Submitted:
29 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Population studied
Patient Population
Control Population
Isolation of Mononuclear Cells from Human Peripheral Blood and Flow Cytometry
Statistical analysis
3. Results
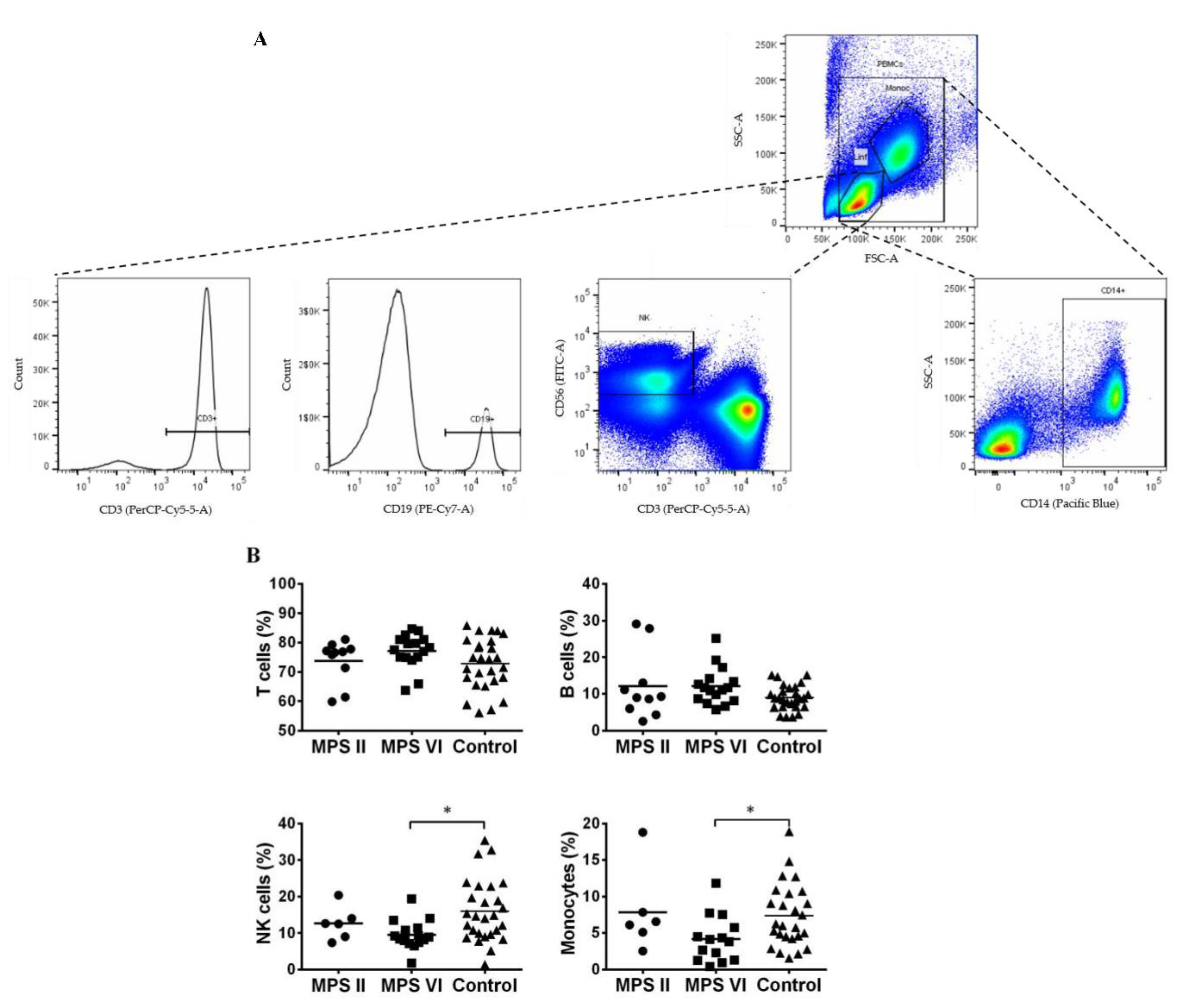
3.1. Major leukocyte populations in MPS II and VI disease patients
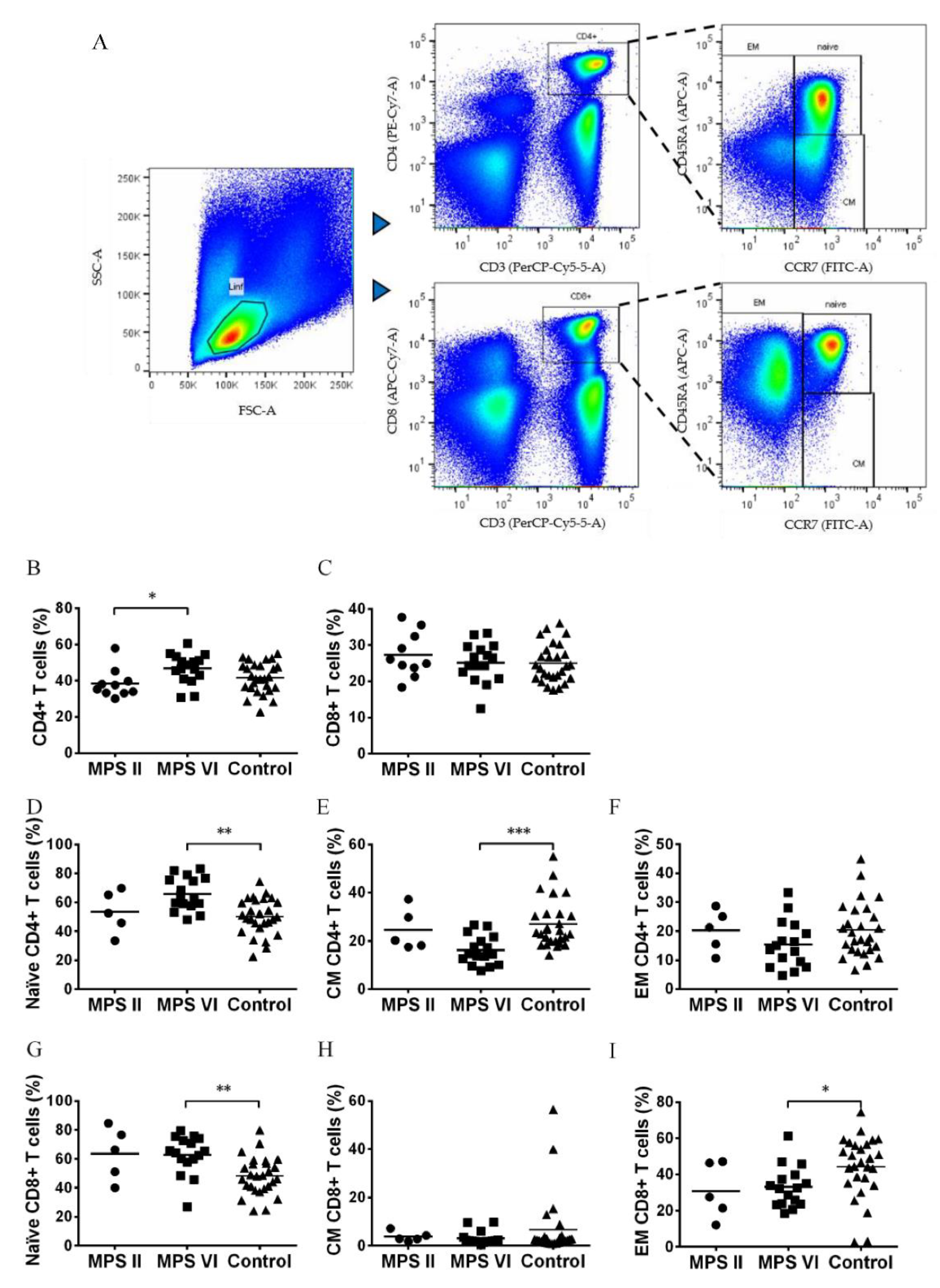
3.2. Naïve vs Memory T cell pool in MPS II and VI disease patients
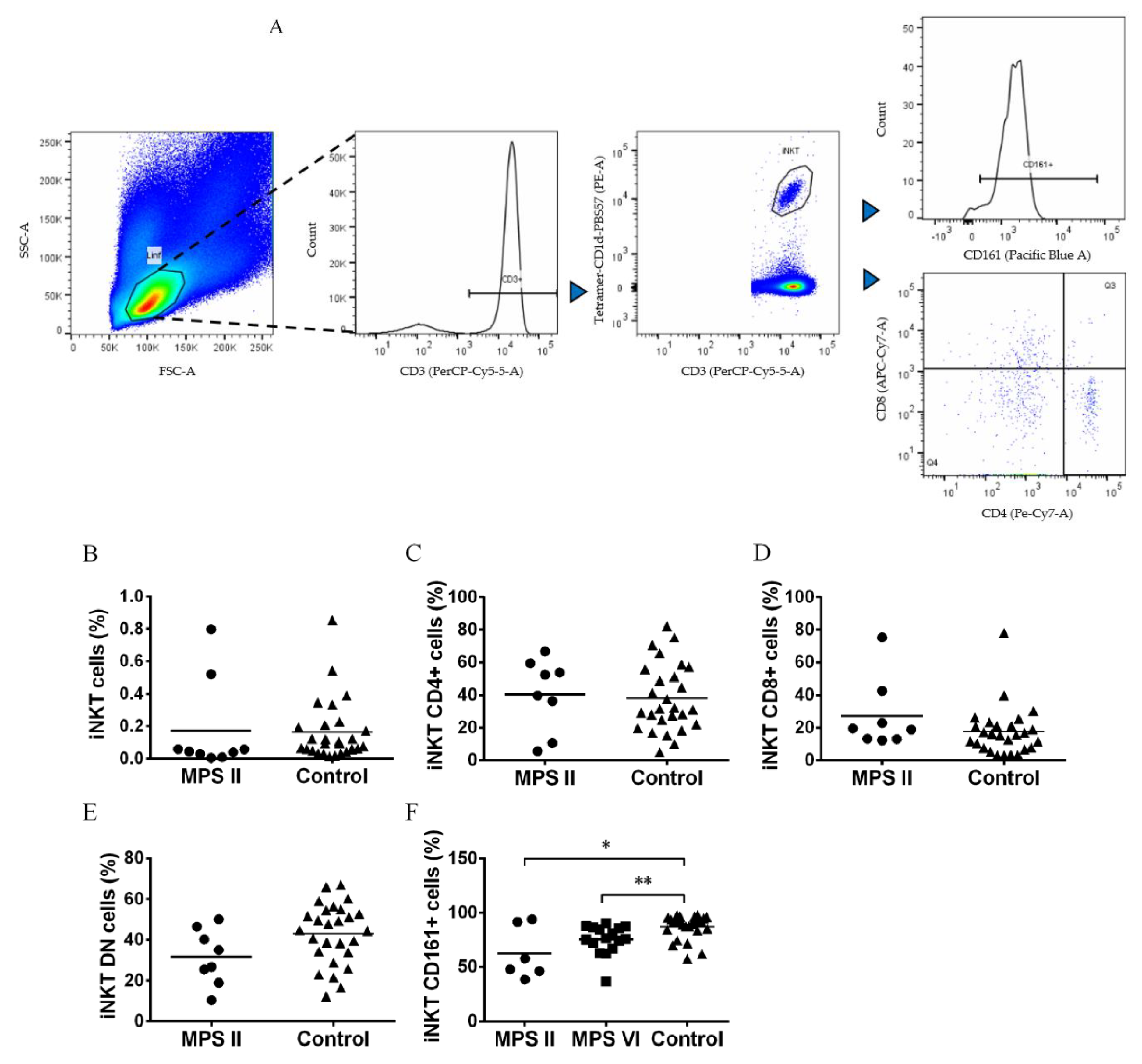
3.3. iNKT cells are normal in number but phenotypically altered in MPS II and MPS VI disease patients
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- M. F. Coutinho, L. Lacerda, and S. Alves, ‘Glycosaminoglycan Storage Disorders: A Review’, Biochem. Res. Int., vol. 2012, pp. 1–16, 2012. [CrossRef]
- S. A. Khan et al., ‘Epidemiology of mucopolysaccharidoses’, Mol. Genet. Metab., vol. 121, no. 3, pp. 227–240, Jul. 2017. [CrossRef]
- ‘Quiagen Site’, QIAGEN Digital Insights. https://my.qiagendigitalinsights.com/ (accessed Feb. 09, 2023).
- R. Giugliani et al., ‘Mucopolysaccharidosis I, II, and VI: brief review and guidelines for treatment’, Genet. Mol. Biol., vol. 33, no. 4, pp. 589–604, Nov. 2010. [CrossRef]
- H. Parker and B. W. Bigger, ‘The role of innate immunity in mucopolysaccharide diseases’, J. Neurochem., p. jnc.14632, Dec. 2018. [CrossRef]
- B. Celik, S. C. Tomatsu, S. Tomatsu, and S. A. Khan, ‘Epidemiology of Mucopolysaccharidoses Update’, Diagnostics, vol. 11, no. 2, p. 273, Feb. 2021. [CrossRef]
- R. Pinto et al., ‘Prevalence of lysosomal storage diseases in Portugal’, Eur. J. Hum. Genet., vol. 12, no. 2, pp. 87–92, Feb. 2004. [CrossRef]
- F. D’Avanzo, A. Zanetti, C. De Filippis, and R. Tomanin, ‘Mucopolysaccharidosis Type VI, an Updated Overview of the Disease’, Int. J. Mol. Sci., vol. 22, no. 24, p. 13456, Dec. 2021. [CrossRef]
- V. Valayannopoulos and F. A. Wijburg, ‘Therapy for the mucopolysaccharidoses’, Rheumatology, vol. 50, no. suppl 5, pp. v49–v59, Dec. 2011. [CrossRef]
- B. K. Burton and D. A. H. Whiteman, ‘Incidence and timing of infusion-related reactions in patients with mucopolysaccharidosis type II (Hunter syndrome) on idursulfase therapy in the real-world setting: A perspective from the Hunter Outcome Survey (HOS)’, Mol. Genet. Metab., vol. 103, no. 2, pp. 113–120, Jun. 2011. [CrossRef]
- P. Harmatz et al., ‘Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: Final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase’, Mol. Genet. Metab., vol. 94, no. 4, pp. 469–475, Aug. 2008. [CrossRef]
- A. Broomfield, S. A. Jones, S. M. Hughes, and B. W. Bigger, ‘The impact of the immune system on the safety and efficiency of enzyme replacement therapy in lysosomal storage disorders’, J. Inherit. Metab. Dis., vol. 39, no. 4, pp. 499–512, Jul. 2016. [CrossRef]
- D. Concolino, F. Deodato, and R. Parini, ‘Enzyme replacement therapy: efficacy and limitations’, Ital. J. Pediatr., vol. 44, no. S2, p. 120, Nov. 2018. [CrossRef]
- B. Donida et al., ‘Oxidative stress and inflammation in mucopolysaccharidosis type IVA patients treated with enzyme replacement therapy’, Biochim. Biophys. Acta BBA - Mol. Basis Dis., vol. 1852, no. 5, pp. 1012–1019, May 2015. [CrossRef]
- G. M. Viana, D. A. Priestman, F. M. Platt, S. Khan, S. Tomatsu, and A. V. Pshezhetsky, ‘Brain Pathology in Mucopolysaccharidoses (MPS) Patients with Neurological Forms’, J. Clin. Med., vol. 9, no. 2, p. 396, Feb. 2020. [CrossRef]
- B. W. Bigger, D. J. Begley, D. Virgintino, and A. V. Pshezhetsky, ‘Anatomical changes and pathophysiology of the brain in mucopolysaccharidosis disorders’, Mol. Genet. Metab., vol. 125, no. 4, pp. 322–331, Dec. 2018. [CrossRef]
- L. D. Archer, K. J. Langford-Smith, B. W. Bigger, and J. E. Fildes, ‘Mucopolysaccharide diseases: A complex interplay between neuroinflammation, microglial activation and adaptive immunity’, J. Inherit. Metab. Dis., vol. 37, no. 1, pp. 1–12, Jan. 2014. [CrossRef]
- E. Fusar Poli et al., ‘Murine neural stem cells model Hunter disease in vitro: glial cell-mediated neurodegeneration as a possible mechanism involved’, Cell Death Dis., vol. 4, no. 11, pp. e906–e906, Nov. 2013. [CrossRef]
- C. M. Simonaro, M. E. Haskins, and E. H. Schuchman, ‘Articular Chondrocytes from Animals with a Dermatan Sulfate Storage Disease Undergo a High Rate of Apoptosis and Release Nitric Oxide and Inflammatory Cytokines: A Possible Mechanism Underlying Degenerative Joint Disease in the Mucopolysaccharidoses’, Lab. Invest., vol. 81, no. 9, pp. 1319–1328, Sep. 2001. [CrossRef]
- i. Mandolfo, H. Parker, and B. Bigger, ‘Innate Immunity in Mucopolysaccharide Diseases’, Int. J. Mol. Sci., vol. 23, no. 4, p. 1999, Feb. 2022. [CrossRef]
- R. Parini and F. Deodato, ‘Intravenous Enzyme Replacement Therapy in Mucopolysaccharidoses: Clinical Effectiveness and Limitations’, Int. J. Mol. Sci., vol. 21, no. 8, p. 2975, Apr. 2020. [CrossRef]
- G. M. Campo et al., ‘Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes’, J. Cell. Biochem., vol. 106, no. 1, pp. 83–92, Jan. 2009. [CrossRef]
- i. M. Naur, A. Anggraini, B. W. Indraswari, S. Wandita, T. Wibowo, and E. L. Haksari, ‘Immunisation issues in patient with mucopolysaccharidosis: A case report’, Med. J. Malaysia, vol. 75, no. Suppl 1, pp. 51–52, May 2020.
- V. Opoka-Winiarska, A. Jurecka, A. Emeryk, and A. Tylki-Szymańska, ‘Osteoimmunology in mucopolysaccharidoses type I, II, VI and VII. Immunological regulation of the osteoarticular system in the course of metabolic inflammation’, Osteoarthritis Cartilage, vol. 21, no. 12, pp. 1813–1823, Dec. 2013. [CrossRef]
- D. A. Simon Davis and C. R. Parish, ‘Heparan Sulfate: A Ubiquitous Glycosaminoglycan with Multiple Roles in Immunity’, Front. Immunol., vol. 4, 2013. [CrossRef]
- G. B. Johnson, G. J. Brunn, Y. Kodaira, and J. L. Platt, ‘Receptor-Mediated Monitoring of Tissue Well-Being Via Detection of Soluble Heparan Sulfate by Toll-Like Receptor 4’, J. Immunol., vol. 168, no. 10, pp. 5233–5239, May 2002. [CrossRef]
- N. S. Gandhi and R. L. Mancera, ‘The Structure of Glycosaminoglycans and their Interactions with Proteins’, Chem. Biol. Drug Des., vol. 72, no. 6, pp. 455–482, Dec. 2008. [CrossRef]
- A. Tessitore, M. Pirozzi, and A. Auricchio, ‘Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI’, PathoGenetics, vol. 2, no. 1, p. 4, Dec. 2009. [CrossRef]
- L. D. Archer, K. J. Langford-Smith, W. R. Critchley, B. W. Bigger, and J. E. Fildes, ‘Characterisation of the T cell and dendritic cell repertoire in a murine model of mucopolysaccharidosis I (MPS I)’, J. Inherit. Metab. Dis., vol. 36, no. 2, pp. 257–262, Mar. 2013. [CrossRef]
- T. M. Daly, R. G. Lorenz, and M. S. Sands, ‘Abnormal Immune Function In Vivo in a Murine Model of Lysosomal Storage Disease’, Pediatr. Res., vol. 47, no. 6, pp. 757–762, Jun. 2000. [CrossRef]
- J. DiRosario et al., ‘Innate and adaptive immune activation in the brain of MPS IIIB mouse model’, J. Neurosci. Res., vol. 87, no. 4, pp. 978–990, Mar. 2009. [CrossRef]
- S. Kilavuz et al., ‘Real-world patient data on immunity and COVID-19 status of patients with MPS, Gaucher, and Pompe diseases from Turkey’, Arch. Pédiatrie, vol. 29, no. 6, pp. 415–423, Aug. 2022. [CrossRef]
- C. S. Pereira et al., ‘Lipid Antigen Presentation by CD1b and CD1d in Lysosomal Storage Disease Patients’, Front. Immunol., vol. 10, p. 1264, Jun. 2019. [CrossRef]
- S. D. Gadola et al., ‘Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases’, J. Exp. Med., vol. 203, no. 10, pp. 2293–2303, Oct. 2006. [CrossRef]
- M. F. Macedo, R. Quinta, C. S. Pereira, and M. C. Sa Miranda, ‘Enzyme replacement therapy partially prevents invariant Natural Killer T cell deficiency in the Fabry disease mouse model’, Mol. Genet. Metab., vol. 106, no. 1, pp. 83–91, May 2012. [CrossRef]
- C. Pereira, H. Ribeiro, and M. Macedo, ‘From Lysosomal Storage Diseases to NKT Cell Activation and Back’, Int. J. Mol. Sci., vol. 18, no. 3, p. 502, Feb. 2017. [CrossRef]
- E. Melum et al., ‘Control of CD1d-restricted antigen presentation and inflammation by sphingomyelin’, Nat. Immunol., vol. 20, no. 12, pp. 1644–1655, Dec. 2019. [CrossRef]
- A. M. Zahran et al., ‘Upregulation of Cytotoxic T-cells in pediatric patients with Gaucher disease’, Sci. Rep., vol. 12, no. 1, p. 4977, Mar. 2022. [CrossRef]
- Y. Burstein, V. Zakuth, G. Rechavi, and Z. Spirer, ‘Abnormalities of cellular immunity and natural killer cells in Gaucher’s disease’, J. Clin. Lab. Immunol., vol. 23, no. 3, pp. 149–151, Jul. 1987.
- A. O. Speak et al., ‘Altered distribution and function of natural killer cells in murine and human Niemann-Pick disease type C1’, Blood, vol. 123, no. 1, pp. 51–60, Jan. 2014. [CrossRef]
- P. Rozenfeld, E. Agriello, N. De Francesco, P. Martinez, and C. Fossati, ‘Leukocyte perturbation associated with Fabry disease’, J. Inherit. Metab. Dis., vol. 32, no. S1, pp. 67–77, Dec. 2009. [CrossRef]
- N. Bettman, I. Avivi, H. Rosenbaum, L. Bisharat, and T. Katz, ‘Impaired migration capacity in monocytes derived from patients with Gaucher disease’, Blood Cells. Mol. Dis., vol. 55, no. 2, pp. 180–186, Aug. 2015. [CrossRef]
- M. Zahran, A. A. Eltayeb, K. I. Elsayh, K. Saad, F.-A. Ahmad, and A. I. M. Ibrahim, ‘Activated and Memory T Lymphocytes in Children with Gaucher Disease’, Arch. Immunol. Ther. Exp. (Warsz.), vol. 65, no. 3, pp. 263–269, Jun. 2017. [CrossRef]
- D. Rigante, C. Cipolla, U. Basile, F. Gulli, and M. C. Savastano, ‘Overview of immune abnormalities in lysosomal storage disorders’, Immunol. Lett., vol. 188, pp. 79–85, Aug. 2017. [CrossRef]
- B. C. Kieseier, K. E. Wisniewski, and H. H. Goebel, ‘The monocyte-macrophage system is affected in lysosomal storage diseases: an immunoelectron microscopic study’, Acta Neuropathol. (Berl.), vol. 94, no. 4, pp. 359–362, Oct. 1997. [CrossRef]
- J. S. Orange, ‘Natural killer cell deficiency’, J. Allergy Clin. Immunol., vol. 132, no. 3, pp. 515–525, Sep. 2013. [CrossRef]
- J. S. Orange, ‘Human natural killer cell deficiencies’:, Curr. Opin. Allergy Clin. Immunol., vol. 6, no. 6, pp. 399–409, Dec. 2006. [CrossRef]
- C.-L. Small et al., ‘NK Cells Play a Critical Protective Role in Host Defense against Acute Extracellular Staphylococcus aureus Bacterial Infection in the Lung’, J. Immunol., vol. 180, no. 8, pp. 5558–5568, Apr. 2008. [CrossRef]
- V. Valayannopoulos, H. Nicely, P. Harmatz, and S. Turbeville, ‘Mucopolysaccharidosis VI’, Orphanet J. Rare Dis., vol. 5, no. 1, p. 5, Dec. 2010. [CrossRef]
- D. Malm, D. S. Halvorsen, L. Tranebjærg, and H. Sjursen, ‘Immunodeficiency in alpha-mannosidosis: a matched case-control study on immunoglobulins, complement factors, receptor density, phagocytosis and intracellular killing in leucocytes’, Eur. J. Pediatr., vol. 159, no. 9, pp. 699–703, Aug. 2000. [CrossRef]
- T. Otomo et al., ‘Mannose 6 phosphorylation of lysosomal enzymes controls B cell functions’, J. Cell Biol., vol. 208, no. 2, pp. 171–180, Jan. 2015. [CrossRef]
- Y. Sagiv et al., ‘Cutting Edge: Impaired Glycosphingolipid Trafficking and NKT Cell Development in Mice Lacking Niemann-Pick Type C1 Protein’, J. Immunol., vol. 177, no. 1, pp. 26–30, Jul. 2006. [CrossRef]
- N. Schrantz, Y. Sagiv, Y. Liu, P. B. Savage, A. Bendelac, and L. Teyton, ‘The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells’, J. Exp. Med., vol. 204, no. 4, pp. 841–852, Apr. 2007. [CrossRef]
- J. Schümann et al., ‘Differential alteration of lipid antigen presentation to NKT cells due to imbalances in lipid metabolism’, Eur. J. Immunol., vol. 37, no. 6, pp. 1431–1441, Jun. 2007. [CrossRef]
- F. W. McNab et al., ‘The Influence of CD1d in Postselection NKT Cell Maturation and Homeostasis’, J. Immunol., vol. 175, no. 6, pp. 3762–3768, Sep. 2005. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or properdty resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




