The skin is the largest human organ and functions as a tight physical barrier, protecting the organism from external environmental factors. The skin is mainly composed of the epidermis, dermis and subcutaneous tissue. The epidermis is divided into the stratum corneum, which is the uppermost layer of the epidermis, and the living epidermis, which produces the skin's main defence function and consists mainly of flat keratin-rich keratinocytes and intercellular lamellar lipid bilayers. Although only 10 to 20 μm thick, the stratum corneum is considered to be the main barrier limiting drug penetration. The living epidermis is 70 to 150 μm thick and consists of keratin-forming cells, Langerhans cells (antigen-presenting immune cells) and melanocytes. The dermis contains fibrin, such as collagen, elastin, fibronectin and a mucopolysaccharide matrix; there are also fibroblasts, mast cells and dendritic cells, hair follicles, sebaceous glands, and sweat glands. The subcutaneous tissue acts as a support, connecting the skin to the muscles beneath [
1].
Transdermal drug delivery (TDD) is a route of drug delivery for treating or preventing disease by absorbing drugs through the skin, permeating into skin and further into the blood circulation. TDD avoids first-pass effects, prolongs the action of drugs with short half-lives through slow release and avoids fluctuations in blood levels, reduces side effects and improves patient compliance. The stratum corneum barrier plays a key role in TDD and many methods have been used to improve the efficiency of TDD, including the use of chemical penetration enhancers and different physical enhancement approaches, such as microneedling [
2,
3], iontophoresis [
4], electroporation [
5], laser ablation [
6] and ultrasound facilitation [
7,
8,
9].
In recent years, microneedles have gained widespread interest in drug delivery and have shown brilliant achievements in delivering both chemical small molecules and biomacromolecules with minimally invasive and painless [
10,
11,
12,
13,
14,
15,
16,
17]. Microneedles usually consist of micrometer-sized needles (50-900 μm in length) in the form of microneedle arrays that can successfully penetrate the stratum corneum and deliver drugs in a minimally invasive manner below the stratum corneum without damaging blood vessels and nerves in the dermis [
18,
19], improving patient compliance and allowing drugs exposed in the epidermis or dermis to be rapidly absorbed by surrounding capillaries and lymph nodes [
20,
21,
22].
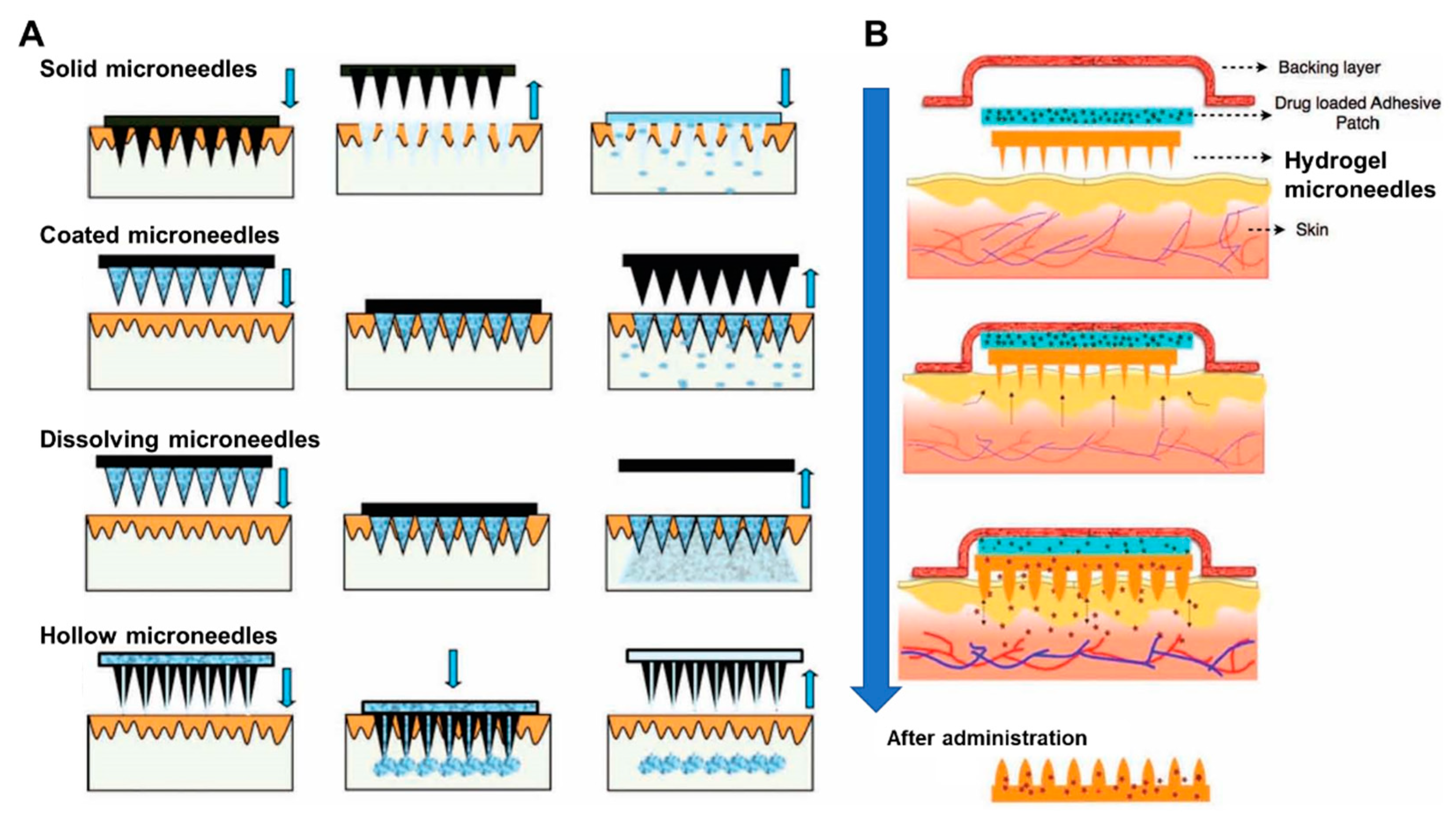
Microneedles can be fabricated with different materials and can be classified into five main types (
Figure 1), namely solid microneedles, coated microneedles, hollow microneedles, dissolving microneedles and hydrogel-forming microneedles (HFMNs) [
23,
24,
25]. Among these, HFMNs, an attractive type of microneedles first reported in 2012, consist of a swellable polymer (cross-linked hydrogel) that enables the sustained delivery of drugs for long periods of time by either incorporating the drug into the polymer structure during preparation or by loading the drug into a separate reservoir and attaching it to the HFMNs [
26]. In the following, the application and evaluation methods of HFMNs in TDD were analyzed and discussed in detail.
1. Characteristics of HFMNs as TDD system
HFMNs are safe, have no residual matrix material and are suitable for long-term, multiple drug delivery. HFMNs have advantages of resisting the closure of skin tissue pores after puncture into the skin, and no matrix material remains when HFMNs patch is removed due to the inherent swelling insolubility and viscoelastic properties of the matrix material [
26,
27]. HFMNs also bring benefits from avoiding drug deposition after microneedle tip penetration [
28].
HFMNs are characterized by water absorption and swelling, sustainable and controlled drug release. Drugs can be loaded in HFMNs in two ways, either by incorporating the drug into the microneedle matrix during preparation or by loading the drug into a separate reservoir and then attaching it to the hydrogel microneedle as a substrate [
29]. Both methods of preparation allow for the continuous delivery of the drug over a long period of time. Materials used to prepare HFMNs are non-toxic, degradable and biocompatible [
30], and commonly used materials include natural compounds such as gelatin and polymer copolymers such as poly (methyl vinyl ether-co-maleic acid) cross-linked with polyethylene glycol (PMVE / MAPEG) [
31,
32]. Among these, PMVE / MAPEG has excellent water absorption capacity and allows the preparation of super-swollen HFMNs that can absorb fluids and swell up to 20 times their original size [
11,
33]. HFMNs pierce the skin and rapidly absorb interstitial fluid, causing the hydrogel to swell, creating a continuous, unobstructed hydrogel conduit for drug permeating into the skin [
34].
HFMNs are able to control the drug release behaviour through the crosslinking density of the hydrogel microneedle matrix material, thus achieving controlled drug delivery kinetics [
35]. Similar to other types of microneedles, the common preparation method for HFMNs is mainly casting, where a microneedle matrix in its flowing hydrogel state is injected into the mould using centrifugal and decompression methods, and then dried. The release and permeation behaviour of the drug is mainly controlled by the nature of the polymers that make up the microneedle matrix, independent of the microneedle preparation process. Some cross-linked polymers, such as those prepared through esterification reactions, have different degrees of polymerisation, resulting in widely varying structures and therefore different properties of water absorption and swelling. This results in different rates of swelling after the microneedle tips are inserted into the skin, leading to different drug release profiles, such as burst and sustained drug release [
11]. For example, HFMNs based on the 'super-swelling' polymer PMVE / MAPEG consisted of drug reservoirs and microneedle tips which did not contain the drug [
11]. When the tips were inserted into the skin, the microneedle tips rapidly absorbed the interstitial fluid, creating continuous conduits between the dermal microcirculation and the attached patch-type drug reservoirs, which sustainedly released the drug. In another report, HFMNs were used to achieve a slow release of metformin with a Tmax of 24 h [
12]. In addition, on-demand drug release can be achieved using HFMNs through stimulation control. Hardy et al. prepared HFMNs using the light responsive materials 2-hydroxyethyl methacrylate (HEMA) and ethylene glycol dimethacrylate (EGDMA) to achieve on-demand delivery of ibuprofen [
36].
2. Evaluation methods for HFMNs
The materials, design, and preparation process of HFMNs are important parameters in determining the properties of microneedles, while effective drug delivery also depends on mechanical strength, skin penetration and release kinetics of HFMNs.
2.1. Appearance and morphology
The morphology and dimensions of HFMNs (including tip radius, height, width, length and spacing) can be characterised by optical microscopy, scanning electron microscopy (SEM) or optical coherence tomography (OCT), confocal laser scanning microscopy (CLSM), and multiphoton microscopy (MPM).
Optical microscopy and SEM are commonly used to image and measure the morphology of HFMNs arrays and the height, width and spacing of microneedles [
37]. OCT imaging is highly accurate, has a certain imaging depth and imaging speed, and is often used to observe in situ the penetration depth of microneedle patches after puncturing into isolated or in vivo skin or to record the process of microneedle changes within the skin [
38,
39].
2.2. Swellability and water insolubility
The swelling properties of HFMNs were determined by placing the microneedle array in distilled water or PBS and removing and weighing at specific time intervals to calculate the percentage of swelling [
40]. Ex vivo skin such as porcine skin was also be used, with the subcutaneous tissue layer carefully removed and the skin placed on tissue paper equilibrated with PBS (pH 7.4). HFMNs patches were punctured into the isolated skin and then removed at specific time intervals and their base-width swelling capacity was measured using digital microscopy [
41].
Water insolubility is an important property of HFMNs. The solubility of HFMNs was calculated by swelling them sufficiently and then placing them at 90°C to dry completely to a constant weight and comparing the weight before swelling with the weight after swelling and drying to a constant weight [
40].
2.3. Mechanical strength
The shape of the microneedle determines how much force can be applied to the microneedle before the needle breaks. The diameter and angle of the needle tip, as well as the height and basal measurement of the microneedle, determine whether the microneedle can be safely and reliably inserted into the skin [
42]. In general, smaller tip diameters, smaller tip angles and higher tip height to width ratios facilitate successful skin penetration. Mechanical strength is generally tested using a texturizer or a motorised force measuring table [
43,
44]. For fracture testing, arrays of microneedles are microscopically observed before and after testing to determine height differences.
2.4. Skin piercing and transdermal permeation properties
Microneedles act on the skin surface, puncturing the epidermis and creating microscopic pores through which the drug diffuses into the dermal microcirculation. The success of microneedle puncture can be assessed using a paraffin membrane or porcine skin. The porcine skin has similar physical properties to human skin and can be used as a simulated human skin model [
43,
45,
46]. When conducting relevant experiments, the skin was first washed with PBS (pH 7.4) and then the skin was placed dermally downwards on a wax sheet [
47]. The HFMNs were then pressed into the skin with the thumb for 30 s. The microneedle arrays were removed from the skin and stained with 150 µL of 1% methylene blue solution for 5 min to assess the position of the stained microneedle pinholes. Excess staining solution was gently washed away with PBS. The stained skin was imaged with a digital microscope and the percentage of stained blue microneedles was calculated to assess the skin puncture performance of the microneedle arrays. The 100% success rate indicated that all microneedle arrays would be observed in the skin [
48]. In general, parameters such as microneedle tip diameter, basal width, length, type of microneedle and its mechanical strength play a crucial role in forming the size of the microchannel in the skin [
49].
Fluorescence microscopy can be used to examine the distribution and accumulation of the drug in the skin. Using fluorescence imaging, Aljuffali et al. observed that after transdermal administration, fluorescence was only detected on the skin surface in the free fluorescent probe group and only a weak fluorescent signal was present in the hair follicles, whereas fluorescence was significantly enhanced in the skin of the fluorescently labelled nanocarriers group, suggesting a pro-permeation effect of the nanocarriers [
50].
When the drug itself is fluorescent or the drug delivery system is labelled with fluorescence, CLSM is often used to observe fluorescence at different skin depths, allowing verification of the depth of penetration of the agent into the skin tissue and visualisation of the accumulation of the agent in the skin tissue. Alvarez-Roman et al. used CLSM to determine the penetration, distribution and accumulation of polymeric nanoparticles in isolated porcine skin [
51].
MPM is suitable for the characterisation of human skin and allows the assessment of skin morphology and layers at a subcellular level. The two-photon excitation principle overcomes the limitations of fluorescence imaging and allows for in vivo non-toxic manipulation. Excitation occurs almost exclusively at the target inspection site without damaging surrounding tissue [
52]. MPM also extends the applicability of fluorescence lifetime imaging microscopy (FLIM), and MPM-FLIM allows non-invasive, high-resolution examination of human skin for in vitro, ex vivo and even clinical in vivo applications [
53]. MPM has been used to evaluate the pathophysiological features of inflamed skin, skin permeation and delivery of drugs [
54,
55,
56].
2.5. In vitro release and transdermal behaviour
The in vitro TDD can be assessed by Franz diffusion in the donor compartment of the diffusion cell, with the stratum corneum of porcine skin fixed face up to the receiving cell, with PBS (pH 7.4) constanted at 37°C as the receiving medium [
57]. The microneedle arrays were applied to the isolated skin and samples were taken from the receiving cell at set intervals. For measuring in vitro drug release, microneedles are placed in PBS (pH 7.4, 37°C), and samples are taken at set intervals to determine drug concentrations. Skin permeation of drug can also be evaluated by in vivo animal models, often in suitable rats or mice. The hair of the anaesthetized animal is removed and the skin is then punctured using a microneedle patch, whilst other parameters associated with drug efficacy can be assessed, such as microneedle strength, permeation efficiency and irritation [
58].
It has been noted that skin structure and immune responses in animal models differ significantly from those in humans. In addition, the biochemical properties of ex vivo human skin are different compared to in vivo human skin [
59]. Therefore, human trials need to be included in the study when conducting pharmacodynamic studies [
60].
3. Application of HFMNs in disease treatment
HFMNs have been widely used for the treatment of various diseases, such as cardiovascular diseases, metabolism-related diseases and cancer, due to their outstanding advantages mentioned above.
3.1. Anticancer
With HFMNs for transdermal anticancer drug delivery can overcome the disadvantages of low bioavailability and side effects of oral administration and can also be used for the local administration of drugs for the treatment of superficial tumours such as melanoma, improving bioavailability while avoiding systemic exposure of the drug. Taking advantage of the abundant immune cells including antigen-presenting cells and Langerhans cells in the epidermis and dermis, the activation of the skin's immune microenvironment can act synergistically with the drugs delivered by HFMNs.
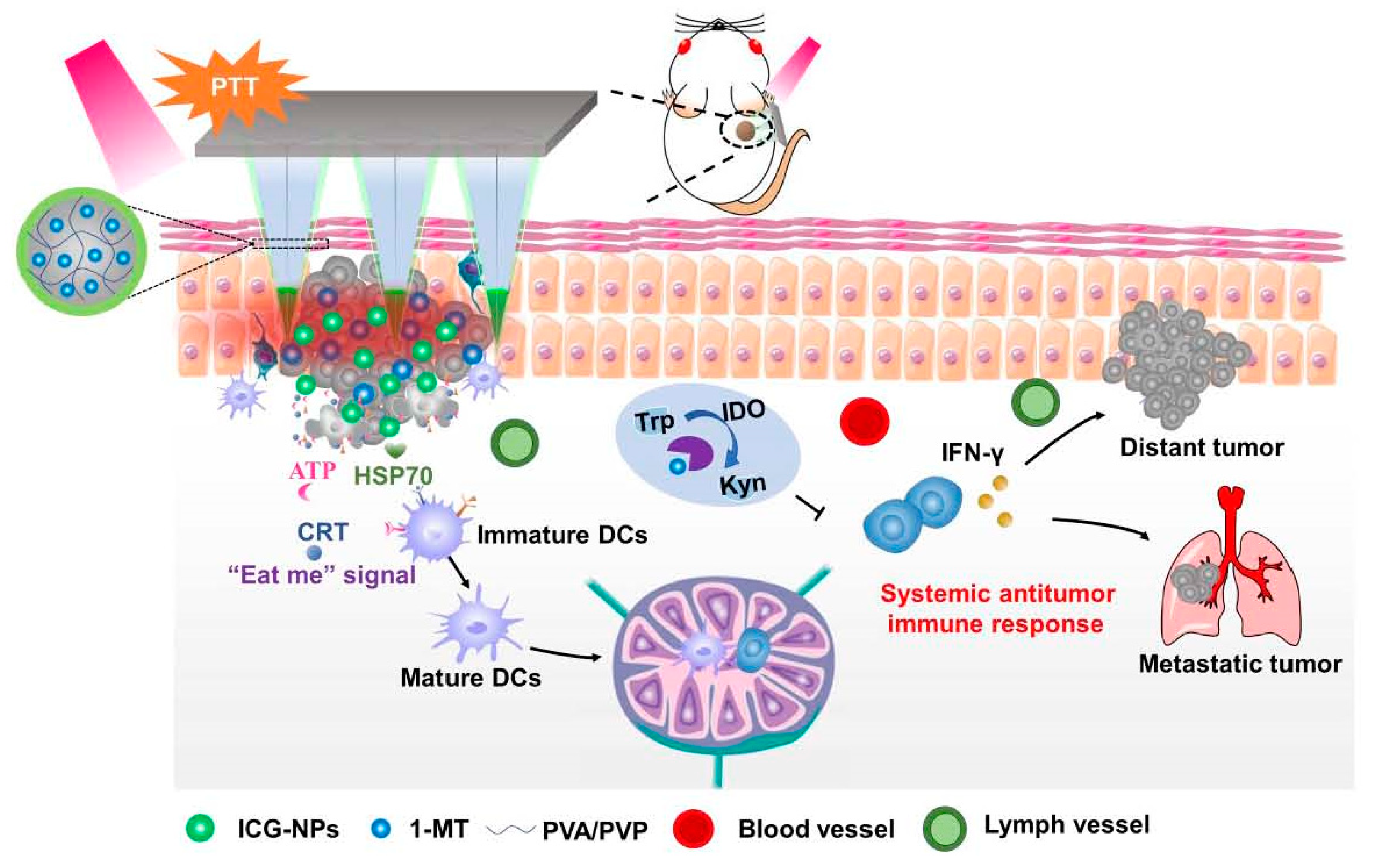
Chen et al. prepared HFMNs with cross-linking polyvinylpyrrolidone (PVP) and polyvinyl alcohol (PVA) as matrix, loaded with 1-methyltryptophan and indocyanine green-encapsulated nanoparticles for the treatment of melanoma [
61]. This system successfully induced immunogenic cell death, enhanced immune response and provided a promising melanoma treatment (
Figure 2).
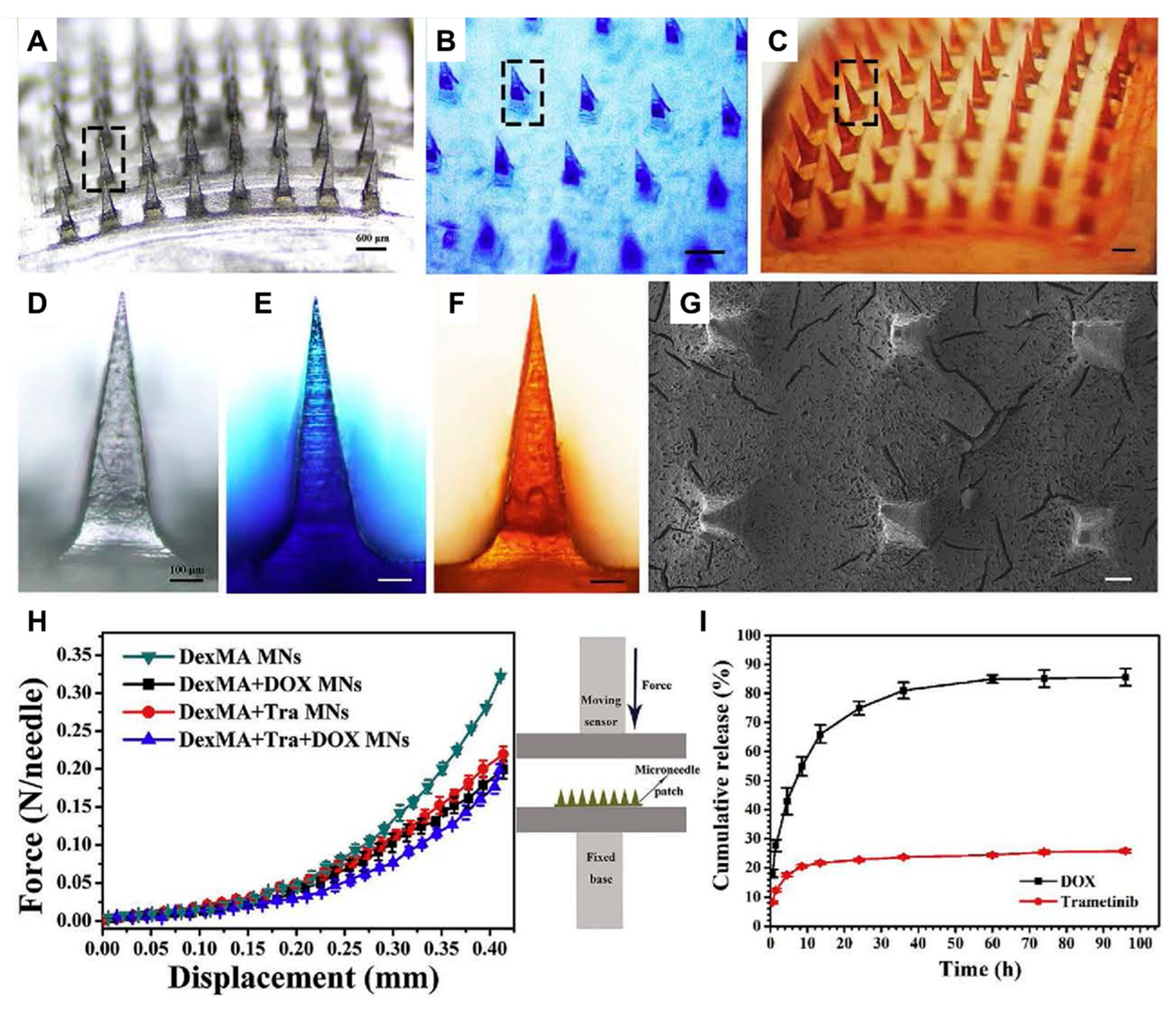
Huang et al. prepared HFMNs loaded with doxorubicin (DOX) and trametinib (Tra) using photo-cross-linked dextrose methacrylate (DexMA) as the microneedle matrix, and successfully achieved the slow release of the drugs and exploited the synergistic effect of DOX and Tra (
Figure 3) [
62].
3.2. Treating diabetes
HFMNs are a promising drug delivery system for the treatment of diabetes because they are minimally invasive, painless, have no microneedle matrix residue and can be repeatedly administered multiple times.
Chen et al. used silk protein and phenylboronic acid/acrylamide as microneedle matrix loaded with insulin to prepare glucose-responsive smart HFMNs [
63]. After the microneedles penetrated the skin, insulin was released autonomously to control the blood glucose concentration when the glucose concentration in the skin tissue increased. The HFMNs also retain their original needle shape after a week in water, offering the potential for safe, residue-free and sustained drug release.
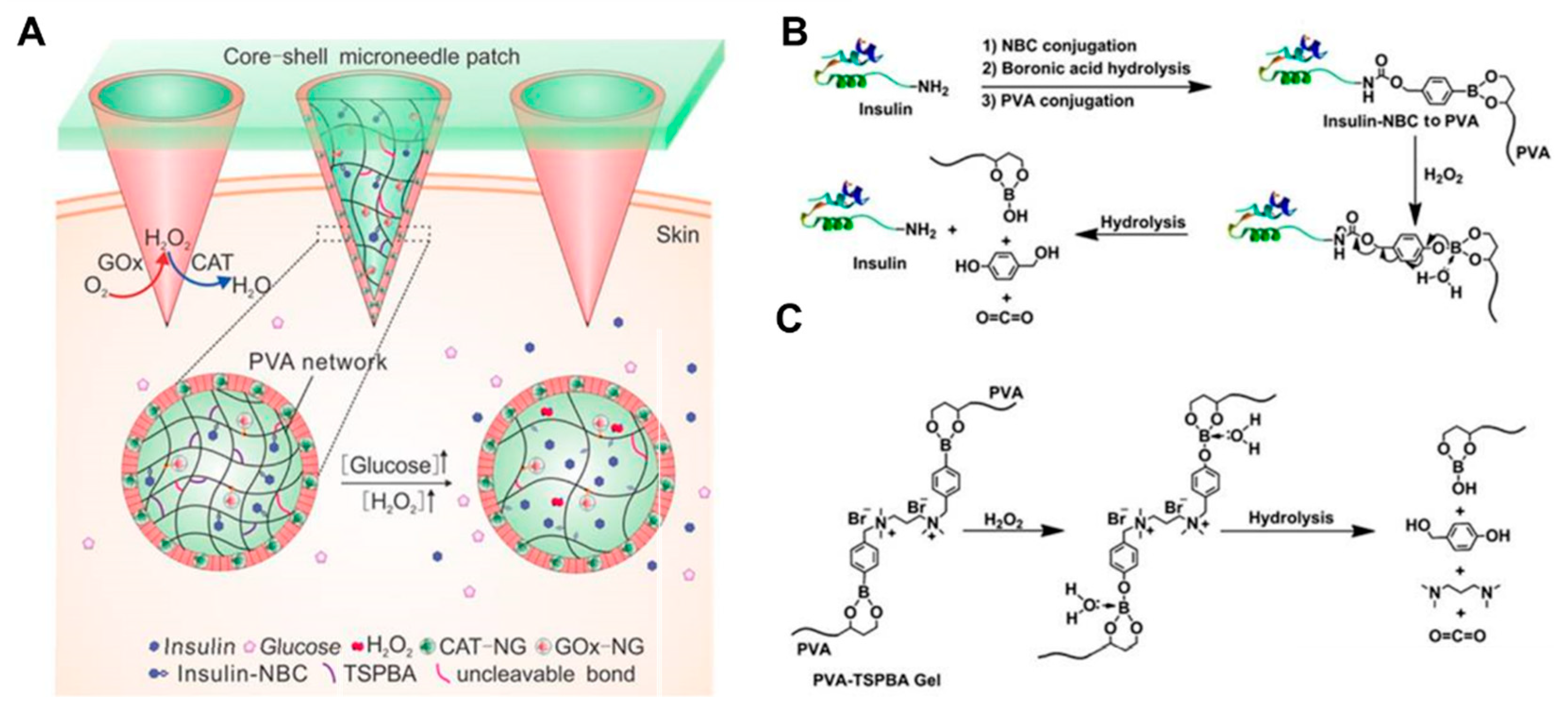
Wang et al. fabricated HFMNs by using PVA as a microneedle matrix, loaded with glucose oxidase (core) and catalase (shell) and loaded 4-nitrophenyl 4-(4,4,5,5-tetramethyl-1,3,2-dioxol-2yl) benzyl carbonate (insulin NBC) modified insulin into PVA, and PVA was further gelated by an H
2O
2-labile linker: N
1-(4-boronobenzyl)-N
3-(4-boronophenyl)-N
1,N
1,N
3,N
3-tetramethylpropane-1,3-diaminium (TSPBA) [
64]. When microneedles were exposed to high glucose concentrations, local high levels of H
2O
2 were produced and insulin NBC was oxidised and hydrolysed, leading to rapid release of free insulin and control of blood glucose concentrations (
Figure 4).
3.3. Treating rheumatoid arthritis
Rheumatoid arthritis is a systemic disease involving multiple joints. Early drug treatment is mostly oral administration, but long-term oral anti-rheumatoid arthritis medication often brings about serious side effects, while local injection drug treatment methods not only require specialist handling, but may also pose the risk of joint damage and infection. HFMNs are suitable for the delivery of drugs for this disease because of their painless and minimally invasive delivery of various active molecules.
Cao et al. designed modified hyaluronic acid-fabricated HFMNs, loaded with reverse deoxythymidine and cholesterol-modified deoxythymidine, which had a protective effect against cartilage/bone erosion in mice joints [
65]. Compared to dissolving microneedles, HFMNs not only increased the loading of nucleic acid aptamers into the cavity of the microneedle mould, but also allowed the loading of HFMNs to be controlled by adjusting the concentration of the aptamer solution, avoiding waste and loss of aptamers during preparation.
4. Summary and outlook
As a new type of TDD method, HFMNs have been increasingly researched for TDD, mainly in the treatment of cardiovascular diseases, tumours and diabetes mellitus, with the greatest advantage being the controlled release of the drug and the targeted drug delivery in the original lesion. In addition, the HFMNs are prepared with certain functional modifications, such as some photothermal materials (e.g., gold nanorods, Prussian blue, and indocyanine green) and photosensitizers (e.g., protoporphyrin, zinc titanocyanine, and titanium dioxide), which induce the HFMNs to produce exogenous stimuli (changes in temperature, magnetic fields, and light) or endogenous stimuli (changes in pH, enzymes, and redox gradients), while combining the delivered drug molecules, antibodies, nucleic acids, etc., to achieve targeted treatment of diseases. However, HFMNs development still face potential biosafety issues during prolonged use, batch industrial production and sterilisation challenges, which pose a huge challenge for their clinical application and are a hot topic for future research.
Author Contributions
Conceptualization, Y.Z. and N.F.; methodology, X.H. and J.L.; software, X.H., J.L. and H.R.; validation, J.L. and M.L.; investigation; Y.Z., N.F., and Y.H.; writing—original draft preparation, X.H. and J.L.; writing—review and editing, Y.Z., N.F. and Y.H.; Supervision, Y.Z., N.F., H.R., and M.L.; project administration, X.H. and J.L. funding acquisition, Y.Z., N.F., and Y.H.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 82074031), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (Grant number TP2020054), and the Program for Shanghai High-Level Local University Innovation Team (SZY20220315).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Menon, G.K. New insights into skin structure: scratching the surface. Adv. Drug Deliv. Rev. 2002, 54, S3–S17. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev 2004, 56, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, F.J.; Bal, S.M.; Van Den Berg, D.J.; et al. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J Control Release 2007, 117, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kanikkannan, N. Iontophoresis-based transdermal delivery systems. BioDrugs 2002, 16, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Denet, A.R.; Vanbever, R.; Preat, V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev 2004, 56, 659–674. [Google Scholar] [CrossRef]
- Wenande, E.; Tam, J.; Bhayana, B.; Schlosser, S.K.; Ishak, E.; Farinelli, W.A.; Chlopik, A.; Hoang, M.P.; Pinkhasov, O.R.; Caravan, P.; et al. Laser-assisted delivery of synergistic combination chemotherapy in in vivo skin. J. Control. Release 2018, 275, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Svenskaya, Y.I.; Genina, E.A.; Parakhonskiy, B.V.; Lengert, E.V.; Talnikova, E.E.; Terentyuk, G.S.; Utz, S.R.; Gorin, D.A.; Tuchin, V.V.; Sukhorukov, G.B. A Simple Non-Invasive Approach toward Efficient Transdermal Drug Delivery Based on Biodegradable Particulate System. ACS Appl. Mater. Interfaces 2019, 11, 17270–17282. [Google Scholar] [CrossRef]
- Azagury, A.; Khoury, L.; Enden, G.; Kost, J. Ultrasound mediated transdermal drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 127–143. [Google Scholar] [CrossRef]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Control. Release 2011, 152, 330–348. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McCrudden, M.T.C.; McAvoy, K.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-Mediated Transdermal Delivery of Bevacizumab. Mol. Pharm. 2018, 15, 3545–3556. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Mccrudden, M.T.C.; Alkilani, A.Z.; et al. Hydrogel-Forming Microneedles Prepared from "Super Swelling'' Polymers Combined with Lyophilised Wafers for Transdermal Drug Deliv. PLoS One 2014, 9, e111547. [Google Scholar] [CrossRef] [PubMed]
- Migdadi, E.M.; Courtenay, A.J.; Tekko, I.A.; McCrudden, M.T.; Kearney, M.-C.; McAlister, E.; McCarthy, H.O.; Donnelly, R.F. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release 2018, 285, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Koutsonanos, D.G.; del Pilar Martin, M.; Zarnitsyn, V.G.; Sullivan, S.P.; Compans, R.W.; Prausnitz, M.R.; Skountzou, I. Transdermal Influenza Immunization with Vaccine-Coated Microneedle Arrays. PLoS ONE 2009, 4, e4773. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, H.S.; Hwang, Y.H.; et al. Enhanced anti-tumor immunotherapy by dissolving microneedle patch loaded ovalbumin. PLoS One 2019, 14, e0220382. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.J.; Ramöller, I.K.; McKenna, P.E.; Abbate, M.T.; Volpe-Zanutto, F.; Vora, L.K.; Kilbourne-Brook, M.; Jarrahian, C.; Moffatt, K.; Zhang, C.; et al. Microarray patches: Breaking down the barriers to contraceptive care and HIV prevention for women across the globe. Adv. Drug Deliv. Rev. 2021, 173, 331–348. [Google Scholar] [CrossRef]
- Volpe-Zanutto, F.; Ferreira, L.T.; Permana, A.D.; Kirkby, M.; Paredes, A.J.; Vora, L.K.; Bonfanti, A.P.; Charlie-Silva, I.; Raposo, C.; Figueiredo, M.C.; et al. Artemether and lumefantrine dissolving microneedle patches with improved pharmacokinetic performance and antimalarial efficacy in mice infected with Plasmodium yoelii. J. Control. Release 2021, 333, 298–315. [Google Scholar] [CrossRef]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Thakur, R.R.S.; Donnelly, R.F. Microneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 44–76. [Google Scholar] [CrossRef]
- Donnelly, R.; Douroumis, D. Microneedles for drug and vaccine delivery and patient monitoring. Drug Deliv. Transl. Res. 2015, 5, 311–312. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in transdermal insulin delivery. Adv. Drug Deliv. Rev. 2019, 139, 51–70. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Singh, T.R.R.; Donnelly, R.F.; Anissimov, Y.G. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J. Control. Release 2018, 270, 184–202. [Google Scholar] [CrossRef]
- Huang, Y.; Park, Y.S.; Moon, C.; David, A.E.; Chung, H.S.; Yang, V.C. Synthetic Skin-Permeable Proteins Enabling Needleless Immunization. Angew. Chem. Int. Ed. 2010, 49, 2724–2727. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef]
- McAlister, E.; Dutton, B.; Vora, L.K.; Zhao, L.; Ripolin, A.; Zahari, D.S.Z.B.P.H.; Quinn, H.L.; Tekko, I.A.; Courtenay, A.J.; Kelly, S.A.; et al. Directly Compressed Tablets: A Novel Drug-Containing Reservoir Combined with Hydrogel-Forming Microneedle Arrays for Transdermal Drug Delivery. Adv. Heal. Mater. 2021, 10, e2001256. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Vora, L.K.; Peng, K.; Donnelly, R.F. Trilayer microneedle array assisted transdermal and intradermal delivery of dexamethasone. Int. J. Pharm. 2022, 612, 121295. [Google Scholar] [CrossRef]
- Yadav, P.R.; Dobson, L.J.; Pattanayek, S.K.; Das, D.B. Swellable microneedles based transdermal drug delivery: Mathematical model development and numerical experiments. Chem. Eng. Sci. 2022, 247. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef]
- Lutton, R.E.; Larrañeta, E.; Kearney, M.-C.; Boyd, P.; Woolfson, A.; Donnelly, R.F. A novel scalable manufacturing process for the production of hydrogel-forming microneedle arrays. Int. J. Pharm. 2015, 494, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Mooney, K.; Mccrudden, M.T.; Vicente-Pérez, E.M.; Belaid, L.; González-Vázquez, P.; Mcelnay, J.C.; Woolfson, A.D. Hydrogel-Forming Microneedles Increase in Volume During Swelling in Skin, but Skin Barrier Function Recovery is Unaffected. J. Pharm. Sci. 2014, 103, 1478–1486. [Google Scholar] [CrossRef]
- Martin, C.; Allender, C.; Brain, K.; Morrissey, A.; Birchall, J. Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications. J. Control. Release 2012, 158, 93–101. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McCrudden, M.T.C.; McAvoy, K.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-Mediated Transdermal Delivery of Bevacizumab. Mol. Pharm. 2018, 15, 3545–3556. [Google Scholar] [CrossRef] [PubMed]
- Rajoli, R.K.; Flexner, C.; Chiong, J.; Owen, A.; Donnelly, R.F.; Larrañeta, E.; Siccardi, M. Modelling the intradermal delivery of microneedle array patches for long-acting antiretrovirals using PBPK. Eur. J. Pharm. Biopharm. 2019, 144, 101–109. [Google Scholar] [CrossRef]
- Eltayib, E.; Brady, A.J.; Caffarel-Salvador, E.; Gonzalez-Vazquez, P.; Alkilani, A.Z.; McCarthy, H.O.; McElnay, J.C.; Donnelly, R.F. Hydrogel-forming microneedle arrays: Potential for use in minimally-invasive lithium monitoring. Eur. J. Pharm. Biopharm. 2016, 102, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Larrañeta, E.; Donnelly, R.F.; McGoldrick, N.; Migalska, K.; McCrudden, M.T.C.; Irwin, N.J.; Donnelly, L.; McCoy, C.P. Hydrogel-Forming Microneedle Arrays Made from Light-Responsive Materials for On-Demand Transdermal Drug Delivery. Mol. Pharm. 2016, 13, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Arsalan, A.; Raya, D.; Hala, D.; et al. Preparation and characterization of flexible furosemide-loaded biodegradable microneedles for intradermal drug delivery. Biomater Sci 2022, 10, 6486–6499. [Google Scholar]
- Yang, L.; Yang, Y.; Chen, H.; Mei, L.; Zeng, X. Polymeric microneedle-mediated sustained release systems: Design strategies and promising applications for drug delivery. Asian J. Pharm. Sci. 2022, 17, 70–86. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McAlister, E.; McCrudden, M.T.; Vora, L.; Steiner, L.; Levin, G.; Levy-Nissenbaum, E.; Shterman, N.; Kearney, M.-C.; McCarthy, H.O.; et al. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery. J. Control. Release 2020, 322, 177–186. [Google Scholar] [CrossRef]
- Suksaeree, J.; Charoenchai, L.; Madaka, F.; Monton, C.; Sakunpak, A.; Charoonratana, T.; Pichayakorn, W. Zingiber cassumunar blended patches for skin application: Formulation, physicochemical properties, and in vitro studies. Asian J. Pharm. Sci. 2015, 10, 341–349. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Pamornpathomkul, B.; Opanasopit, P. Fabrication, characterization and comparison of α-arbutin loaded dissolving and hydrogel forming microneedles. Int. J. Pharm. 2020, 586, 119508. [Google Scholar] [CrossRef]
- Bal, S.M.; Caussin, J.; Pavel, S.; Bouwstra, J.A. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 2008, 35, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Courtenay, A.J.; Tekko, I.A.; Larrañeta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2020, 146, 290–298. [Google Scholar] [CrossRef]
- He, M.C.; Chen, B.Z.; Ashfaq, M.; et al. Assessment of mechanical stability of rapidly separating microneedles for transdermal drug delivery. Drug Deliv Transl Res 2018, 8, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Z.; Ashfaq, M.; Zhang, X.P.; Zhang, J.N.; Guo, X.D. In vitro and in vivo assessment of polymer microneedles for controlled transdermal drug delivery. J. Drug Target. 2018, 26, 720–729. [Google Scholar] [CrossRef]
- Lee, I.C.; He, J.S.; Tsai, M.T.; et al. Fabrication of a novel partially dissolving polymer microneedle patch for transdermal drug delivery. J Mater Chem B 2015, 3, 276–285. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.J.; McCrudden, M.T.C.; Alkilani, A.Z.; Vicente-Pérez, E.M.; O'Mahony, C.; González-Vázquez, P.; McCarron, P.A.; Woolfson, A.D. Hydrogel-Forming and Dissolving Microneedles for Enhanced Delivery of Photosensitizers and Precursors. Photochem. Photobiol. 2014, 90, 641–647. [Google Scholar] [CrossRef]
- Donadei, A.; Kraan, H.; Ophorst, O.; Flynn, O.; O'Mahony, C.; Soema, P.C.; Moore, A.C. Skin delivery of trivalent Sabin inactivated poliovirus vaccine using dissolvable microneedle patches induces neutralizing antibodies. J. Control. Release 2019, 311-312, 96–103. [Google Scholar] [CrossRef]
- Kalluri, H.; Banga, A.K. Formation and Closure of Microchannels in Skin Following Microporation. Pharm. Res. 2011, 28, 82–94. [Google Scholar] [CrossRef]
- Roy, R.K.; Thakur, M.; Dixit, V.K. Hair growth promoting activity of Eclipta alba in male albino rats. Arch. Dermatol. Res. 2008, 300, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Aljuffali, I.A.; Sung, C.T.; Shen, F.M.; et al. Squarticles as a Lipid Nanocarrier for Delivering Diphencyprone and Minoxidil to Hair Follicles and Human Dermal Papilla Cells. AAPS J 2014, 16, 140–150. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carriers. Aaps Pharmscitech 2017, 18, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Yazdani-Arazi, S.N.; Ghanbarzadeh, S.; Adibkia, K.; Kouhsoltani, M.; Hamishehkar, H. Histological evaluation of follicular delivery of arginine via nanostructured lipid carriers: a novel potential approach for the treatment of alopecia. Artif. Cells, Nanomedicine, Biotechnol. 2017, 45, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.W.; Yu, W.W.; Colvin, V.L.; Monteiroriviere, N. Biological interactions of quantum dot nanoparticles in skin and in human epidermal keratinocytes. Toxicol. Appl. Pharmacol. 2008, 228, 200–211. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, C.; Song, G.; Jin, X.; Xu, Z. In vivo skin penetration and metabolic path of quantum dots. Sci. China Life Sci. 2013, 56, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Filipe, P.; Silva, J.; Silva, R.; de Castro, J.C.; Gomes, M.M.; Alves, L.; Santus, R.; Pinheiro, T. Stratum Corneum Is an Effective Barrier to TiO2 and ZnO Nanoparticle Percutaneous Absorption. Ski. Pharmacol. Physiol. 2009, 22, 266–275. [Google Scholar] [CrossRef]
- González-Vázquez, P.; Larrañeta, E.; McCrudden, M.T.C.; Jarrahian, C.; Rein-Weston, A.; Quintanar-Solares, M.; Zehrung, D.; McCarthy, H.; Courtenay, A.J.; Donnelly, R.F. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J. Control. Release 2017, 265, 30–40. [Google Scholar] [CrossRef]
- Shende, P.; Sardesai, M.; Gaud, R.S. Micro to nanoneedles: a trend of modernized transepidermal drug delivery system. Artif. Cells, Nanomedicine, Biotechnol. 2018, 46, 19–25. [Google Scholar] [CrossRef]
- Coulman, S.A.; Birchall, J.C.; Alex, A.; Pearton, M.; Hofer, B.; O’mahony, C.; Drexler, W.; Považay, B. In Vivo, In Situ Imaging of Microneedle Insertion into the Skin of Human Volunteers Using Optical Coherence Tomography. Pharm. Res. 2011, 28, 66–81. [Google Scholar] [CrossRef]
- Arya, J.; Henry, S.; Kalluri, H.; McAllister, D.V.; Pewin, W.P.; Prausnitz, M.R. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials 2017, 128, 1–7. [Google Scholar] [CrossRef]
- Chen, M.; Quan, G.; Wen, T.; Yang, P.; Qin, W.; Mai, H.; Sun, Y.; Lu, C.; Pan, X.; Wu, C. Cold to Hot: Binary Cooperative Microneedle Array-Amplified Photoimmunotherapy for Eliciting Antitumor Immunity and the Abscopal Effect. ACS Appl. Mater. Interfaces 2020, 12, 32259–32269. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, H.; Huang, S.; et al. Dextran methacrylate hydrogel microneedles loaded with doxorubicin and trametinib for continuous transdermal administration of melanoma. Carbohydr Polym 2020, 246, 116650. [Google Scholar] [CrossRef]
- Chen, S.; Matsumoto, H.; Moro-Oka, Y.; Tanaka, M.; Miyahara, Y.; Suganami, T.; Matsumoto, A. Smart Microneedle Fabricated with Silk Fibroin Combined Semi-interpenetrating Network Hydrogel for Glucose-Responsive Insulin Delivery. ACS Biomater. Sci. Eng. 2019, 5, 5781–5789. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Yu, J.; Kahkoska, A.R.; Zhang, X.; Wang, C.; Sun, W.; Corder, R.D.; Chen, Z.; Khan, S.A.; et al. Core–Shell Microneedle Gel for Self-Regulated Insulin Delivery. ACS Nano 2018, 12, 2466–2473. [Google Scholar] [CrossRef]
- Cao, J.; Su, J.; An, M.; et al. Novel DEK-Targeting Aptamer Delivered by a Hydrogel Microneedle Attenuates Collagen-Induced Arthritis. Mol Pharm 2021, 18, 305–316. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).