junctional Adhesion Molecule 2 (JAM2) |
F- CTGCTCCTCGGGTACTTGG R- CCCTTTTGAAAATTTGTGCTTGC |
XM_015299112.3 |
| Juncitonal Adhesion Molecule 3 (JAM3) |
F- CCAGAGTGTTGAGCTGTCCT R- AGAATTTCTGCCCGAGTTGC |
XM_417876.6 |
| Zonula Occluden 1 (ZO1) |
F- GGAGTACGAGCAGTCAACATAC R- GAGGCGCACGATCTTCATAA |
XM_413773 |
| Zonula Occluden (ZO2) |
F- GCGTCCCATCCTGAGAAATAC R- CTTGTTCACTCCCTTCCTCTTC |
NM_204918.1 |
| AMP activated protein kinase (AMPK) |
F- ATCTGTCTCGCCCTCATCCT R- CCACTTCGCTCTTCTTACACCTT |
NM_001039603.1 |
| Mammalian target of rapamycin (mTOR) |
F- CATGTCAGGCACTGTGTCTATTCTC R- CTTTCGCCCTTGTTTCTTCACT |
XM_417614.6 |
| Mucin 2 (MUC2) |
F- TTCATGATGCCTGCTCTTGTG R- CCTGAGCCTTGGTACATTCTTGT |
XM_421035 |
| Annexin 1 (ANXA1) |
F- CTGCCTGACTGCCCTTGTGA R- GTTTGTGTCGTGTTCCACTCCC |
NM_206906.1 |
| TNF Receptor Associated Factor 3 (TRAF3) |
F- CTGAGAAAAGATTTGCCAGACCA R- CATGAAACCATGACACACGGG |
XM_040672268.1 |
| Excitatory amino acid transporter 3 (EAAT3) |
F- GGTGAAGGCGGACAGGAA R- TGCTGAGCAGGAGCCAGTT |
XM_424930.7 |
| Glucose transporter 2 (GLUT2) |
F- GAAGGTGGAGGAGGCCAAA R- TTTCATCGGGTCACAGTTTCC |
NM_207178.1 |
| Glucose transporter (GLUT5) |
F- CCTCAGCATAGTGTGTGTCATCATT R- GGATCGGACTGGCTCCAA |
XM_040689119.1 |
| Peptide transporter 1 (PepT1) |
F- GACAACTTTTCTACAGCCATCTACCA R- CCCAGGATGGGCGTCAA |
NM_204365.1 |
| Peptide transporter 2 (PepT2) |
F- TGAAAAACCGCTCCCATCA R- TGTTCCGATGCCCAGTCAA |
NM_001319028.1 |
| Sodium dependent glucose cotransporter 1 (SGLT1) |
F- AGCATTTCAGCATGGTGTGTCT R- TGCTCCTATCTCAGGGCAGTTC |
NM_001293240.1 |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) |
F- CCTAGGATACACAGAGGACCAGGTT R- GGTGGAGGAATGGCTGTCA |
NM_204305.1 |
| Dietary Treatments1 | Statistics | |||||
|---|---|---|---|---|---|---|
| NC | PC | CQ15 | CQ30 | SEM2 | P-value | |
| Days 0 to 8* | ||||||
| BW | 175.22 | 176.23 | 180.16 | 176.02 | 2.09 | 0.3593 |
| ADG | 21.93 | 22.35 | 22.55 | 22.42 | 0.23 | 0.2752 |
| ADFI | 18.85 | 19.59 | 20.34 | 19.50 | 0.37 | 0.0625 |
| FCR | 0.86 | 0.88 | 0.90 | 0.87 | 0.02 | 0.3593 |
| Days 9 to 14 | ||||||
| ADG | 34.02 | 36.07 | 34.70 | 32.42 | 1.30 | 0.2724 |
| ADFI | 45.26b | 46.11ab | 48.51a | 45.07b | 0.88 | 0.0373 |
| FCR | 1.34 | 1.29 | 1.42 | 1.39 | 0.06 | 0.3687 |
| Days 0 to 14 | ||||||
| BW | 364.13 | 376.94 | 379.01 | 365.46 | 5.72 | 0.1698 |
| ADG | 31.03 | 31.93 | 31.56 | 31.55 | 0.56 | 0.7272 |
| ADFI | 34.16b | 34.59b | 36.60a | 35.45ab | 0.49 | 0.0081 |
| FCR | 1.10 | 1.09 | 1.16 | 1.12 | 0.02 | 0.1278 |
| Days 15 to 28 | ||||||
| ADG | 69.02 | 69.77 | 72.32 | 69.70 | 1.34 | 0.3370 |
| ADFI | 105.99 | 106.41 | 108.88 | 107.80 | 1.51 | 0.5229 |
| FCR | 1.54 | 1.53 | 1.51 | 1.55 | 0.01 | 0.1982 |
| Days 0 to 28 | ||||||
| BW | 1348.78 | 1370.61 | 1381.27 | 1389.33 | 22.61 | 0.1679 |
| ADG | 53.74ab | 55.59a | 55.81a | 53.09b | 0.78 | 0.0468 |
| ADFI | 75.14 | 76.90 | 78.00 | 75.06 | 0.90 | 0.0782 |
| FCR | 1.40 | 1.38 | 1.40 | 1.41 | 0.01 | 0.1705 |
| Days 29 to 42 | ||||||
| ADG | 111.73 | 112.65 | 114.45 | 111.66 | 3.02 | 0.9061 |
| ADFI | 177.38 | 182.62 | 182.93 | 175.35 | 2.25 | 0.0553 |
| FCR | 1.60 | 1.62 | 1.60 | 1.57 | 0.03 | 0.7096 |
| Days 0 to 42 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




