Submitted:
27 June 2023
Posted:
28 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Extraction of Total RNA and cDNA Synthesis
2.3. Molecular cloning and characterization
2.4. siRNA synthesis
2.5. Preparation of AG homogenate and recombinant IAG
2.6. In vitro experiments
2.7. Gene expression analysis
3. Results
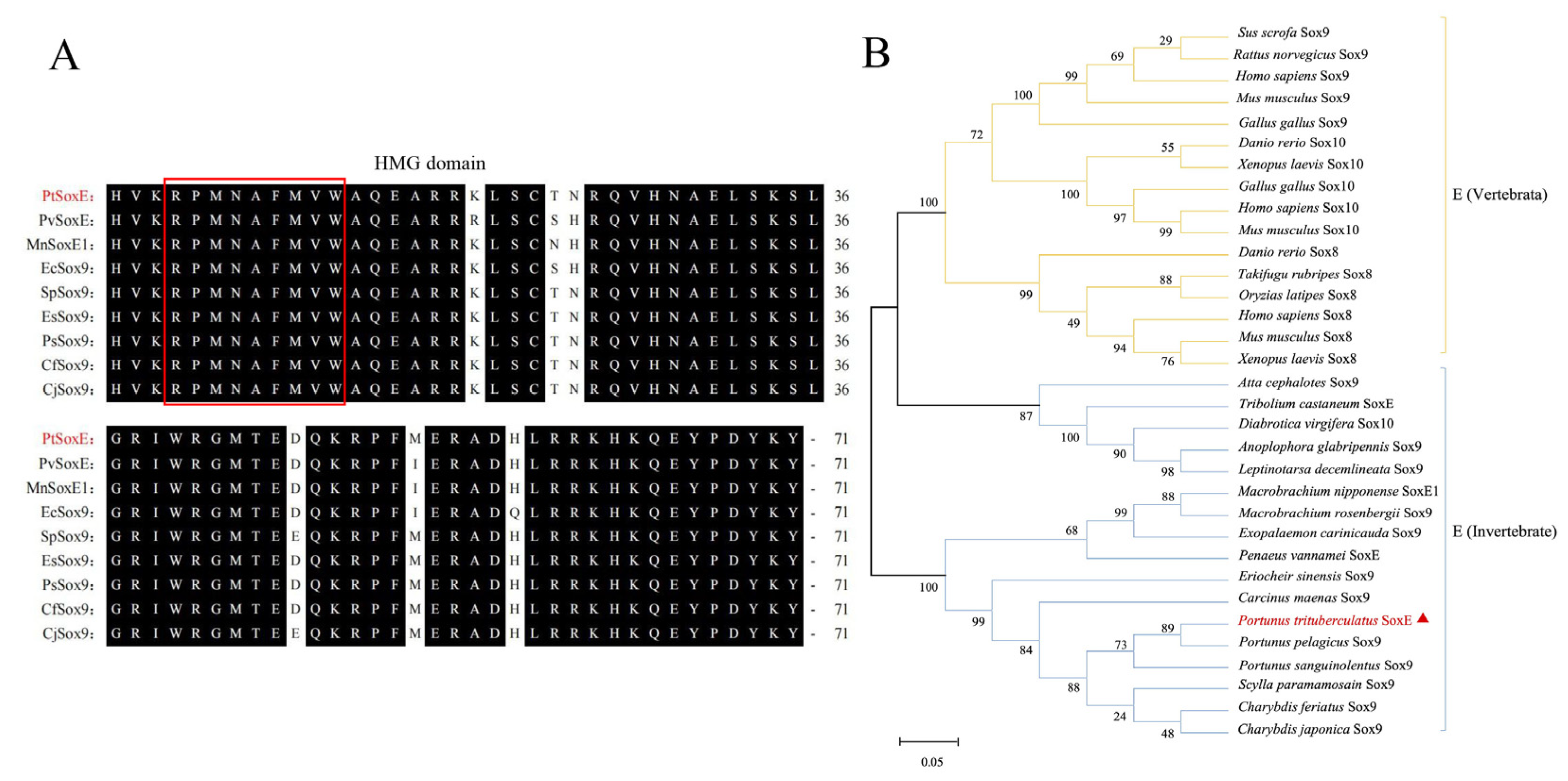
3.1. Molecular characterization of PtSoxE
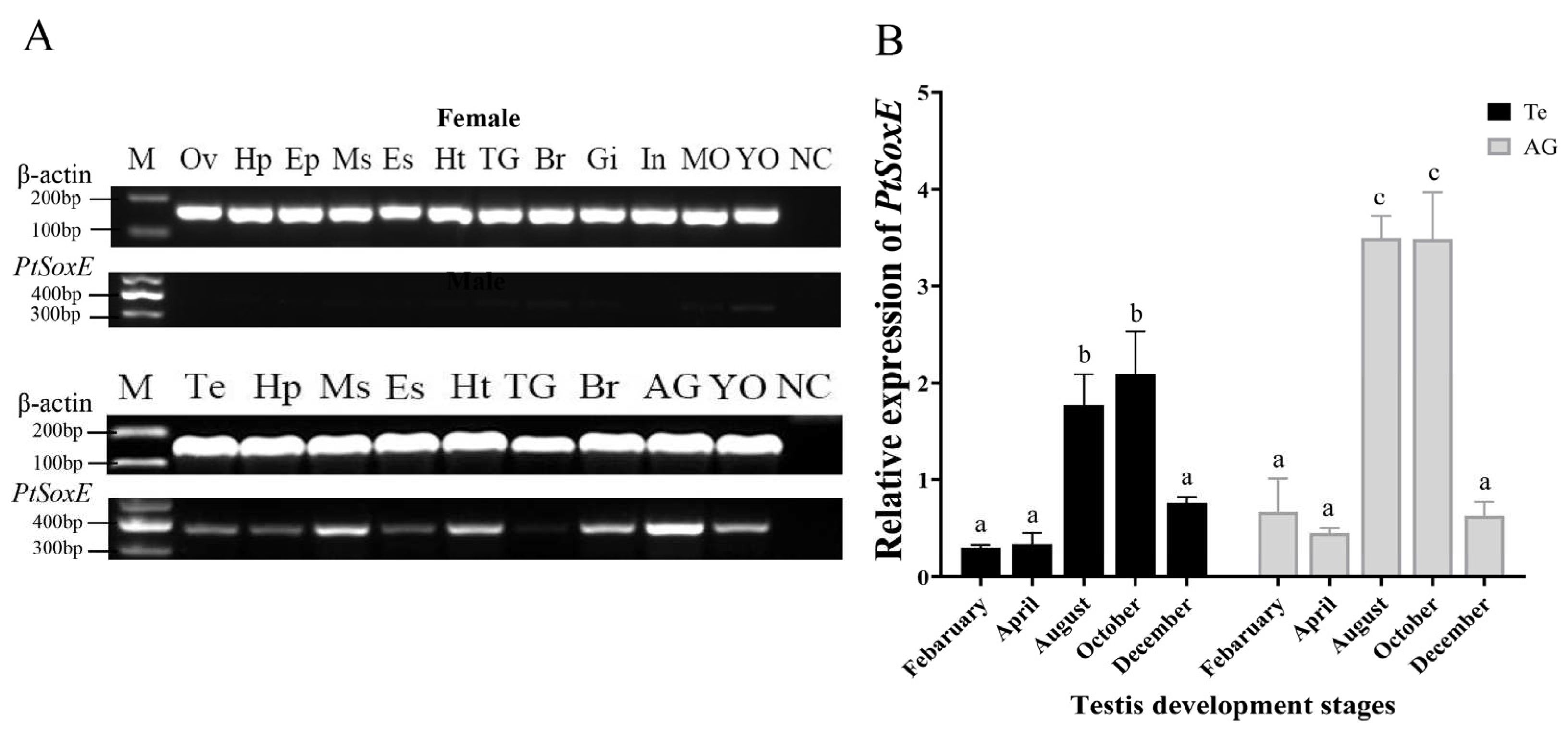
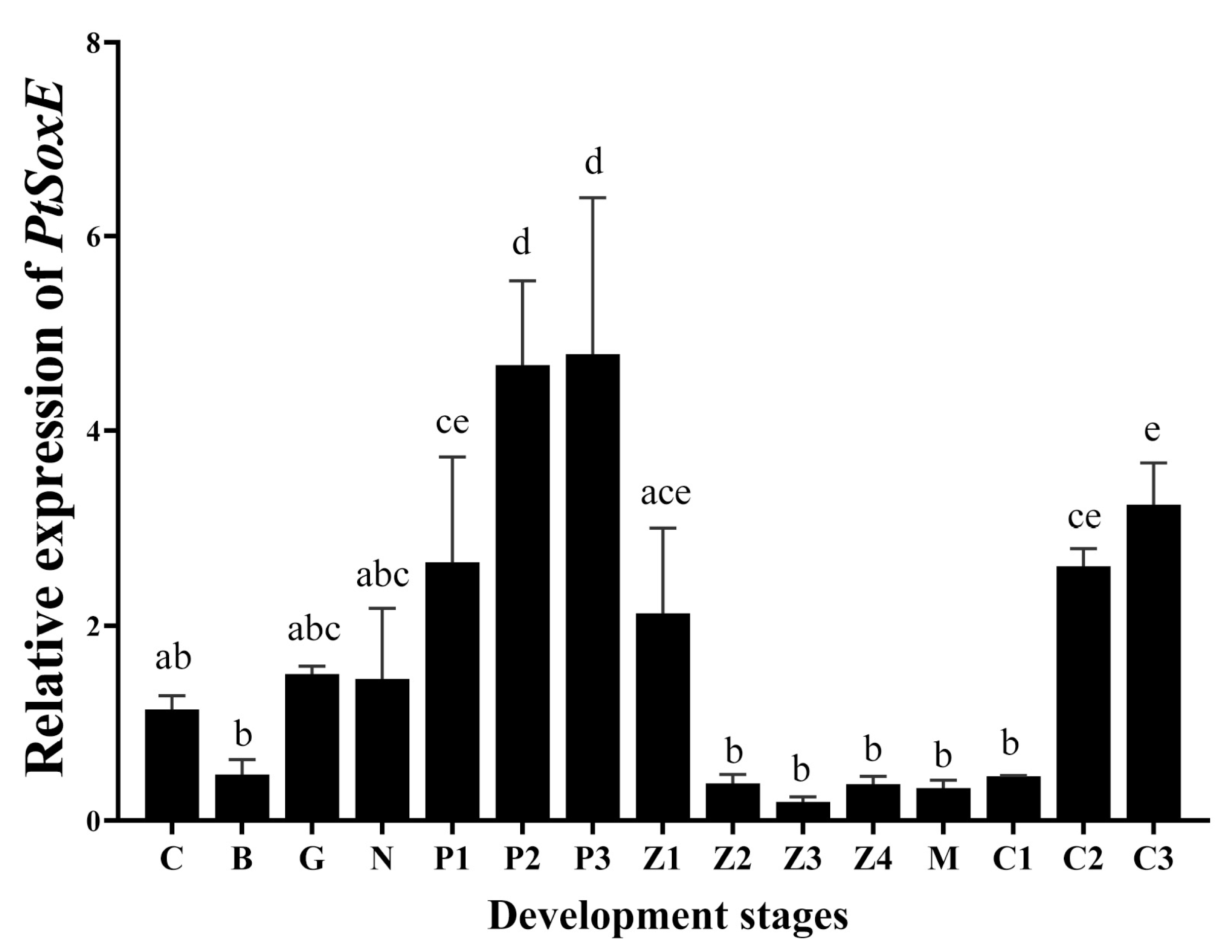
3.2. Spatial and temporal patterns of PtSoxE expression
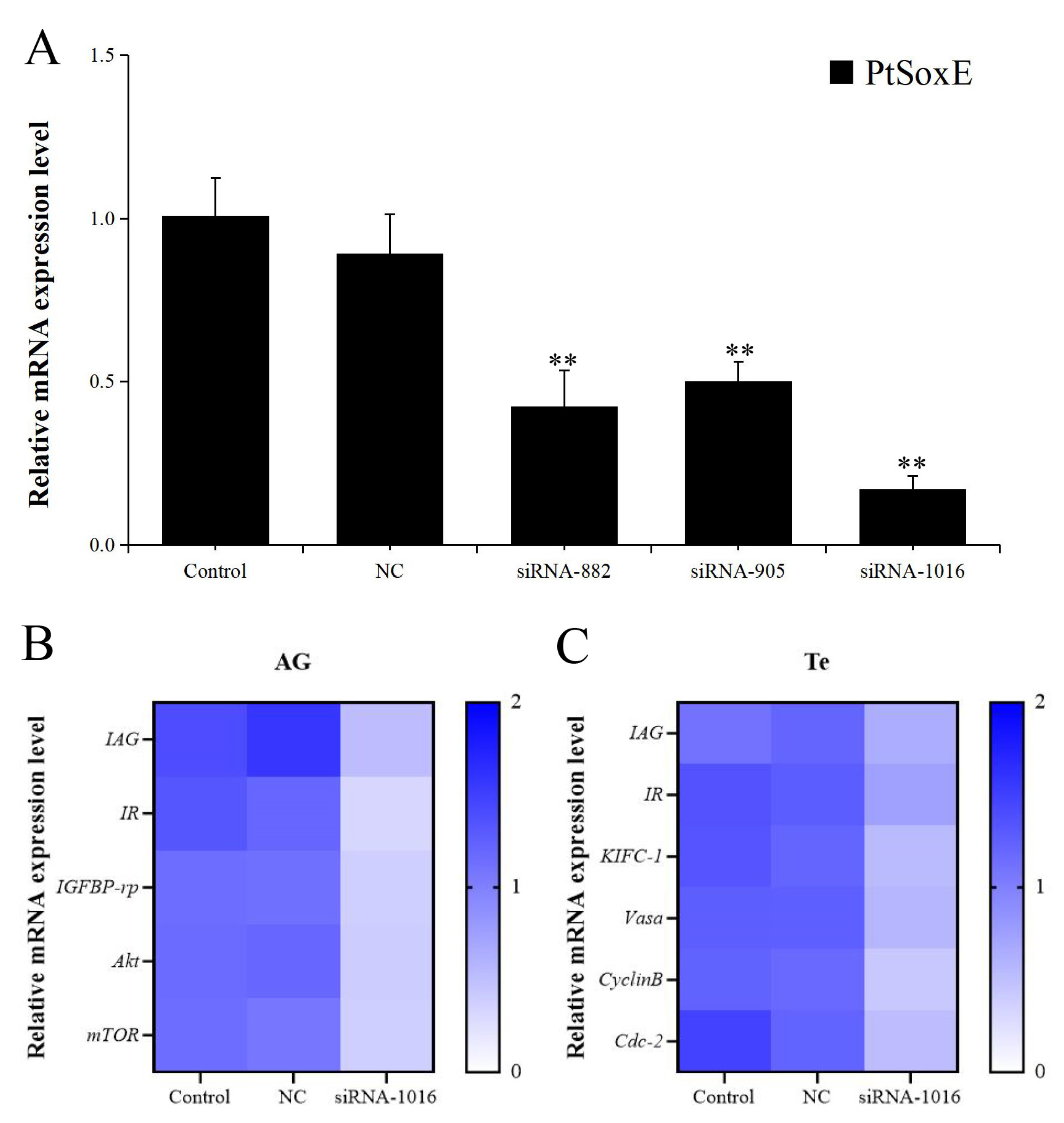
3.3. Effects of PtSoxE siRNA on gene expression in AG and testis
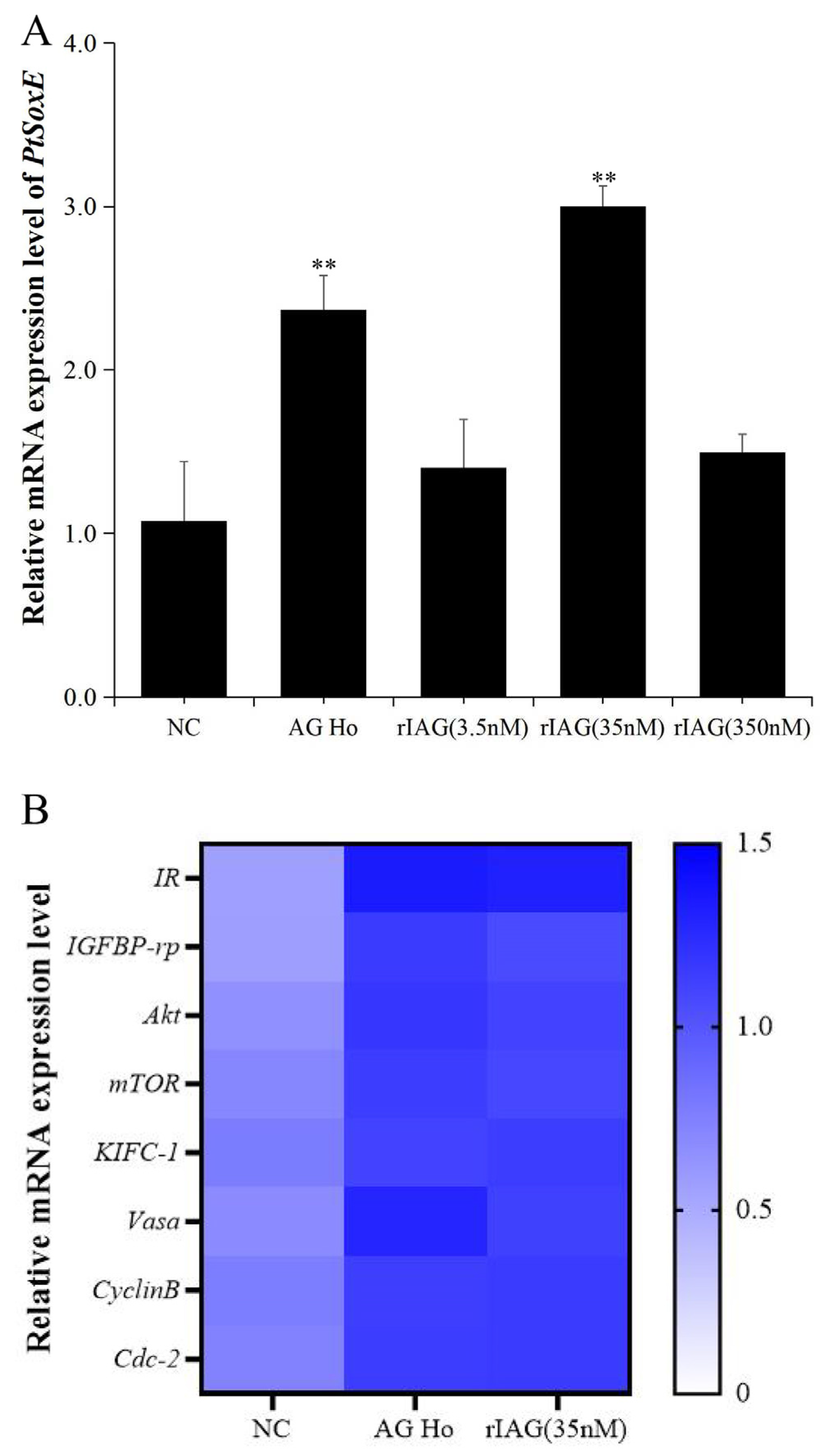
3.4. Effects of AG homogenate and rIAG on PtSoxE expression in testis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kamachi, Y.; Kondoh, H. Sox proteins: regulators of cell fate specification and differentiation. Development 2013, 140, 4129–4144. [Google Scholar] [CrossRef]
- Gubbay, J.; Collignon, J.; Koopman, P.; Capel, B.; Economou, A.; Münsterberg, A.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990, 346, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.-M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Bonatto Paese, C.L.; Leite, D.J.; Schönauer, A.; McGregor, A.P.; Russell, S. Duplication and expression of Sox genes in spiders. BMC Evolutionary Biology 2018, 18, 205. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Wang, M.; Zhang, W.; Pan, J.; Qin, Q.; Zhong, L.; Shao, J.; Sun, M.; Jiang, H.; Bian, W. Genome-wide identification, phylogeny and expressional profile of the Sox gene family in channel catfish (Ictalurus punctatus). Comparative Biochemistry and Physiology Part D: Genomics and Proteomics 2018, 28, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, B.; Du, H. A review on Sox genes in fish. Reviews in Aquaculture 2021, 13, 1986–2003. [Google Scholar] [CrossRef]
- Mei, J.; Gui, J.F. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Science China Life Sciences 2015, 58, 124–136, http://ir.ihb.ac.cn/handle/342005/20209. [Google Scholar] [CrossRef]
- Nitzan, G.; Chris, R. F.; Sophie, W. ; S. Alexandra, G.M.; Isabella, M.S.; Shiela, C.S.; Ryohei, S.; Francis, P.; Danielle, M.M.; Robin, L.B. Sex reversal following deletion of a single distal enhancer of Sox9. Science 2018, 360, 1469–1473. [Google Scholar] [CrossRef]
- Takehana, Y.; Matsuda, M.; Myosho, T.; Suster, M.L.; Kawakami, K.; Shin, I.T. et al. Co-option of Sox3 as the male-determining factor on the Y chromosome in the fish Oryzias dancena. Nature Communication 2014, 5, 4157. [Google Scholar] [CrossRef]
- Schartl, M.; Schories, S.; Wakamatsu, Y.; et al. Sox5 is involved in germ-cell regulation and sex determination in medaka following co-option of nested transposable elements. BMC Biology 2018, 16, 1–17. [Google Scholar] [CrossRef]
- Canning, C.A.; Lovell-Badge, R. Sry and sex determination: how lazy can it be? Trends in Genetics 2002, 18, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Koopman, P. Sex determination: a tale of two Sox genes. Trends in Genetics 2005, 21, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Polanco, J.C.; Wilhelm, D.; Davidson, T.L.; Knight, D.; Koopman, P. Sox10 gain-of-function causes XX sex reversal in mice: implications for human 22q-linked disorders of sex development. Human Molecular Genetics 2010, 19, 506–516. [Google Scholar] [CrossRef]
- Adolfi, M.C.; Carreira, A.C.O.; Jesus, L.W.O.; Bogerd, J.; Funes, R.M.; Schartl, M.; Sogayar, M.C.; Borella, M.I. Molecular cloning and expression analysis of dmrt1 and Sox9 during gonad development and male reproductive cycle in the lambari fish, Astyanax altiparanae. Reproductive Biology and Endocrinology 2015, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, H.; Tveiten, H.; Torgersen, J.S.; Andersen, Ø. Divergent and sex-dimorphic expression of the paralogs of the Sox9-Amh-Cyp19a1 regulatory cascade in developing and adult atlantic cod (Gadus morhua L. ). Molecular Reproduction and Development 2013, 80, 358–370. [Google Scholar] [CrossRef]
- Zheng, J.; Jia, Y.; Liu, S.; Chi, M.; Cheng, S.; Gu, Z. Molecular characterization and expression profiles of transcription factor Sox gene family in Culter alburnus. Gene Expression Patterns 2020, 36, 119112. [Google Scholar] [CrossRef]
- Luo, Y.S.; Hu, W.; Liu, X.C.; Lin, H.R.; Zhu, Z.Y. Molecular cloning and mRNA expression pattern of Sox9 during sex reversal in orange-spotted grouper (Epinephelus coioides). Aquaculture 2010, 306, 322–328. [Google Scholar] [CrossRef]
- Xia, X.; Chen, J.; Zhang, L.; Du, Q.; Sun, J.; Chang, Z. Molecular cloning and mRNA expression pattern of Sox10 in Paramisgurnus dabryanus. Molecular Biology Reports 2013, 40, 3123–3134. [Google Scholar] [CrossRef]
- Ventura, T.; Sagi, A. The insulin-like androgenic gland hormone in crustaceans: From a single gene silencing to a wide array of sexual manipulation-based biotechnologies. Biotechnology Advances 2012, 30, 1543–1550. [Google Scholar] [CrossRef]
- Li, F.J.; Jiang, F.W.; Bai, H.K.; Fu, H.T.; Jin, S.B.; Sun, S.M.; Qiao, H.; Zhang, W.Y. Genomic cloning, expression, and single nucleotide polymorphism association analysis of the insulin-like androgenic gland hormone gene in the oriental river prawn (Macrobrachium nipponense). Genetics and Molecular Research 2015, 14, 5910–5921. [Google Scholar] [CrossRef]
- Ma, K.Y.; Li, J.L.; Qiu, G.F. Identification of putative regulatory region of insulin-like androgenic gland hormone gene (IAG) in the prawn Macrobrachium nipponense and proteins that interact with IAG by using yeast two-hybrid system. General and comparative endocrinology 2016, 229, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jin, S.; Fu, H.; Qiao, H.; Zhang, W.; Jiang, S.; Gong, Y.; Xiong, Y.; Wu, Y. Functional analysis of a SoxE gene in the oriental freshwater prawn, Macrobrachium nipponense by molecular cloning, expression pattern analysis, and in situ hybridization (de Haan, 1849). 3 Biotech 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, Z.; Jia, X.; Zou, Z.; Liang, K.; Wang, Y. Transcriptional Regulation of Vih by Oct4 and Sox9 in Scylla paramamosain. Frontiers in Endocrinology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, R.; Tu, S.; Yu, Q.; Xie, X.; Zhu, D. Putative Role of CFSH in the eyestalk-AG-testicular endocrine axis of the swimming crab Portunus trituberculatus. Animals 2023, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, X.; Wang, M.; Zheng, H.; Zheng, L.; Zhu, D. Molecular characterization and expression analysis of the inverbrate Dmrt1 homologs in the swimming crab, Portunus trituberculatus (Miers, 1876) (Decapoda, Portunidae). Crustaceana 2020, 93, 851–866. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, H.; Lo, T.S.; Chu, K.H. Inhibitory effects of the androgenic gland on ovarian development in the mud crab Scylla paramamosain. Comparative Biochemistry & Physiology Part A Molecular & Integrative Physiology 2005, 140, 343–348. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the Sox family of developmental transcription factors based on sequence and structural indicators. Developmental Biology 2000, 227, 239–255. [Google Scholar] [CrossRef]
- Wan, H.; Liao, J.; Zhang, Z.; Zeng, X.; Liang, K.; Wang, Y. Molecular cloning, characterization, and expression analysis of a sex-biased transcriptional factor Sox9 gene of mud crab Scylla paramamosain. Gene 2021, 774, 145423. [Google Scholar] [CrossRef]
- Chen, Y.L.; Wang, Y.M.; Xu, H.J.; Li, J.W.; Luo, J.Y.; Wang, M.-R.; Ma, W.-M. The characterization and knockdown of a male gonad-specific insulin-like receptor gene in the white shrimp Penaeus vannamei. Aquaculture Reports 2022, 27, 101345. [Google Scholar] [CrossRef]
- Herran, B.; Bertaux, J.; Grève, P. Divergent evolution and clade-specific duplications of the Insulin-like Receptor in malacostracan crustaceans. General and comparative endocrinology 2018, 268, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Li, Y.; Zhou, M.; Wang, W. siRNA knockdown of MrIR induces sex reversal in Macrobrachium rosenbergii. Aquaculture 2020, 523, 735172. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, D.F.; Yang, J.F.; Qi, Y. Microstructure and ultrastructure of androgenic gland in wwimming crab Portunus trituberculatus. Fish. Sci. In Chinese. 2010, 29, 193–197. (In Chinese) [Google Scholar] [CrossRef]
- Wang, M.E.; Zheng, H.; Xie, X.; Xu, R.; Zhu, D. Molecular identification and putative role of insulin growth factor binding protein-related protein (IGFBP-rp) in the swimming crab Portunus trituberculatus. Gene 2022, 833, 146551. [Google Scholar] [CrossRef]
- Hou, C.C.; Yang, W.X. Acroframosome-Dependent KIFC1 facilitates acrosome formation during spermatogenesis in the caridean shrimp Exopalaemon modestus. PloS one 2013, 8, e76065. [Google Scholar] [CrossRef]
- He, X.Y.; Fang, X.; Luo, B.Y.; Qiu, G.F. Identification and characterization of a new germline-specific marker vasa gene and its promoter in the giant freshwater prawn Macrobrachium rosenbergii. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2022, 259, 110716. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Xiong, Y.; Chen, T.; Jiang, S.; Qiao, H.; Gong, Y.; Wu, Y.; Jin, S.; Fu, H. RNA Interference analysis of the functions of cyclin B in male reproductive development of the oriental river prawn (Macrobrachium nipponense). Genes 2022, 13(11), 2079. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, W.; Xiong, Y.; Chen, T.; Jiang, S.; Qiao, H.; Gong, Y.; Wu, Y.; Jin, S.; Fu, H. RNA interference analysis of the potential functions of cyclin-dependent kinase 2 in sexual reproduction of male oriental river prawns (Macrobrachium nipponense). Aquaculture International 2023. [Google Scholar] [CrossRef]
| Name | Sequence (5′-3′) | PCR Objective | Tm (°C), GC (%), ΔG (kcal / mol) |
|---|---|---|---|
| PtSoxE-F1 | ATGGAAACTGTGAAAAAGGAACG | cDNA Clone | 60.3, 39.1, -42.6 |
| PtSoxE-R1 | TCAGTGCCACATGGTGGC | cDNA Clone | 58.3, 61.1 -35.9 |
| PtSoxE-F2 | ATGGAAACTGTGAAAAAGGAACG | RT-PCR | 60.3, 39.1, -42.6 |
| PtSoxE-R2 | GTCTTCCAGTATCTTGGTCACGG | RT-PCR | 60.4, 52.2, -41.5 |
| PtSoxE-QF | TGACGGAGGACCAAAAGCG | qPCR | 61.6, 57.9, -39.9 |
| PtSoxE-QR | TTGCCCACAGTCTTCACATTCTC | qPCR | 61.6, 47.8, -41.5 |
| β-actin-F | CGAAACCTTCAACACTCCCG | RT-PCR qPCR | 60.4, 55, -40.1 |
| β-actin-R | GGATAGCGTGAGGAAGGGCATA | RT-PCR qPCR | 63.2, 54.5, -43.8 |
| PtIAG-QF | CGCTTCACGCTCTCCTAGT | qPCR | 55.4, 57.9, -36.8 |
| PtIAG-QR | TCCTTCTTCCTATCCACTGAGT | qPCR | 54.9, 45.5, -38 |
| PtIGFBP-rp-QF | TTACCACTATTGACGGCACCT | qPCR | 57.4, 47.6, -39.2 |
| PtIGFBP-rp-QR | TCATTATCTGTACCCATCCTGTT | qPCR | 55.8, 39.1, -39.2 |
| PtIR-QF | CTGATGCGTTTGTCGTATTT | qPCR | 53.8, 40, -36.7 |
| PtIR-QR | GAAGCGTGGTGCCTATTT | qPCR | 53.2, 50, -35.7 |
| PtAkt-QF | CTCAACCAGGAACGCTTCTTC | qPCR | 59.1, 52.4, -40 |
| PtAkt-QR | TGTGTCCATCAGCATCCAGTAA | qPCR | 58.7, 45.5, -38.8 |
| PtmTOR-QF | TCTCCTGGCTGTTGCTGTC | qPCR | 59.6, 55, -37.9 |
| PtmTOR-QR | GCTTCTTGCTTGGTGTATCCTT | qPCR | 58.2, 45.5, -40.7 |
| PtKIFC-1-QF | TCCAATCGCCATCTACCTCAG | qPCR | 60, 52.4, -40.1 |
| PtKIFC-1-QR | CGTCTTCAGCATCTCCAGAATG | qPCR | 59.9, 50, -40.1 |
| PtVasa-QF | GCTTGCCATCCAGATATTCCAT | qPCR | 60.7, 45.5, -42 |
| PtVasa-QR | TGCTCCTTCATACGCCTCAA | qPCR | 59.1, 50, -39.1 |
| PtCyclinB-QF | ATGTGCCACTACAAGGCGTCT | qPCR | 59.7, 52.4, -40 |
| PtCyclinB-QR | ATCAGCGTGTCATTCCAATCC | qPCR | 59.6, 47.6, -39.6 |
| PtCdc2-QF | CCGTCAAGCAGATGGACAGTG | qPCR | 61.2, 57.1, -39.6 |
| PtCdc2-QR | CCAGGTCGTCAAAGTAAGGGTG | qPCR | 61.2, 54.5, -41.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





