Submitted:
12 June 2023
Posted:
13 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
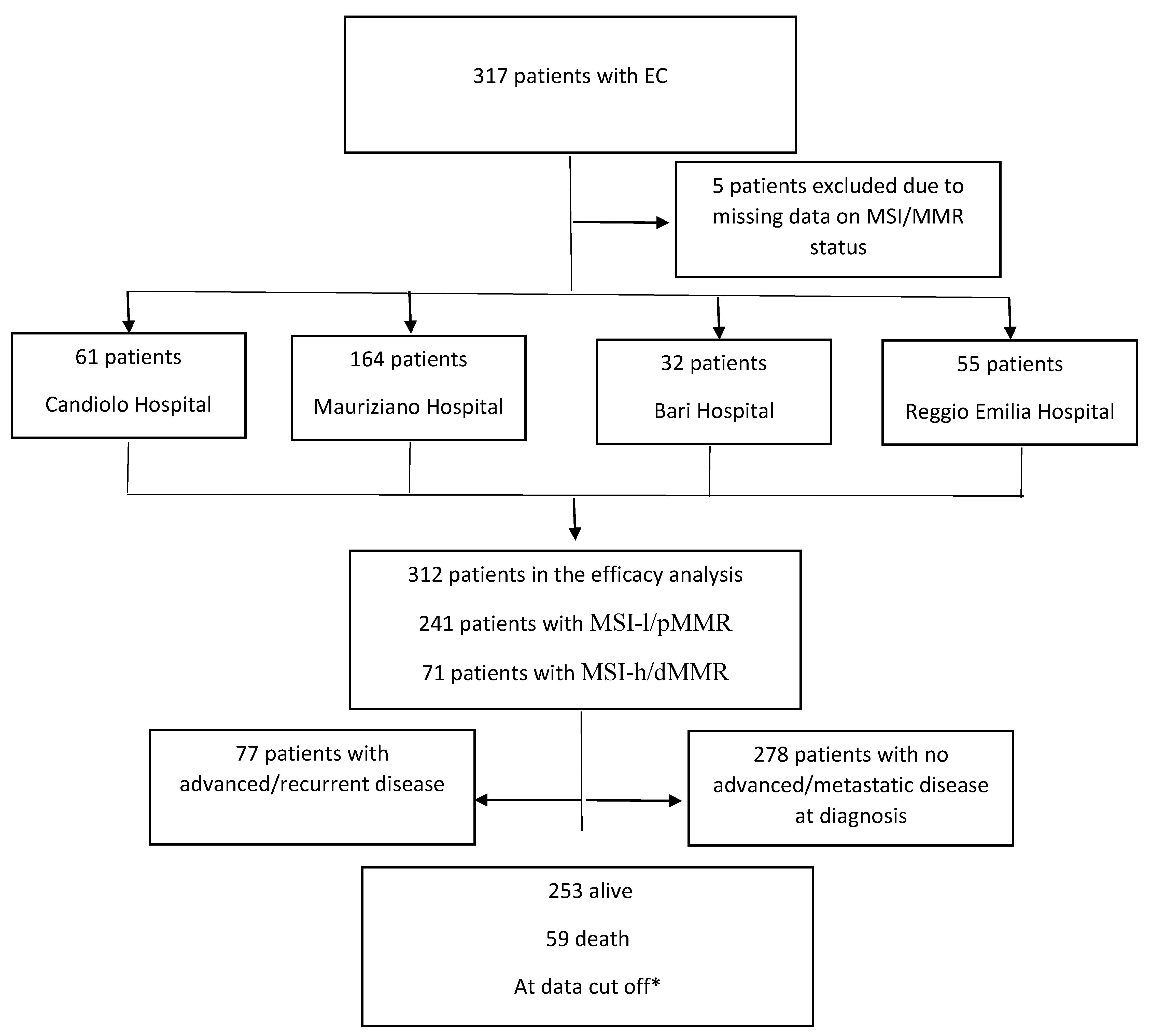
Patients
Efficacy endpoints
MMR/MSI testing
Statistical analysis
Results
Discussion
Conclusions
Supplementary Materials
Author contributions
Institutional Review Board Statement
Informed Consent Statement
Conflict of Interest
The abstract was submitted to ESGO Congress 2023.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Son J, Chambers LM, Carr C, Michener CM, Yao M, Beavis A, Yen TT, Stone RL, Wethington SL, Fader AN et al. Adjuvant treatment improves overall survival in women with high-intermediate risk early-stage endometrial cancer with lymphovascular space invasion. Int J Gynecol Cancer 2020, 30, 1738–1747. [Google Scholar] [CrossRef] [PubMed]
- Miller DS, Filiaci VL, Mannel RS, Cohn DE, Matsumoto T, Tewari KS, DiSilvestro P, Pearl ML, Argenta PA, Powell MA et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J Clin Oncol 2020, 38, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Makker V, MacKay H, Ray-Coquard I, Levine DA, Westin SN, Aoki D, Oaknin A. Endometrial cancer. Nat Rev Dis Primers 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Passarello K, Kurian S, Villanueva V. Endometrial Cancer: An Overview of Pathophysiology, Management, and Care. Semin Oncol Nurs 2019, 35, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Bokhman, JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Wilczyński M, Danielska J, Wilczyński J. An update of the classical Bokhman’s dualistic model of endometrial cancer. Prz Menopauzalny 2016, 15, 63–68. [Google Scholar]
- Talhouk A, McAlpine JN. New classification of endometrial cancers: the development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract 2016, 3, 14. [Google Scholar] [CrossRef]
- Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Moore K, Brewer MA. Endometrial Cancer: Is This a New Disease? Am Soc Clin Oncol Educ Book 2017, 37, 435–442. [Google Scholar] [CrossRef]
- Zhao S, Chen L, Zang Y, Liu W, Liu S, Teng F, Xue F, Wang Y. Endometrial cancer in Lynch syndrome. Int J Cancer 2022, 150, 7–17. [Google Scholar] [CrossRef]
- Li Z, Cheng B, Liu S, Ding S, Liu J, Quan L, Hao Y, Xu L, Zhao H, Guo J et al. Non-classical phenotypes of mismatch repair deficiency and microsatellite instability in primary and metastatic tumors at different sites in Lynch syndrome. Front Oncol 2022, 12, 1004469. [Google Scholar] [CrossRef] [PubMed]
- Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, Stover E, Strickland KC, D’Andrea AD, Wu CJ et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol 2015, 1, 1319–1323. [Google Scholar] [CrossRef]
- Yen TT, Wang TL, Fader AN, Shih IM, Gaillard S. Molecular Classification and Emerging Targeted Therapy in Endometrial Cancer. Int J Gynecol Pathol 2020, 39, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Oaknin A, Gilbert L, Tinker AV, Brown J, Mathews C, Press J, Sabatier R, O’Malley DM, Samouelian V, Boni V et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer 2022, 10. [Google Scholar]
- Meyer LA, Broaddus RR, Lu KH. Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control 2009, 16, 14–22. [Google Scholar] [CrossRef] [PubMed]
- O’Malley DM, Bariani GM, Cassier PA, Marabelle A, Hansen AR, De Jesus Acosta A, Miller WH, Safra T, Italiano A, Mileshkin L et al. Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J Clin Oncol 2022, 40, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay S, Ray-Coquard I et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med 2022, 386, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Mirza MR, Chase DM, Slomovitz BM, dePont Christensen R, Novák Z, Black D, Gilbert L, Sharma S, Valabrega G, Landrum LM et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N Engl J Med 2023. [Google Scholar]
- Eskander RN, Sill MW, Beffa L, Moore RG, Hope JM, Musa FB, Mannel R, Shahin MS, Cantuaria GH, Girda E et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N Engl J Med 2023. [Google Scholar]
- de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA, Khaw P, Colombo A et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2018, 19, 295–309. [Google Scholar] [CrossRef]
- Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, Leary A, Edmondson RJ, Powell ME, Crosbie EJ et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 2015, 28, 836–844. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo A, de Boer SM, Powell ME, Mileshkin LR, Mackay HJ, Leary A, Nijman HW, Singh N, Pollock PM, Bessette P et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J Clin Oncol 2020, 38, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- de Boer SM, Wortman BG, Bosse T, Powell ME, Singh N, Hollema H, Wilson G, Chowdhury MN, Mileshkin L, Pyman J et al. Clinical consequences of upfront pathology review in the randomised PORTEC-3 trial for high-risk endometrial cancer. Ann Oncol 2018, 29, 424–430. [Google Scholar] [CrossRef]
- Soumerai TE, Donoghue MTA, Bandlamudi C, Srinivasan P, Chang MT, Zamarin D, Cadoo KA, Grisham RN, O’Cearbhaill RE, Tew WP et al. Clinical Utility of Prospective Molecular Characterization in Advanced Endometrial Cancer. Clin Cancer Res 2018, 24, 5939–5947. [Google Scholar] [CrossRef]
- Sovak MA, Dupont J, Hensley ML, Ishill N, Gerst S, Abu-Rustum N, Anderson S, Barakat R, Konner J, Poyner E et al. Paclitaxel and carboplatin in the treatment of advanced or recurrent endometrial cancer: a large retrospective study. Int J Gynecol Cancer 2007, 17, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Pectasides D, Xiros N, Papaxoinis G, Pectasides E, Sykiotis C, Koumarianou A, Psyrri A, Gaglia A, Kassanos D, Gouveris P et al. Carboplatin and paclitaxel in advanced or metastatic endometrial cancer. Gynecol Oncol 2008, 109, 250–254. [Google Scholar] [CrossRef]
- Zighelboim I, Goodfellow PJ, Gao F, Gibb RK, Powell MA, Rader JS, Mutch DG. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol 2007, 25, 2042–2048. [Google Scholar] [CrossRef]
- van den Heerik ASVM, Horeweg N, de Boer SM, Bosse T, Creutzberg CL. Adjuvant therapy for endometrial cancer in the era of molecular classification: radiotherapy, chemoradiation and novel targets for therapy. Int J Gynecol Cancer 2021, 31, 594–604. [Google Scholar] [CrossRef]
| All patients | MSI-l/pMMR patients | MSI-h/dMMR patients | p-value | |
|---|---|---|---|---|
| Center (N. of patients) | 312 | 241/312 (77.2%) | 71/312 (22.8%) | |
| -Mauriziano | 164 (52.6%) | 132 (54.8%) | 32 (45.1%) | |
| -Candiolo | 61 (19.6%) | 44 (18.3%) | 17 (23.9%) | |
| - Reggio Emilia | 55 (17.6%) | 40 (16.6%) | 15 (21.1%) | |
| -Bari | 32 (10.3) | 25 (10.4%) | 7 (9.9%) | |
| Median age at diagnosis(years, CI) | 65.3 (31.5–90.9) | 65.3 (31.5-90.9) | 64.6 (42.4-89.8) | p=0.26 |
| Comorbidities | p=0.66 | |||
| -Yes | 222 (71.2%) | 170 (70.5%) | 52(73.1%) | |
| -No | 90(28.8%) | 71(29.5%) | 19(26.8%) | |
| Diabetes | p=0.57 | |||
| -Yes | 42 (13.5%) | 31 (12.9%) | 11 (15.5%) | |
| -No | 270 (86.5%) | 210 (87.1%) | 60 (84.5%) | |
| Hypertension | p=0.73 | |||
| -Yes | 135 (43.3%) | 103 (42.7%) | 32 (45.1%) | |
| -No | 177 (56.7%) | 138 (57.3%) | 39 (54.9%) | |
| Cardiopathy | p=0.95 | |||
| Yes | 27 (8.7%) | 21 (8.7%) | 6 (8.5%) | |
| No | 285 (91.3%) | 220 (91.3%) | 65(91.5%) | |
| Hystology | p=0.002 | |||
| Endometrioid | 239 (76.6%) | 175 (72.6%) | 64 (90.1%) | |
| Other | 73 (23.4%) | 66 (27.4%) | 7 (9.9%) | |
| FIGO stage at diagnosis | p=0.43 | |||
| I | 151 (48.4%) | 115 (47.7%) | 36 (50.7%) | |
| II | 32 (10.3%) | 23 (9.5%) | 9 (12.7%) | |
| III | 93(29.8%) | 73 (30.3%) | 20 (28.2%) | |
| IV | 33 (10.6%) | 28 (11.6%) | 5 (7.0%) | |
| Missing data | 3 (0.9%) | 2 (0.8%) | 1 (1.4%) |
| Whole series (N=278) |
MSI-l/pMMR patients (N=212) |
MSI-h/dMMR patients (N=94) |
p value | |
|---|---|---|---|---|
| N. of patients with event (recurrence or death) | 94 (XXX%) | 73/94 (77.6%) | 21/94 (22.3%) | |
| Adjuvant Therapy | p=0.20 | |||
| Yes | 171/278 (61.5%) | 126/212 (54.9%) | 45/66 (68.2%) | |
| No | 107/278 (38.5%) | 86/212 (40.6%) | 21/66 (31.8%) | |
| Radiotherapy | p=0.34 | |||
| Yes | 104/278 (37.4%) | 76/212 (35.8%) | 28/66 (42.4%) | |
| No | 174/278 (62.6%) | 136/212 (64.2%) | 38/66 (57.6%) | |
| Brachitherapy | p=0.34 | |||
| Yes | 96/278 (34.5%) | 70/212 (33.0%) | 26/66 (39.4%) | |
| No | 182/278 (65.5%) | 142/212 (67.0%) | 40/66 (60.6%) | |
| Chemotherapy | p=0.71 | |||
| Yes | 115/278 (41.4%) | 89/212 (42.0%) | 26/66 (39.4%) | |
| No | 163/278 (58.6%) | 123/212 (58.0%) | 40/66 (60.6%) | |
| Hormonotherapy | p=0.21 | |||
| Yes | 5/278 (1.8%) | 5/212 (2.4%) | 0/66 (0%) | |
| No | 273/278 (98.2%) | 207/212 (97.6%) | 66/66 (100%) | |
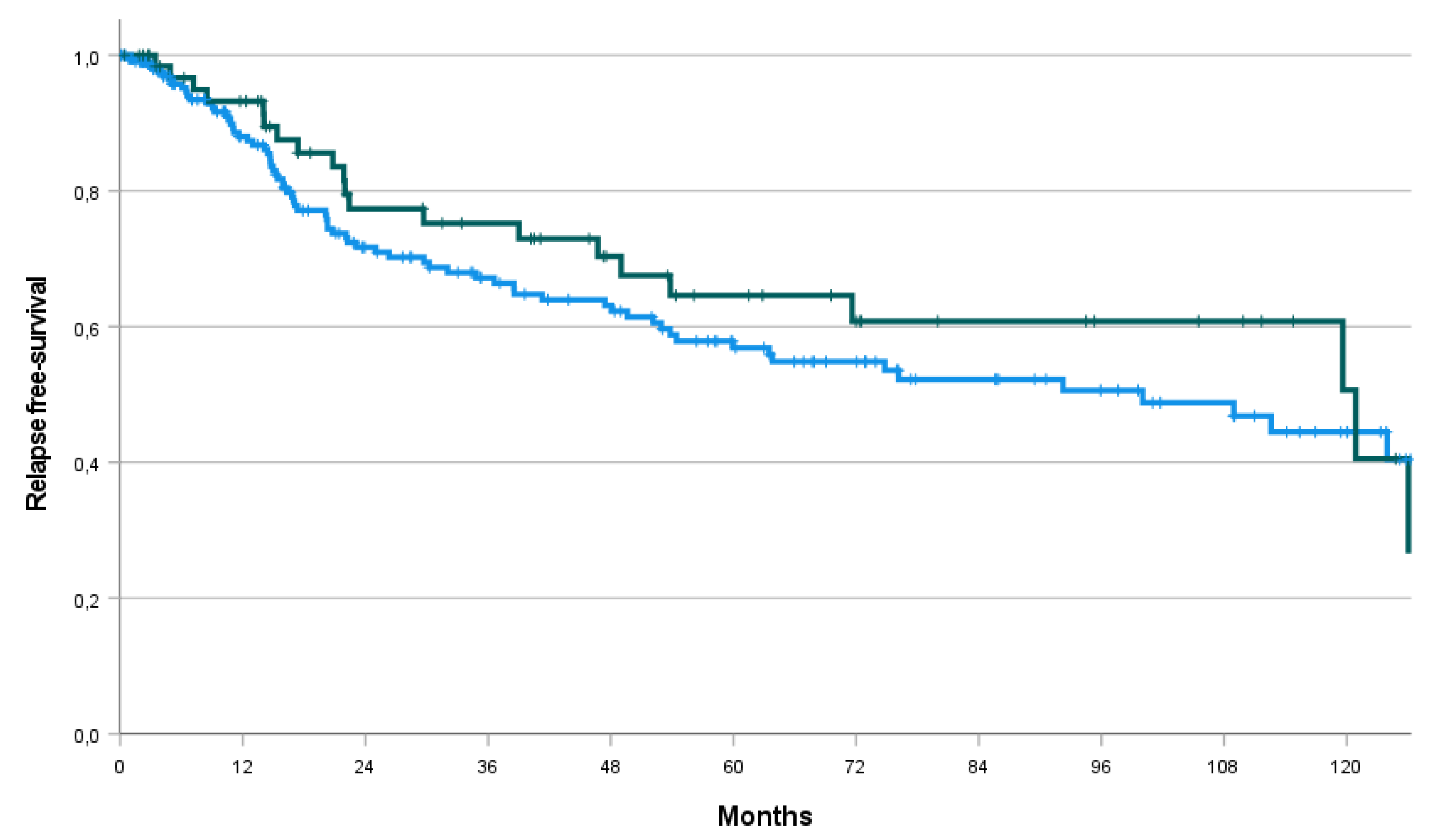
| RFS Median (months, CI) |
112.6 (78.4 – 146.8) | 100.0 (59.4 – 140.7) | 120.9 (60.0 – 181.8) | p=0.39 |
| Rate at 1 year | 89.3% | 88.0% | 93.2% | |
| Rate at 2 years | 73.1% | 71.7% | 77.4% | |
| Rate at 3 years | 69.2% | 67.2% | 75.2% | |
| Rate at 5 years | 58.8% | 56.9% | 64.6% | |
| Rate at 10 years | 46.0% | 44.5% | 50.7% |
| All comers | MSI-l/pMMR patients | MSI-h/dMMR patients | p value | |
|---|---|---|---|---|
| N. of patients | 77 | 62 (80.5%) | 15 (19.5%) | |
| Median age at advanced/metastatic disease (years, CI) | 68.3 (39.8–87.3) | 67.9 (39.8-86.0) | 71.0 (56.8-87.2) | p=0.72 |
| First line CT | ||||
| platinum-based | 59 (76.6%) | 46 (74.2%) | 13 (86.7%) | |
| liposomal doxorubicin | 12/77 (15.6%) | 11/62 (17.4%) | 1/15 (6.7%) | |
| other | 6/77 (6.8%) | 5/62 (8.1%) | 1/15 (6.7%) | |
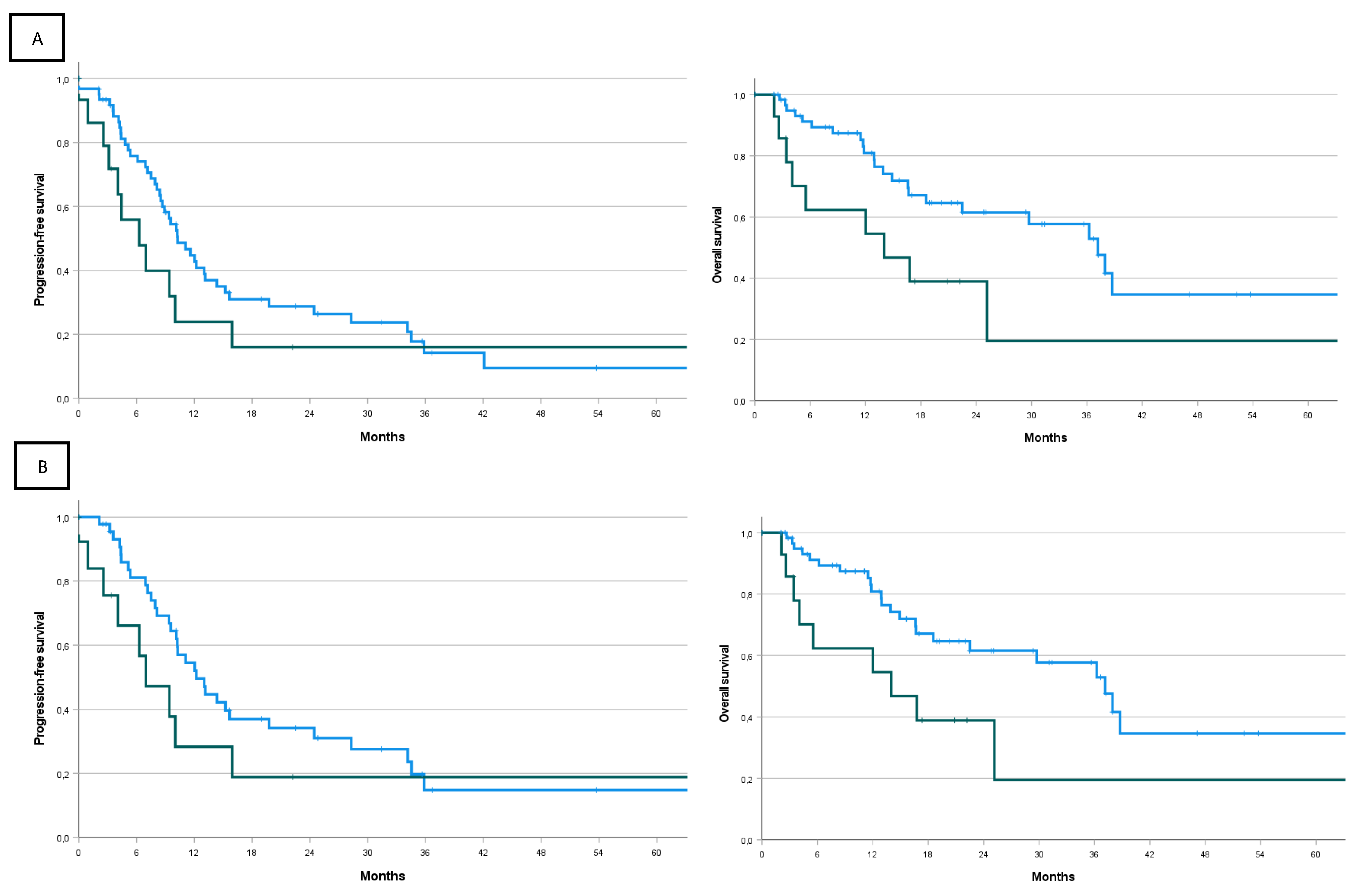
| PFS (months, CI) | 10.0 (8.51– 11.55) | 10.3 (7.7 – 12.8) | 6.3 (2.0 – 10.6) | p=0.21 |
| Rate at 6 months | 72.0% | 75,8% | 55.8% | |
| Rate at 1 year | 40.8% | 44.7% | 23.9% | |
| Rate at 2 years | 26.3% | 28.8% | 16.0% | |
| Rate at 3 years | 14.3% | 14.2% | 16.0% | |
| Rate at 5 years | 10.7% | 9.5% | 16.0% | |
| OS (months, CI) | 36.3 (20.9-51.6) | 37.2 (28.0 – 46.4) | 14.0 (1.0 – 27.1) | p=0.039 |
| Rate at 6 months | 85.8% | 91.2% | 62.3% | |
| Rate at 1 year | 77.5% | 80.9% | 62.3% | |
| Rate at 2 years | 56.9% | 61.6% | 39.0% | |
| Rate at 3 years | 50.7% | 57.7% | 19.5% | |
| Rate at 5 years | 32.4% | 34.7% | 19.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





