Submitted:
31 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
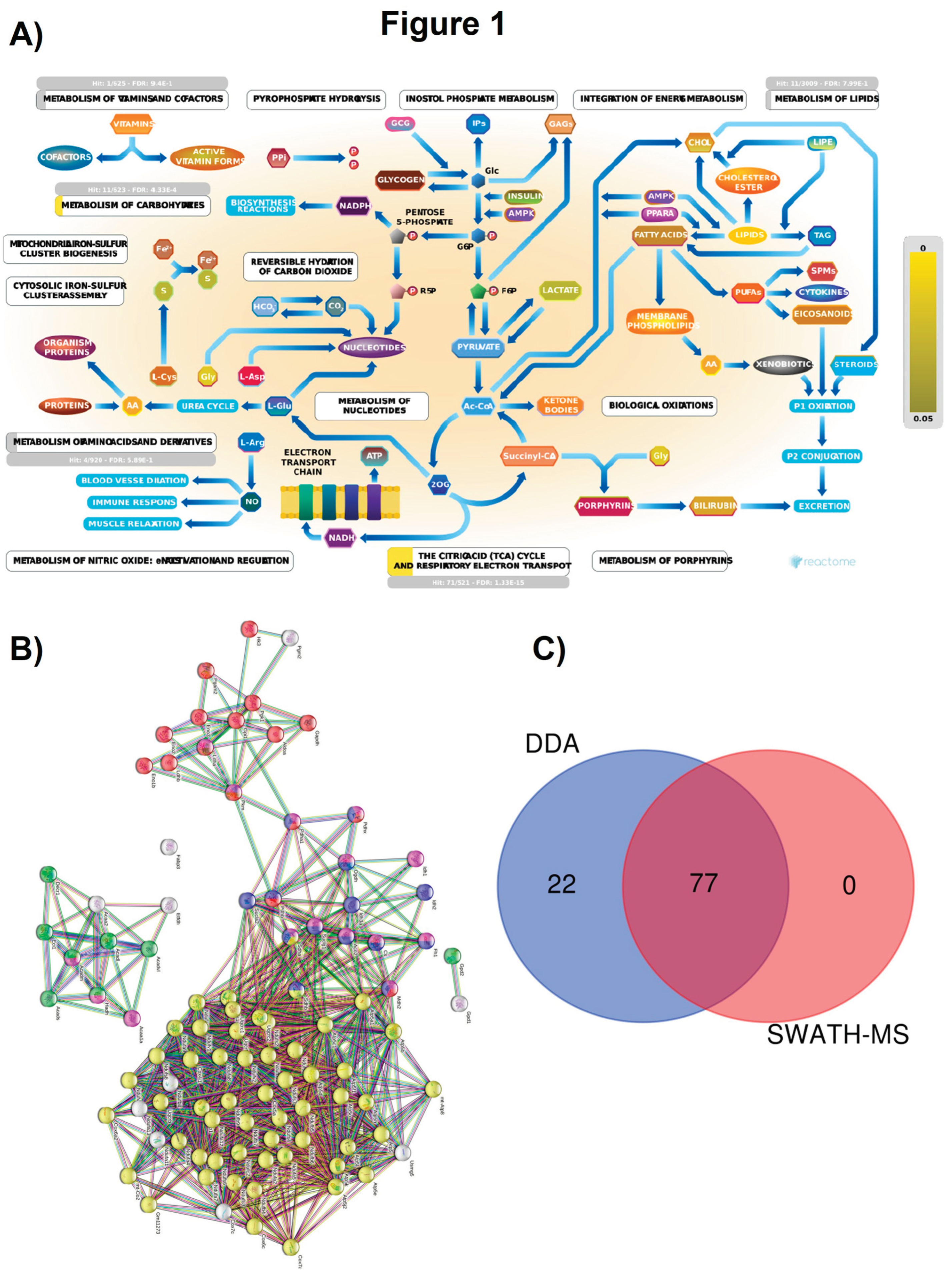
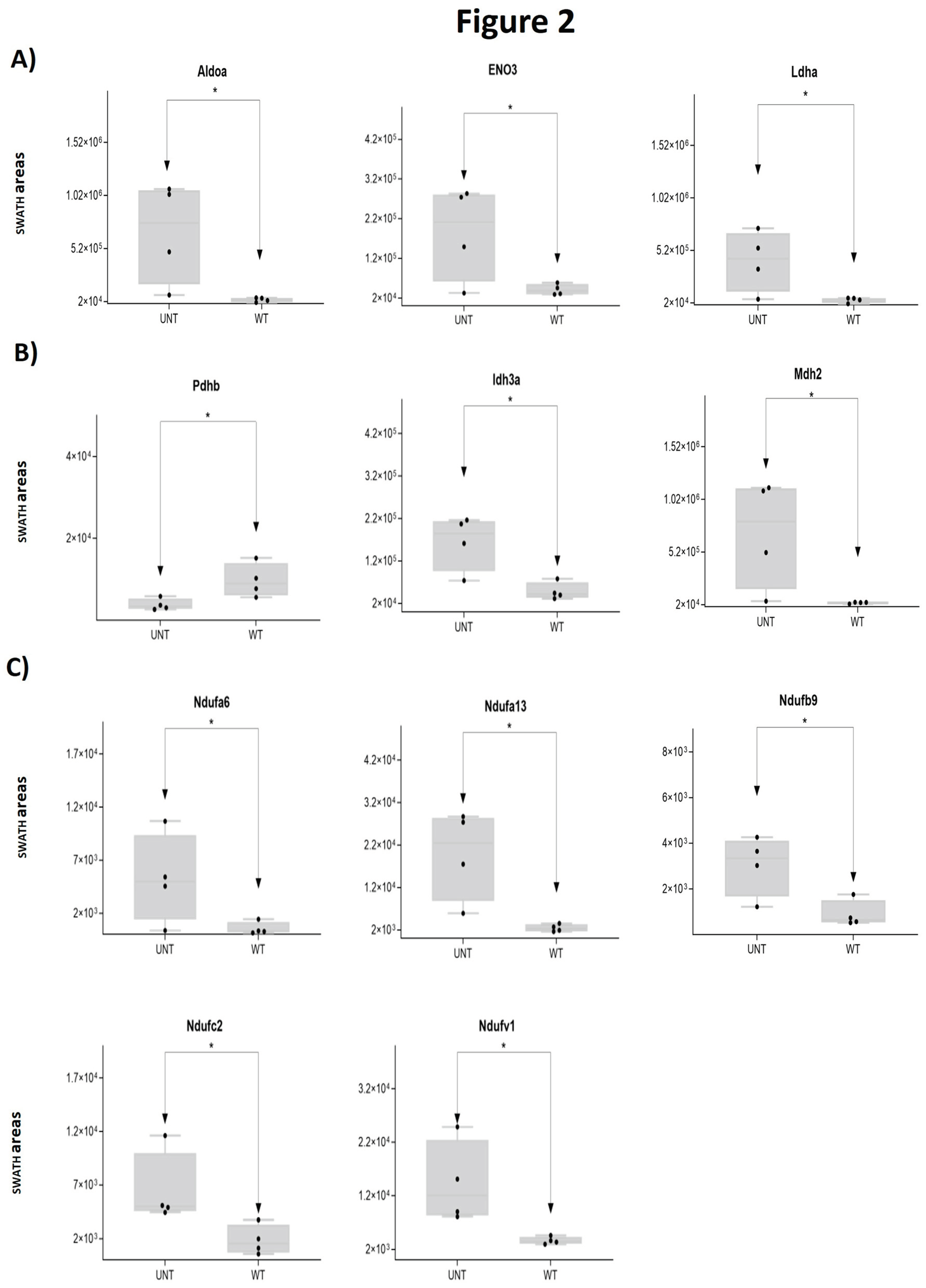
2.1. Section I: Dysregulation of Metabolic Pathways Implicated in Energy Production
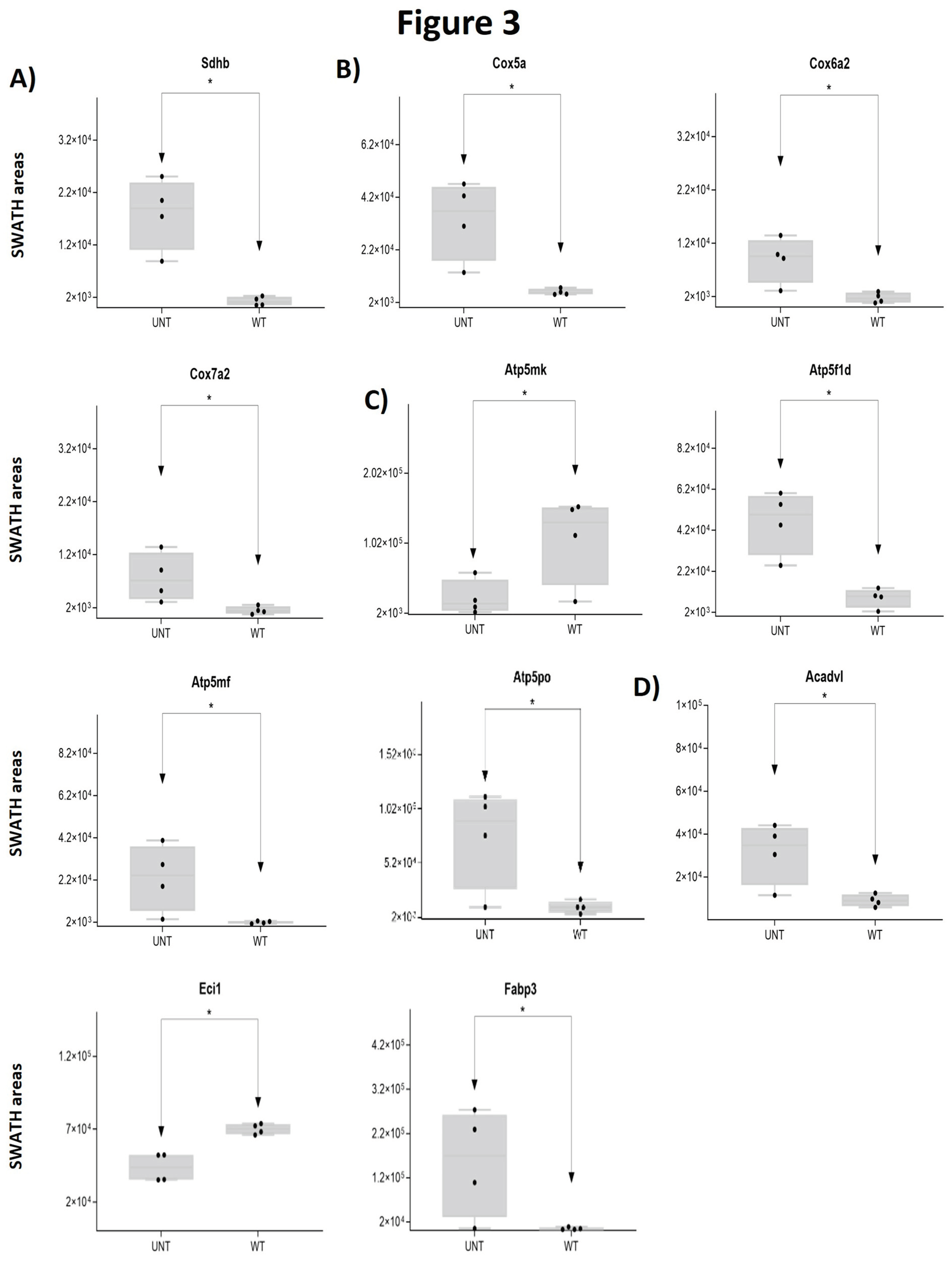
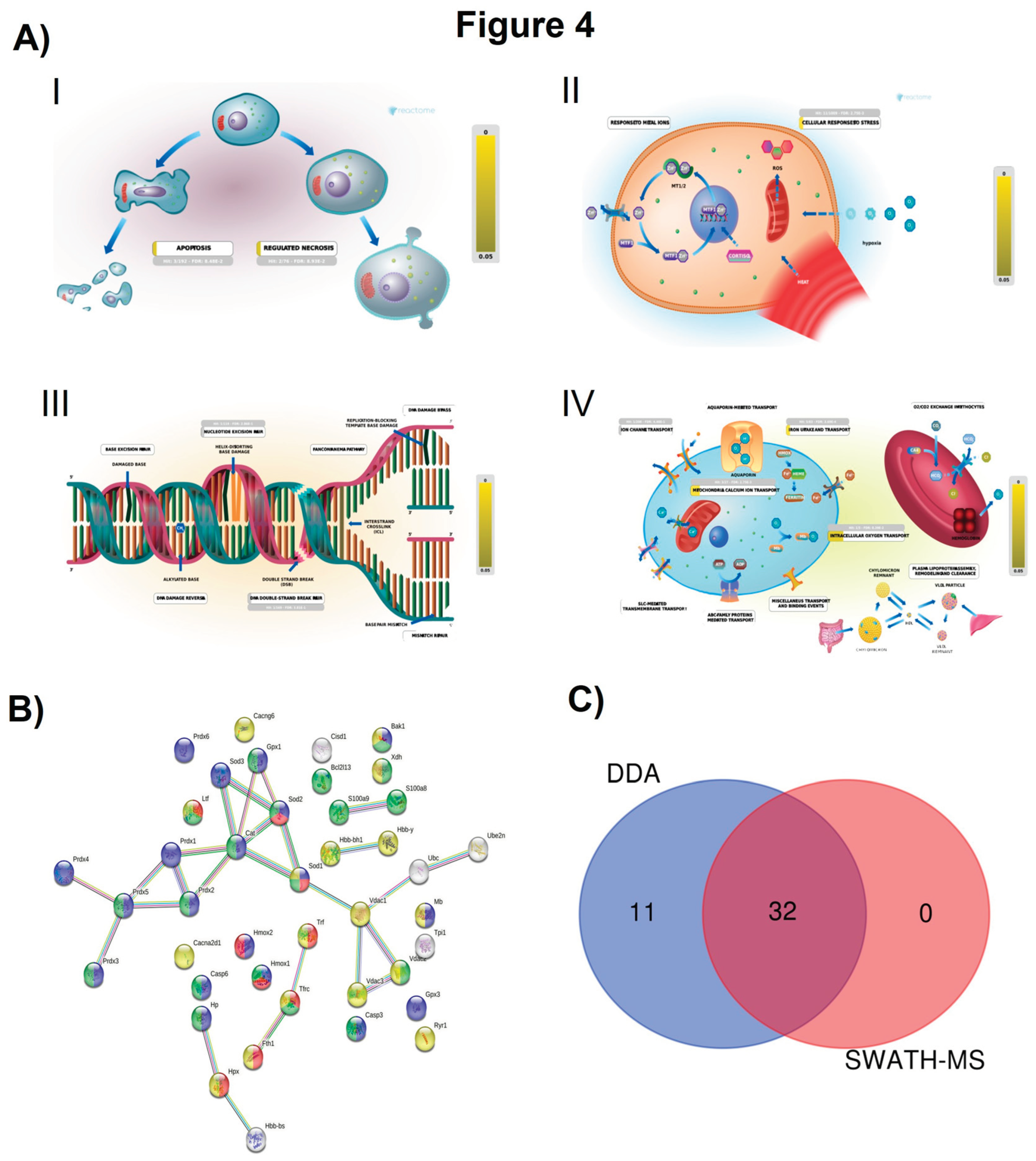
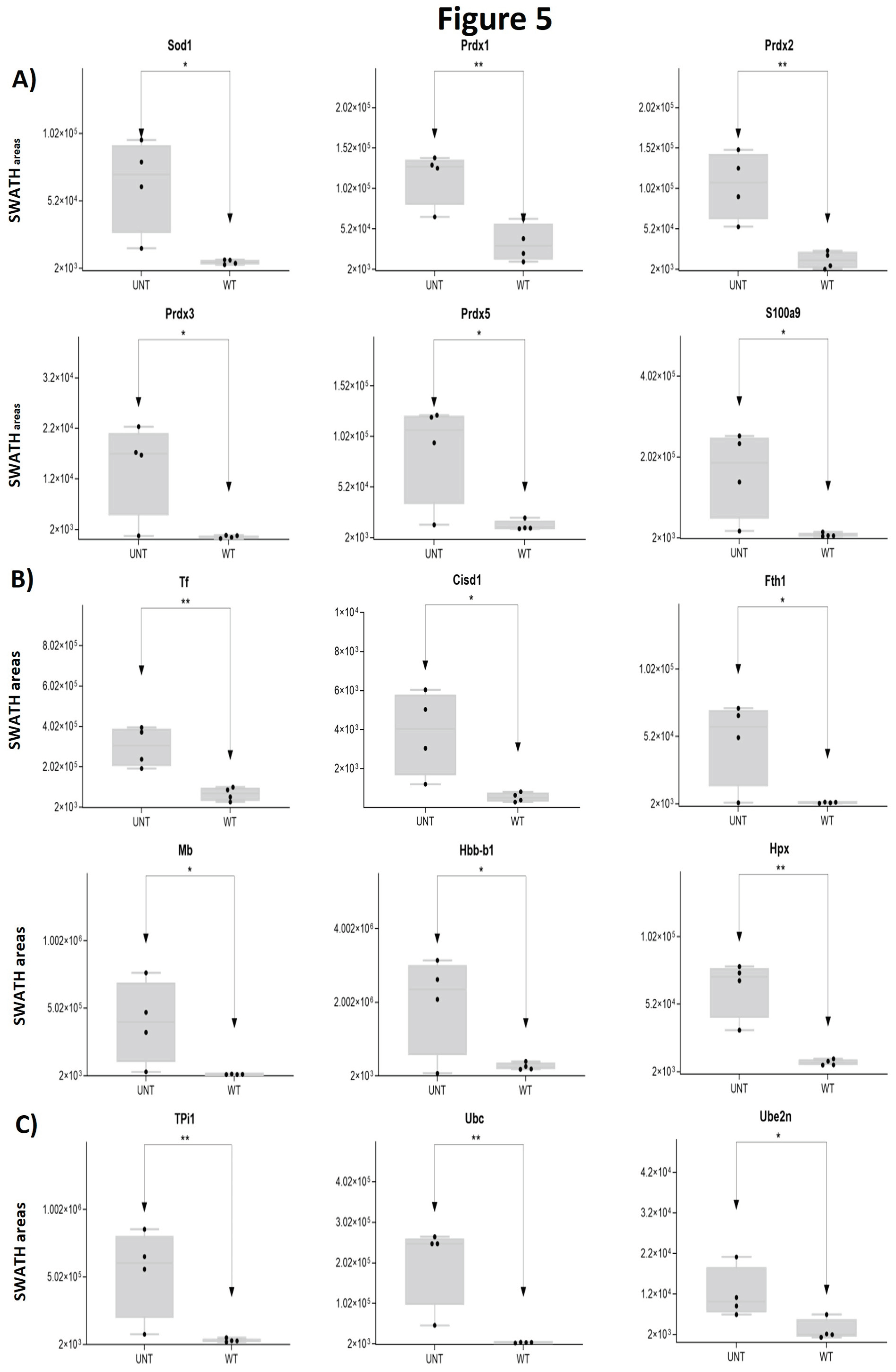
2.2. Section II: Proteins Related to Mitochondria Function: Oxidative Stress, Voltage-Dependent Channels, Apoptosis, Compensatory Mechanisms of Oxidative Stress, and DNA Damage
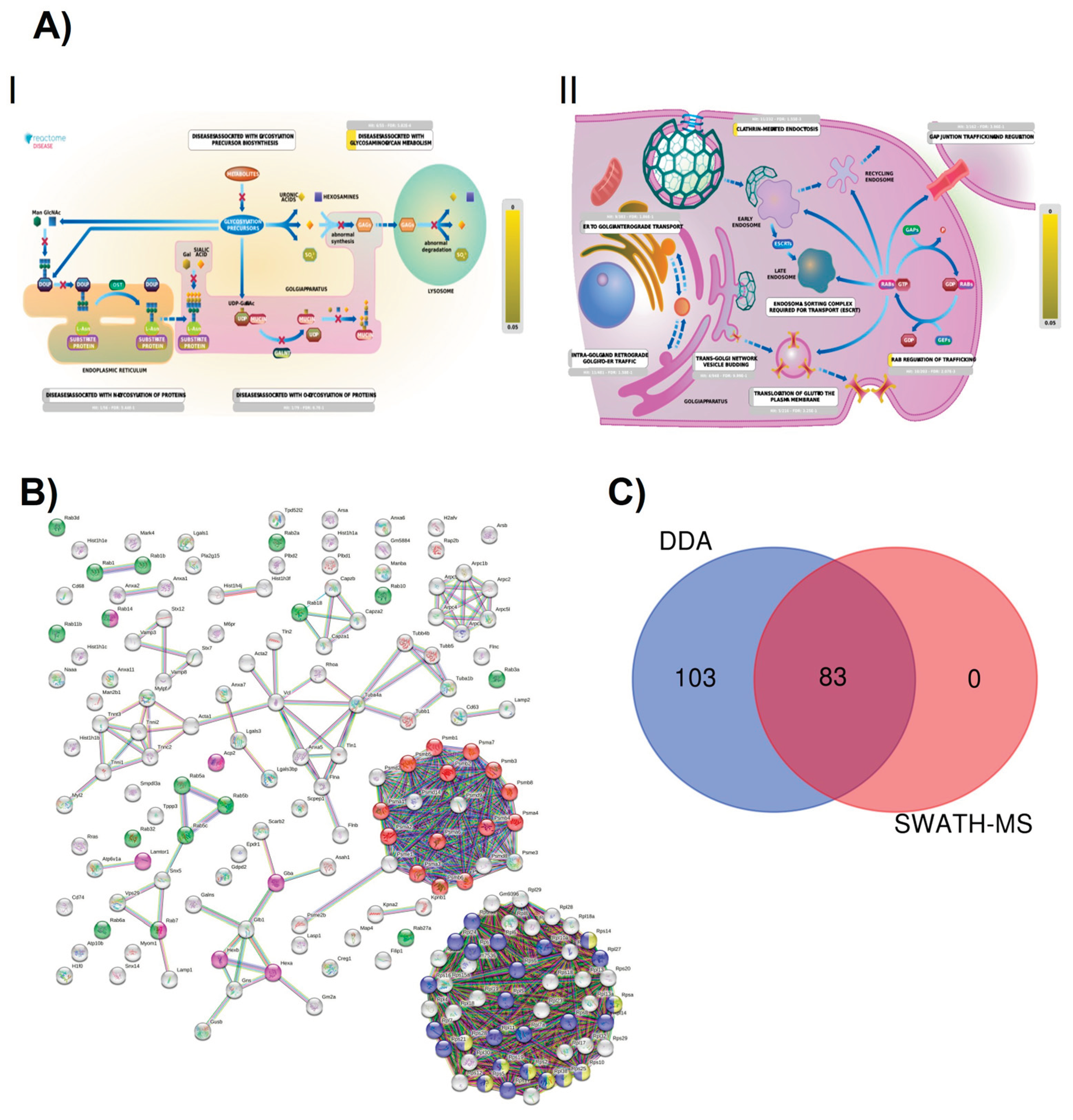
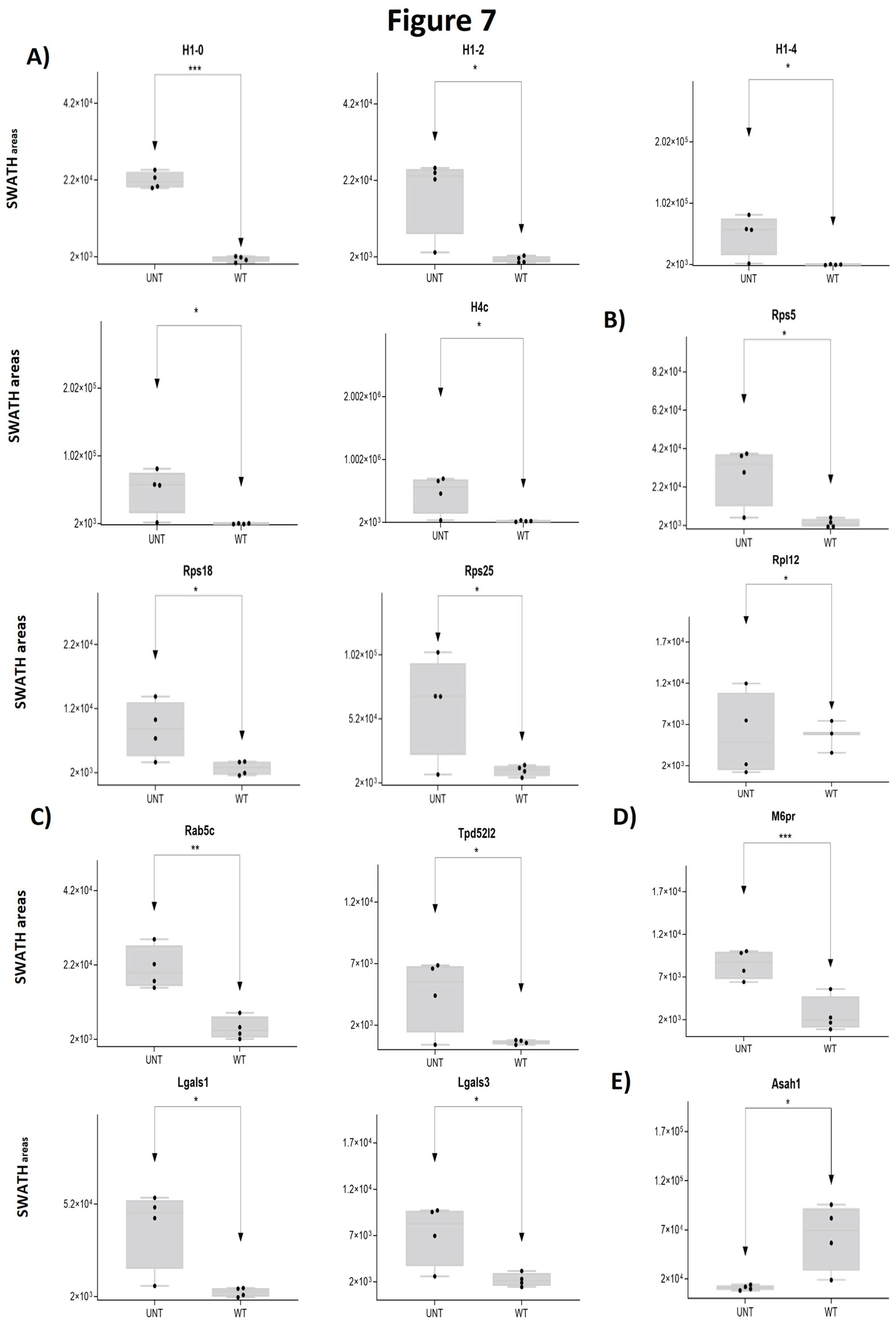
2.3. Section III. Histones, Ribosomes, Proteasomes, Vesicular Transport and Lysosomes
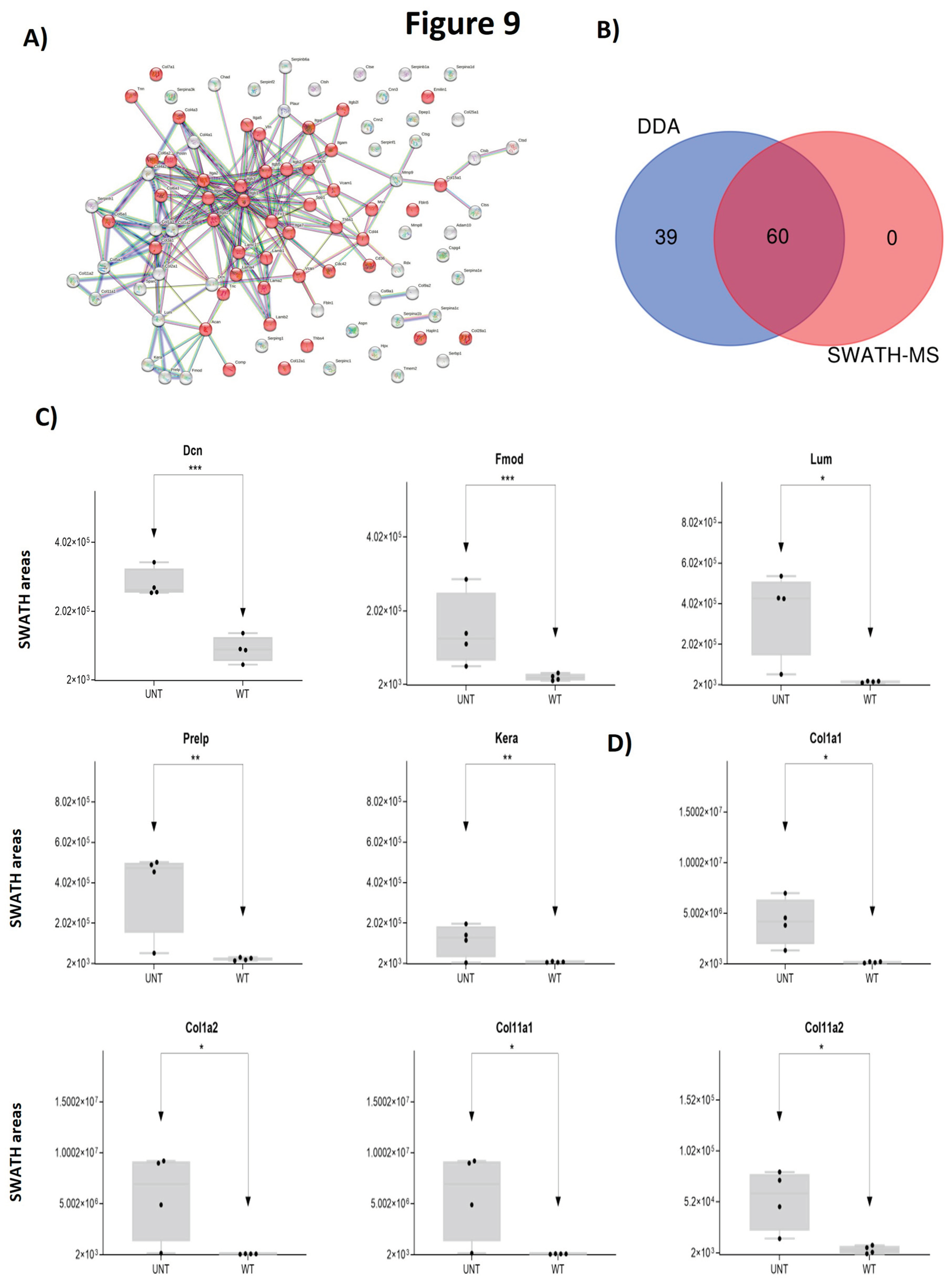
2.4. Section IV. Extracellular matrix.
- Small leucine-rich proteoglycans: DDA identified Class I, II, and IV proteins (Supplementary Table S1).
- ○
- Class I proteins: Dcn was significantly upregulated in UNT mice (FC >3; Figure 9c, Supplementary Table S3, Section 4.1). Aspn was detected in UNT mice only.
- ○
- Class II proteins: DDA identified 4 proteins (Fmod, Lum, Prelp, Kera; Supplementary Table S1), all of which were significantly upregulated in UNT mice with FC >3 (Figure 9c, Table S III, Section 4.1)
- ○
- Class IV: Only Chad was significantly upregulated in UNT mice, with a FC <3 (Table S3, Section 4.1).
- Cell surface proteoglycans: DDA identified proteins related to the cell surface (e.g., Cspg4; Supplementary Table S1), none of which were differentially expressed in UNT versus WT.
- Fibrillar collagen: DDA identified 8 proteins related to fibrillar collagen, 5 of which were significantly upregulated in UNT mice, 4 with an FC <3 (Col1a1, Col1a2, Col11a1, and Col11a2) (Figure 9d, Supplementary Table S3, Section 4.2). Col5a1 was identified only in WT mice.
- Networking-forming collagen: 3 proteins were identified, one of which was present only in WT mice (Col4a1).
- FACITs, Beaded-filament-forming collagens: 3 proteins identified.
- MACITs, anchoring fibrils, multiplexin: 1 protein identified.
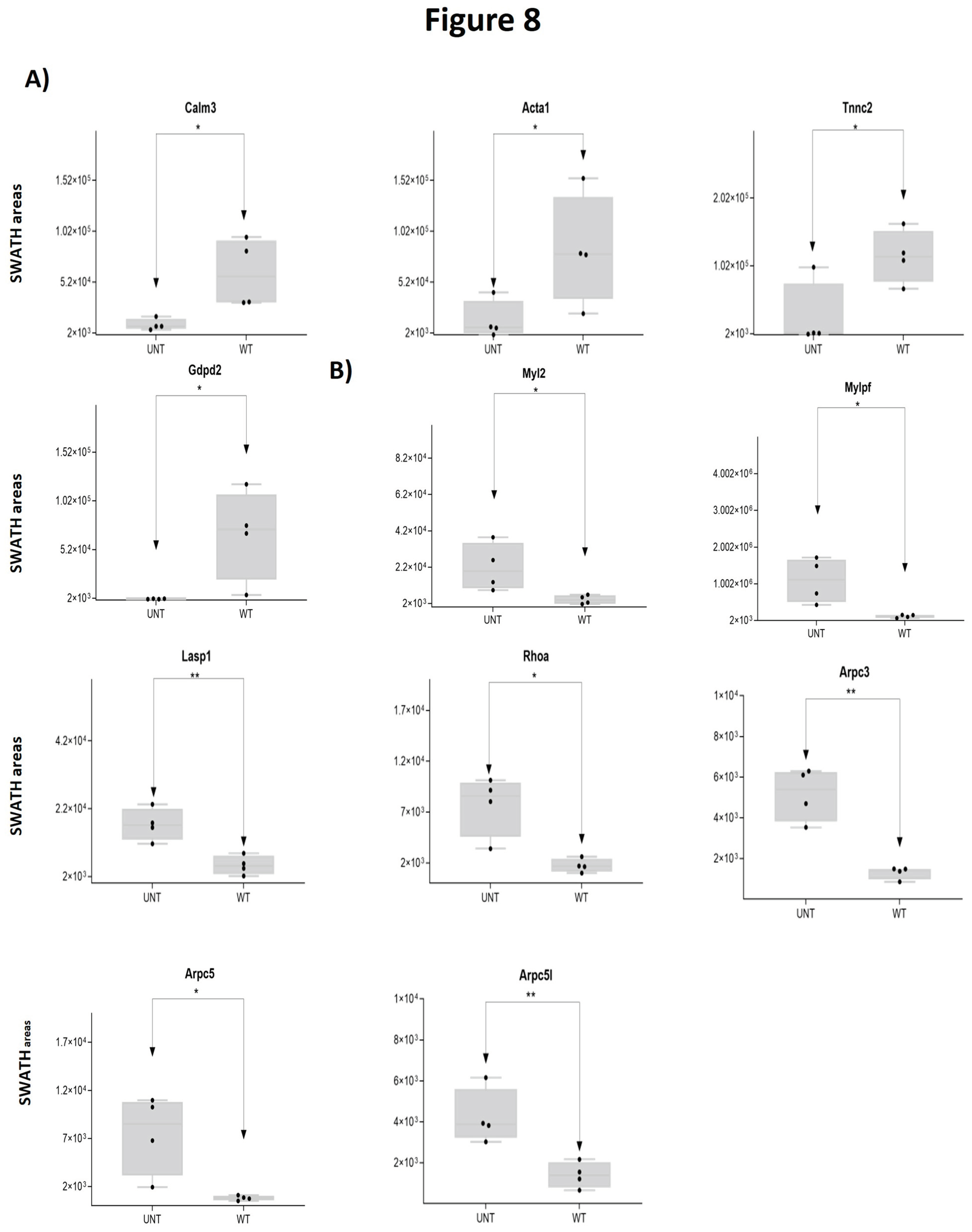
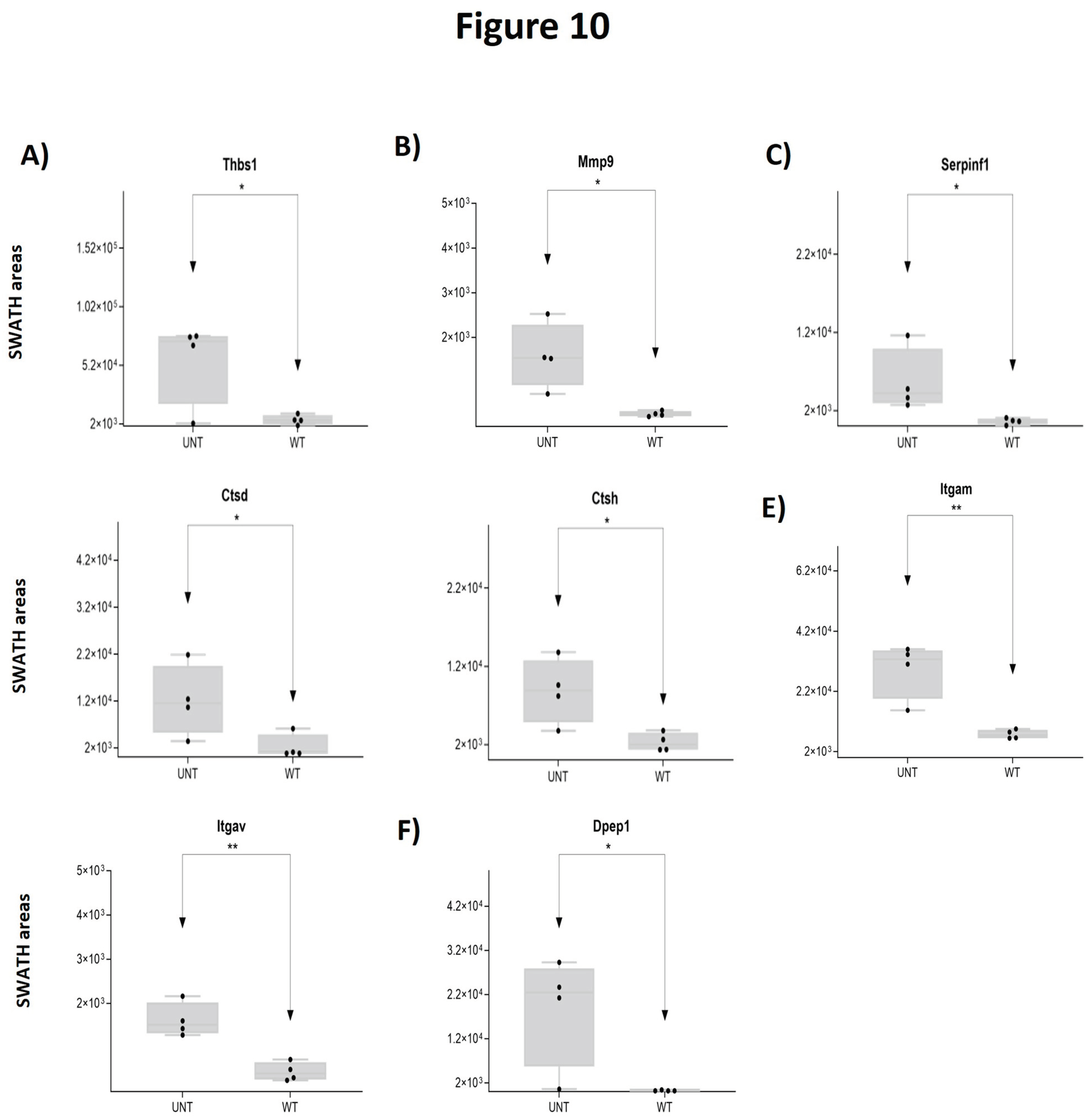
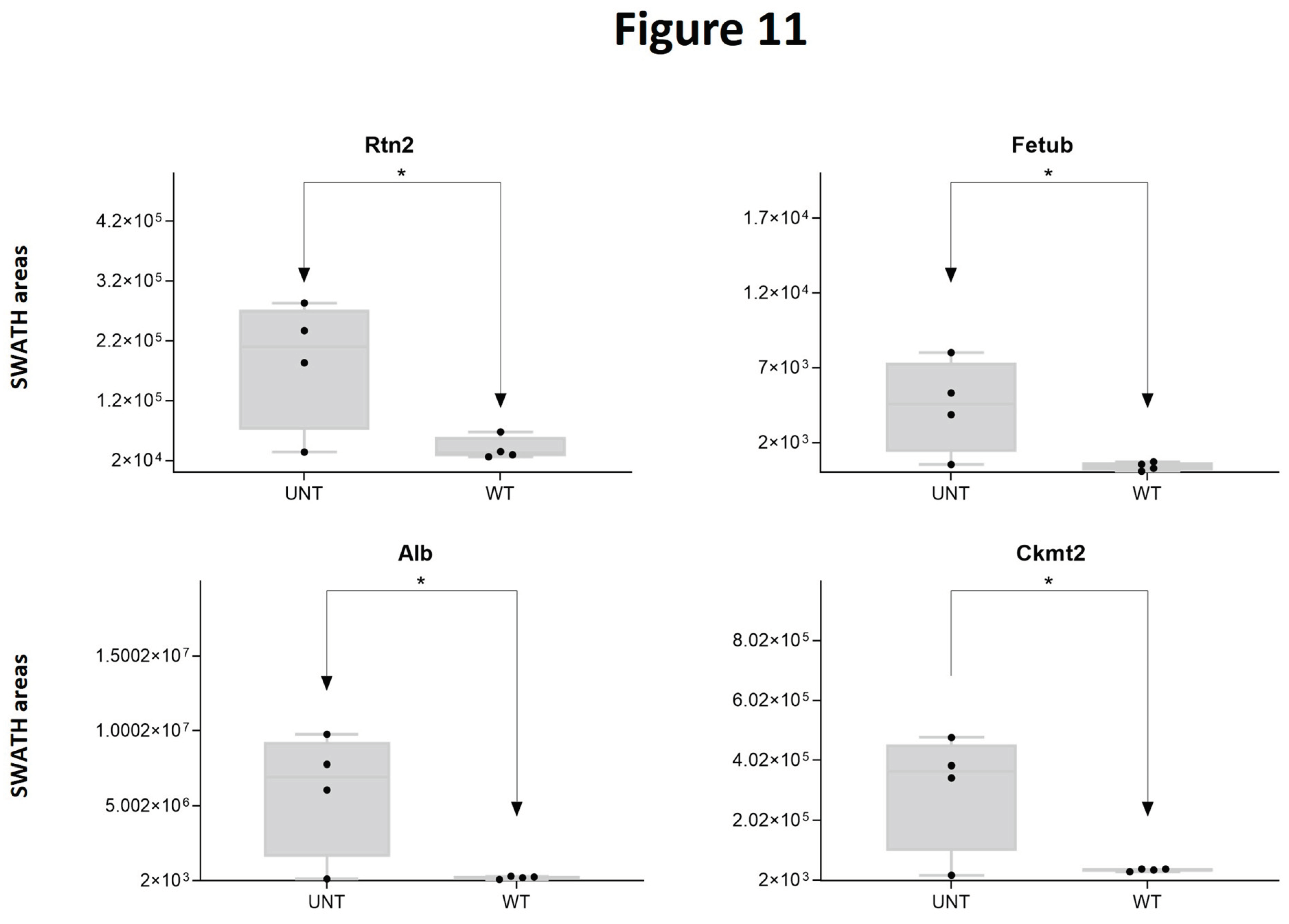
2.5. Section V. Other Proteins of Interest
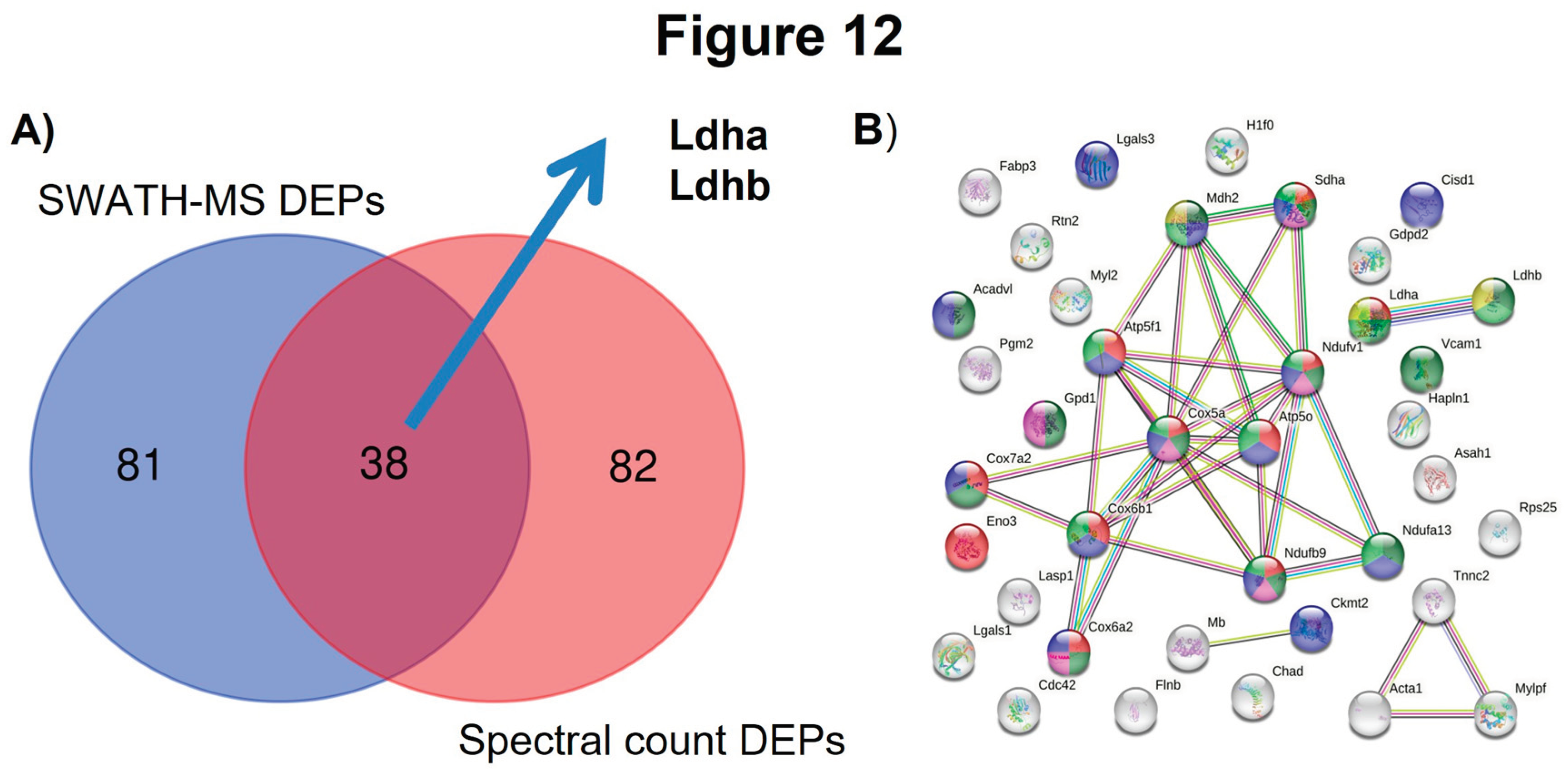
3. Quantitative Validation of DEPs
3.1. Mouse DEPs Validation by Different Proteomic Technology
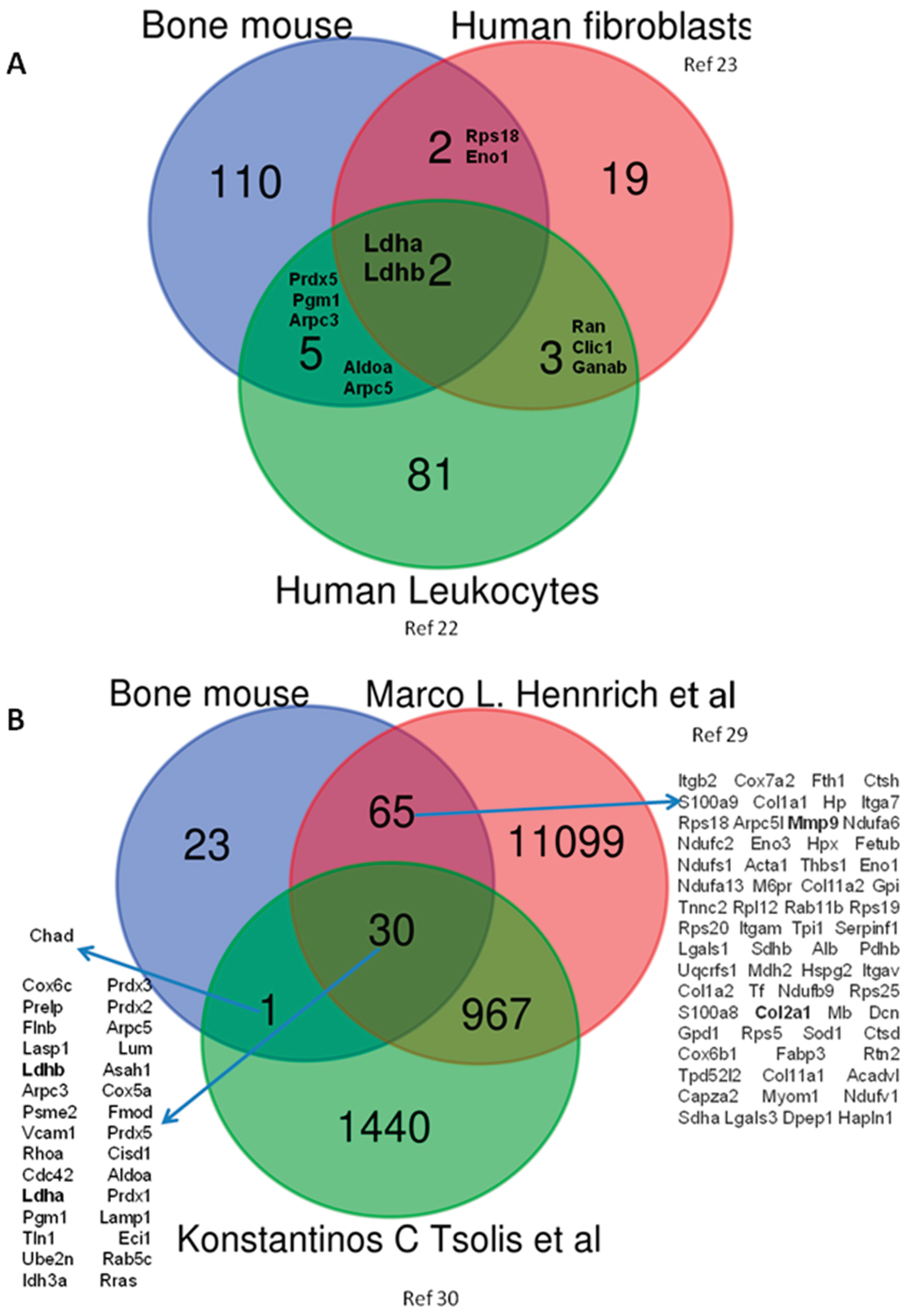
3.2. In Silico Validation of Mouse DEPs Using Data Sets from Human Samples
4. Discussion
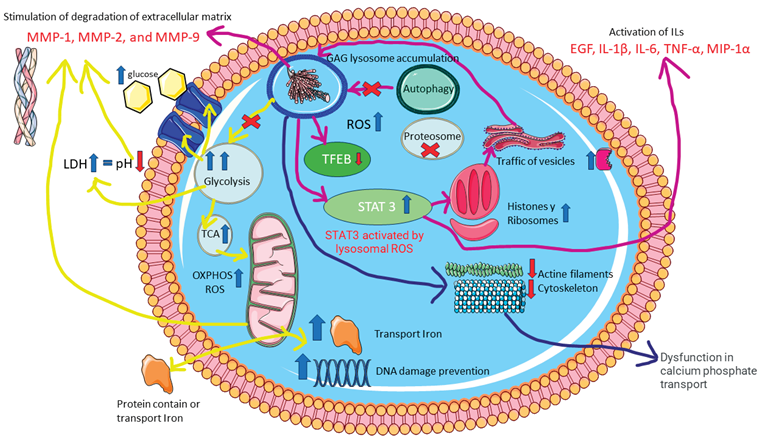
5. Material and Methods
5.1. Experimental Design and Statistical Analysis
5.2. Animal Model and Bone Protein Extraction
5.3. Functional and Pathway Analysis
5.4. In Silico Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability
Conflicts of Interest
Abbreviations:
References
- Khan, S.; Alméciga-Díaz, C.J.; Sawamoto, K.; Mackenzie, W.G.; Theroux, M.C.; Pizarro, C.; Mason, R.W.; Orii, T.; Tomatsu, S. Mucopolysaccharidosis IVA and Glycosaminoglycans. Mol. Genet. Metab. 2017, 120, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, K.; Álvarez González, J.V.; Piechnik, M.; Otero, F.J.; Couce, M.L.; Suzuki, Y.; Tomatsu, S. Mucopolysaccharidosis IVA: Diagnosis, Treatment, and Management. Int. J. Mol. Sci. 2020, 21, 1517. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Averill, L.W.; Sawamoto, K.; Mackenzie, W.G.; Bober, M.B.; Pizarro, C.; Goff, C.J.; Xie, L.; Orii, T.; Theroux, M. Obstructive Airway in Morquio A Syndrome, the Past, the Present and the Future. Mol. Genet. Metab. 2016, 117, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Melbouci, M.; Mason, R.W.; Suzuki, Y.; Fukao, T.; Orii, T.; Tomatsu, S. Growth Impairment in Mucopolysaccharidoses. Mol. Genet. Metab. 2018, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Çelik, B.; Tomatsu, S.C.; Tomatsu, S.; Khan, S.A. Epidemiology of Mucopolysaccharidoses Update. Diagn. Basel Switz. 2021, 11, 273. [Google Scholar] [CrossRef]
- Montaño, A.M.; Tomatsu, S.; Gottesman, G.S.; Smith, M.; Orii, T. International Morquio A Registry: Clinical Manifestation and Natural Course of Morquio A Disease. J. Inherit. Metab. Dis. 2007, 30, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Mackenzie, W.G.; Theroux, M.C.; Mason, R.W.; Thacker, M.M.; Shaffer, T.H.; Montaño, A.M.; Rowan, D.; Sly, W.; Alméciga-Díaz, C.J.; et al. Current and Emerging Treatments and Surgical Interventions for Morquio A Syndrome: A Review. Res. Rep. Endocr. Disord. 2012, 2012, 65–77. [Google Scholar] [CrossRef]
- Lavery, C.; Hendriksz, C. Mortality in Patients with Morquio Syndrome a. JIMD Rep. 2015, 15, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Akyol, M.U.; Alden, T.D.; Amartino, H.; Ashworth, J.; Belani, K.; Berger, K.I.; Borgo, A.; Braunlin, E.; Eto, Y.; Gold, J.I.; et al. Recommendations for the Management of MPS IVA: Systematic Evidence- and Consensus-Based Guidance. Orphanet J. Rare Dis. 2019, 14, 137. [Google Scholar] [CrossRef]
- Tomatsu, S.; M. Montano, A.; Oikawa, H.; J. Rowan, D.; Smith, M.; Barrera, L.; Chinen, Y.; M. Thacker, M.; G. Mackenzie, W.; Suzuki, Y.; et al. Mucopolysaccharidosis Type IVA (Morquio A Disease): Clinical Review and Current Treatment: A Special Review. Curr. Pharm. Biotechnol. 2011, 12, 931–945. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. The Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- De Franceschi, L.; Roseti, L.; Desando, G.; Facchini, A.; Grigolo, B. A Molecular and Histological Characterization of Cartilage from Patients with Morquio Syndrome. Osteoarthritis Cartilage 2007, 15, 1311–1317. [Google Scholar] [CrossRef]
- Hendriksz, C.J.; Lavery, C.; Coker, M.; Ucar, S.K.; Jain, M.; Bell, L.; Lampe, C. Burden of Disease in Patients with Morquio A Syndrome: Results from an International Patient-Reported Outcomes Survey. Orphanet J. Rare Dis. 2014, 9, 32. [Google Scholar] [CrossRef]
- Morrone, A.; Caciotti, A.; Atwood, R.; Davidson, K.; Du, C.; Francis-Lyon, P.; Harmatz, P.; Mealiffe, M.; Mooney, S.; Oron, T.R.; et al. Morquio A Syndrome-Associated Mutations: A Review of Alterations in the GALNS Gene and a New Locus-Specific Database. Hum. Mutat. 2014, 35, 1271–1279. [Google Scholar] [CrossRef]
- Tebani, A.; Mauhin, W.; Abily-Donval, L.; Lesueur, C.; Berger, M.G.; Nadjar, Y.; Berger, J.; Benveniste, O.; Lamari, F.; Laforêt, P.; et al. A Proteomics-Based Analysis Reveals Predictive Biological Patterns in Fabry Disease. J. Clin. Med. 2020, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Hackenberg, Y.; Li, J.; Winter, D. Updates on the Study of Lysosomal Protein Dynamics: Possibilities for the Clinic. Expert Rev. Proteomics 2023, 1–9. [Google Scholar] [CrossRef]
- Tomatsu, S.; Orii, K.O.; Vogler, C.; Nakayama, J.; Levy, B.; Grubb, J.H.; Gutierrez, M.A.; Shim, S.; Yamaguchi, S.; Nishioka, T.; et al. Mouse Model of N-Acetylgalactosamine-6-Sulfate Sulfatase Deficiency (Galns-/-) Produced by Targeted Disruption of the Gene Defective in Morquio A Disease. Hum. Mol. Genet. 2003, 12, 3349–3358. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, V.J.; Bravo, S.B.; Chantada-Vazquez, M.P.; Colón, C.; De Castro, M.J.; Morales, M.; Vitoria, I.; Tomatsu, S.; Otero-Espinar, F.J.; Couce, M.L. Characterization of New Proteomic Biomarker Candidates in Mucopolysaccharidosis Type IVA. Int. J. Mol. Sci. 2020, 22, 226. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, J.V.; Bravo, S.B.; García-Vence, M.; De Castro, M.J.; Luzardo, A.; Colón, C.; Tomatsu, S.; Otero-Espinar, F.J.; Couce, M.L. Proteomic Analysis in Morquio A Cells Treated with Immobilized Enzymatic Replacement Therapy on Nanostructured Lipid Systems. Int. J. Mol. Sci. 2019, 20, 4610. [Google Scholar] [CrossRef]
- Hennrich, M.L.; Romanov, N.; Horn, P.; Jaeger, S.; Eckstein, V.; Steeples, V.; Ye, F.; Ding, X.; Poisa-Beiro, L.; Lai, M.C.; et al. Cell-Specific Proteome Analyses of Human Bone Marrow Reveal Molecular Features of Age-Dependent Functional Decline. Nat. Commun. 2018, 9, 4004. [Google Scholar] [CrossRef]
- Tsolis, K.C.; Bei, E.S.; Papathanasiou, I.; Kostopoulou, F.; Gkretsi, V.; Kalantzaki, K.; Malizos, K.; Zervakis, M.; Tsezou, A.; Economou, A. Comparative Proteomic Analysis of Hypertrophic Chondrocytes in Osteoarthritis. Clin. Proteomics 2015, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.; Gillet, L.; Rosenberger, G.; Amon, S.; Collins, B.C.; Aebersold, R. Data-Independent Acquisition-Based SWATH-MS for Quantitative Proteomics: A Tutorial. Mol. Syst. Biol. 2018, 14, e8126. [Google Scholar] [CrossRef] [PubMed]
- Chantada-Vázquez, M.D.P.; García Vence, M.; Serna, A.; Núñez, C.; Bravo, S.B. SWATH-MS Protocols in Human Diseases. Methods Mol. Biol. Clifton NJ 2021, 2259, 105–141. [Google Scholar] [CrossRef]
- Álvarez, J.V.; Bravo, S.B.; Chantada-Vázquez, M.P.; Barbosa-Gouveia, S.; Colón, C.; López-Suarez, O.; Tomatsu, S.; Otero-Espinar, F.J.; Couce, M.L. Plasma Proteomic Analysis in Morquio A Disease. Int. J. Mol. Sci. 2021, 22, 6165. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, D.H.; Hwang, S.-I.; Wu, L.; Han, D.K. Role of Spectral Counting in Quantitative Proteomics. Expert Rev. Proteomics 2010, 7, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Maor, G.; Vasiliver-Shamis, G.; Hazan-Brill, R.; Wertheimer, E.; Karnieli, E. GLUT4 in Murine Bone Growth: From Uptake and Translocation to Proliferation and Differentiation. Am. J. Physiol.-Endocrinol. Metab. 2011, 300, E613–E623. [Google Scholar] [CrossRef] [PubMed]
- Varzideh, F.; Jankauskas, S.S.; Kansakar, U.; Mone, P.; Gambardella, J.; Santulli, G. Sortilin Drives Hypertension by Modulating Sphingolipid/Ceramide Homeostasis and by Triggering Oxidative Stress. J. Clin. Invest. 132, e156624. [CrossRef]
- Mobasheri, A.; Dobson, H.; Mason, S.; Cullingham, F.; Shakibaei, M.; Moley, J.; Moley, K. Expression of the GLUT1 and GLUT9 Facilitative Glucose Transporters in Embryonic Chondroblasts and Mature Chondrocytes in Ovine Articular Cartilage. Cell Biol. Int. 2005, 29, 249–260. [Google Scholar] [CrossRef]
- Peansukmanee, S.; Vaughan-Thomas, A.; Carter, S.D.; Clegg, P.D.; Taylor, S.; Redmond, C.; Mobasheri, A. Effects of Hypoxia on Glucose Transport in Primary Equine Chondrocytes in Vitro and Evidence of Reduced GLUT1 Gene Expression in Pathologic Cartilage in Vivo. J. Orthop. Res. 2009, 27, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Arra, M.; Swarnkar, G.; Ke, K.; Otero, J.E.; Ying, J.; Duan, X.; Maruyama, T.; Rai, M.F.; O’Keefe, R.J.; Mbalaviele, G.; et al. LDHA-Mediated ROS Generation in Chondrocytes Is a Potential Therapeutic Target for Osteoarthritis. Nat. Commun. 2020, 11, 3427. [Google Scholar] [CrossRef]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase; StatPearls Publishing, 2022;
- De Simoni, S.; Goemaere, J.; Knoops, B. Silencing of Peroxiredoxin 3 and Peroxiredoxin 5 Reveals the Role of Mitochondrial Peroxiredoxins in the Protection of Human Neuroblastoma SH-SY5Y Cells toward MPP+. Neurosci. Lett. 2008, 433, 219–224. [Google Scholar] [CrossRef]
- Donida, B.; Marchetti, D.P.; Biancini, G.B.; Deon, M.; Manini, P.R.; da Rosa, H.T.; Moura, D.J.; Saffi, J.; Bender, F.; Burin, M.G.; et al. Oxidative Stress and Inflammation in Mucopolysaccharidosis Type IVA Patients Treated with Enzyme Replacement Therapy. Biochim. Biophys. Acta 2015, 1852, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta BBA–Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fábregas, J.; Prescott, A.; van Kasteren, S.; Pedrioli, D.L.; McLean, I.; Moles, A.; Reinheckel, T.; Poli, V.; Watts, C. Lysosomal Protease Deficiency or Substrate Overload Induces an Oxidative-Stress Mediated STAT3-Dependent Pathway of Lysosomal Homeostasis. Nat. Commun. 2018, 9, 5343. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Ballabio, A. Lysosomal Adaptation: How the Lysosome Responds to External Cues. Cold Spring Harb. Perspect. Biol. 2014, 6, a016907. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X. Lysosome Biogenesis: Regulation and Functions. J. Cell Biol. 2021, 220, e202102001. [Google Scholar] [CrossRef] [PubMed]
- Frontiers | Proteasome Inhibition Activates Autophagy-Lysosome Pathway Associated With TFEB Dephosphorylation and Nuclear Translocation. Available online: https://www.frontiersin.org/articles/10.3389/fcell.2019.00170/full (accessed on 15 March 2023).
- Jeger, J.L. Endosomes, Lysosomes, and the Role of Endosomal and Lysosomal Biogenesis in Cancer Development. Mol. Biol. Rep. 2020, 47, 9801–9810. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K. The Proteasome: Overview of Structure and Functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 12–36. [Google Scholar] [CrossRef]
- Biomarkers in Patients with Mucopolysaccharidosis Type II and IV | Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S2214426918301265?token=46217FB08DA3E84FBF0C9306F0C27566A213946844D570840EDC9E018BAC9274A5432276AA421658FF010B9A9526A330&originRegion=eu-west-1&originCreation=20230314121158 (accessed on 14 March 2023).
- Xu, X.; Xiong, X.; Sun, Y. The Role of Ribosomal Proteins in the Regulation of Cell Proliferation, Tumorigenesis, and Genomic Integrity. Sci. China Life Sci. 2016, 59, 656–672. [Google Scholar] [CrossRef]
- Induction of Endosomal/Lysosomal Pathways in Differentiating Osteoblasts as Revealed by Combined Proteomic and Transcriptomic Analyses | Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0014579310006174?token=B14F90719184F762442CFBFAF9DB9AC58416545DE7CA05F9BBF6AB241098272EA2376D069536921878F0B2FE38480801&originRegion=eu-west-1&originCreation=20230331095118 (accessed on 31 March 2023).
- Tsukuba, T.; Sakai, E.; Nishishita, K.; Kadowaki, T.; Okamoto, K. New Functions of Lysosomes in Bone Cells. J. Oral Biosci. 2017, 59, 92–95. [Google Scholar] [CrossRef]
- Tumor Protein D54 Defines a New Class of Intracellular Transport Vesicles–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31672706/ (accessed on 16 March 2023).
- Coutinho, M.F.; Prata, M.J.; Alves, S. Mannose-6-Phosphate Pathway: A Review on Its Role in Lysosomal Function and Dysfunction. Mol. Genet. Metab. 2012, 105, 542–550. [Google Scholar] [CrossRef]
- Jia, J.; Claude-Taupin, A.; Gu, Y.; Choi, S.W.; Peters, R.; Bissa, B.; Mudd, M.H.; Allers, L.; Pallikkuth, S.; Lidke, K.A.; et al. Galectin-3 Coordinates a Cellular System for Lysosomal Repair and Removal. Dev. Cell 2020, 52, 69–87.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Ni, S.; Yang, Z.-C.; Liu, R.-P. Oxidative Stress Induces Chondrocyte Apoptosis through Caspase-Dependent and Caspase-Independent Mitochondrial Pathways and the Antioxidant Mechanism of Angelica Sinensis Polysaccharide. Oxid. Med. Cell. Longev. 2020, 2020, 3240820. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Gillet, G.; Prudent, J.; Popgeorgiev, N. Bcl-2 Family of Proteins in the Control of Mitochondrial Calcium Signalling: An Old Chap with New Roles. Int. J. Mol. Sci. 2021, 22, 3730. [Google Scholar] [CrossRef]
- Strubbe-Rivera, J.O.; Schrad, J.R.; Pavlov, E.V.; Conway, J.F.; Parent, K.N.; Bazil, J.N. The Mitochondrial Permeability Transition Phenomenon Elucidated by Cryo-EM Reveals the Genuine Impact of Calcium Overload on Mitochondrial Structure and Function. Sci. Rep. 2021, 11, 1037. [Google Scholar] [CrossRef]
- Marston, S.; Zamora, J.E. Troponin Structure and Function: A View of Recent Progress. J. Muscle Res. Cell Motil. 2020, 41, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. Actin, Myosin, and Cell Movement. Cell Mol. Approach 2nd Ed. 2000. [Google Scholar]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.-P.L. The Role of Reactive Oxygen Species in Homeostasis and Degradation of Cartilage. Osteoarthritis Cartilage 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Garbe, A.I.; Roscher, A.; Schüler, C.; Lutter, A.-H.; Glösmann, M.; Bernhardt, R.; Chopin, M.; Hempel, U.; Hofbauer, L.C.; Rammelt, S.; et al. Regulation of Bone Mass and Osteoclast Function Depend on the F-Actin Modulator SWAP-70. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 2085–2096. [Google Scholar] [CrossRef]
- Takito, J.; Otsuka, H.; Inoue, S.; Kawashima, T.; Nakamura, M. Symmetrical Retrograde Actin Flow in the Actin Fusion Structure Is Involved in Osteoclast Fusion. Biol. Open 2017, 6, 1104–1114. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, N.; Cui, L.; Wu, Z.; Qiu, G. Filamin B: The next Hotspot in Skeletal Research? J. Genet. Genomics 2017, 44, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Erkhembaatar, M.; Gu, D.R.; Lee, S.H.; Yang, Y.-M.; Park, S.; Muallem, S.; Shin, D.M.; Kim, M.S. Lysosomal Ca2+ Signaling Is Essential for Osteoclastogenesis and Bone Remodeling. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Urukova, Y.; Cardelli, M.; Aubin, J.E.; Harrison, R.E. Lysosome Dispersion in Osteoblasts Accommodates Enhanced Collagen Production during Differentiation. J. Biol. Chem. 2008, 283, 19678–19690. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.S.; Feigan, J.; Lowther, D.A. The Mechanism of Chondrocyte Hydrogen Peroxide Damage. Depletion of Intracellular ATP Due to Suppression of Glycolysis Caused by Oxidation of Glyceraldehyde-3-Phosphate Dehydrogenase. J. Rheumatol. 1989, 16, 7–14. [Google Scholar] [PubMed]
- Taskiran, D.; Stefanovic-Racic, M.; Georgescu, H.; Evans, C. Nitric Oxide Mediates Suppression of Cartilage Proteoglycan Synthesis by Interleukin-1. Biochem. Biophys. Res. Commun. 1994, 200, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Häuselmann, H.J.; Oppliger, L.; Michel, B.A.; Stefanovic-Racic, M.; Evans, C.H. Nitric Oxide and Proteoglycan Biosynthesis by Human Articular Chondrocytes in Alginate Culture. FEBS Lett. 1994, 352, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Hickery, M.S.; Bayliss, M.T. Interleukin-1 Induced Nitric Oxide Inhibits Sulphation of Glycosaminoglycan Chains in Human Articular Chondrocytes. Biochim. Biophys. Acta BBA–Gen. Subj. 1998, 1425, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix Metalloproteinases (MMPs), the Main Extracellular Matrix (ECM) Enzymes in Collagen Degradation, as a Target for Anticancer Drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Sasaki, K.; Hattori, T.; Fujisawa, T.; Takahashi, K.; Inoue, H.; Takigawa, M. Nitric Oxide Mediates Interleukin-1-Induced Gene Expression of Matrix Metalloproteinases and Basic Fibroblast Growth Factor in Cultured Rabbit Articular Chondrocytes. J. Biochem. (Tokyo) 1998, 123, 431–439. [Google Scholar] [CrossRef]
- Mantuano, E.; Inoue, G.; Li, X.; Takahashi, K.; Gaultier, A.; Gonias, S.L.; Campana, W.M. The Hemopexin Domain of Matrix Metalloproteinase-9 Activates Cell Signaling and Promotes Migration of Schwann Cells by Binding to Low-Density Lipoprotein Receptor-Related Protein. J. Neurosci. 2008, 28, 11571–11582. [Google Scholar] [CrossRef]
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the Hemopexin Domain of Matrix Metalloproteinases in Cell Migration. J. Cell. Physiol. 2008, 217, 643–651. [Google Scholar] [CrossRef]
- Tomatsu, S.; Yasuda, E.; Patel, P.; Ruhnke, K.; Shimada, T.; Mackenzie, W.G.; Mason, R.; Thacker, M.M.; Theroux, M.; Montaño, A.M.; et al. Morquio A Syndrome: Diagnosis and Current and Future Therapies. Pediatr. Endocrinol. Rev. PER 2014, 12, 141–151. [Google Scholar]
- Karsdal, M.A.; Madsen, S.H.; Christiansen, C.; Henriksen, K.; Fosang, A.J.; Sondergaard, B.C. Cartilage Degradation Is Fully Reversible in the Presence of Aggrecanase but Not Matrix Metalloproteinase Activity. Arthritis Res. Ther. 2008, 10, R63. [Google Scholar] [CrossRef]
- Kohno, T.; Wada, A.; Igarashi, Y. N-Glycans of Sphingosine 1-Phosphate Receptor Edg-1 Regulate Ligand-Induced Receptor Internalization. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 983–992. [Google Scholar] [CrossRef]
- Rainero, E. Extracellular Matrix Endocytosis in Controlling Matrix Turnover and beyond: Emerging Roles in Cancer. Biochem. Soc. Trans. 2016, 44, 1347–1354. [Google Scholar] [CrossRef]
- Redestig, H.; Fukushima, A.; Stenlund, H.; Moritz, T.; Arita, M.; Saito, K.; Kusano, M. Compensation for Systematic Cross-Contribution Improves Normalization of Mass Spectrometry Based Metabolomics Data. Anal. Chem. 2009, 81, 7974–7980. [Google Scholar] [CrossRef]
- Lambert, J.P.; Ivosev, G.; Couzens, A.L.; Larsen, B.; Taipale, M.; Lin, Z.Y.; Zhong, Q.; Lindquist, S.; Vidal, M.; Aebersold, R.; et al. Mapping Differential Interactomes by Affinity Purification Coupled with Data-Independent Mass Spectrometry Acquisition. Nat. Methods 2013, 10, 1239–1245. [Google Scholar] [CrossRef]
- Kuster, B.; Schirle, M.; Mallick, P.; Aebersold, R. Scoring Proteomes with Proteotypic Peptide Probes. Nat. Rev. Mol. Cell Biol. 2005, 6, 577–583. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted Data Extraction of the MS/MS Spectra Generated by Data-Independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Mol. Cell. Proteomics MCP 2012, 11, O111.016717. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Veiga, T.; Bravo, S.; Gómez-Tato, A.; Yáñez-Gómez, C.; Abuín, C.; Varela, V.; Cueva, J.; Palacios, P.; Dávila-Ibáñez, A.B.; Piñeiro, R.; et al. Red Blood Cells Protein Profile Is Modified in Breast Cancer Patients. Mol. Cell. Proteomics MCP 2022, 21, 100435. [Google Scholar] [CrossRef] [PubMed]
- López-López, M.; Regueiro, U.; Bravo, S.B.; Chantada-Vázquez, M.D.P.; Pena, C.; Díez-Feijoo, E.; Hervella, P.; Lema, I. Shotgun Proteomics for the Identification and Profiling of the Tear Proteome of Keratoconus Patients. Invest. Ophthalmol. Vis. Sci. 2022, 63, 12. [Google Scholar] [CrossRef]
- Lambert, J.-P.; Ivosev, G.; Couzens, A.L.; Larsen, B.; Taipale, M.; Lin, Z.-Y.; Zhong, Q.; Lindquist, S.; Vidal, M.; Aebersold, R.; et al. Mapping Differential Interactomes by Affinity Purification Coupled with Data-Independent Mass Spectrometry Acquisition. Nat. Methods 2013, 10, 1239–1245. [Google Scholar] [CrossRef]
- Narasimhan, M.; Kannan, S.; Chawade, A.; Bhattacharjee, A.; Govekar, R. Clinical Biomarker Discovery by SWATH-MS Based Label-Free Quantitative Proteomics: Impact of Criteria for Identification of Differentiators and Data Normalization Method. J. Transl. Med. 2019, 17, 184. [Google Scholar] [CrossRef]
- Multiplex Biomarker Screening Assay for Urinary Extracellular Vesicles Study: A Targeted Label-Free Proteomic Approach | Scientific Reports. Available online: https://www.nature.com/articles/s41598-018-33280-7 (accessed on 30 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




