Submitted:
27 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
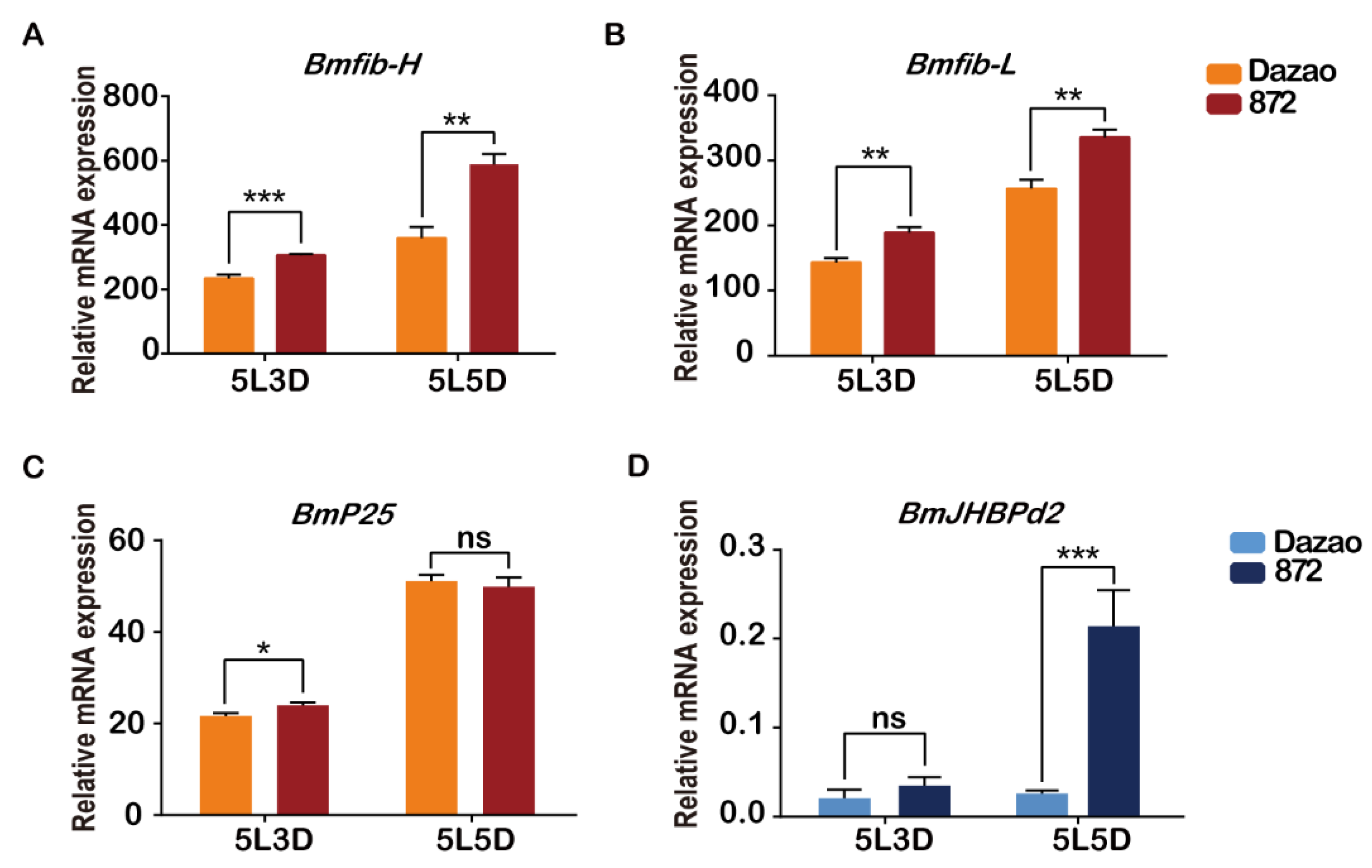
2.1. Expression of BmJHBPd2 in different silk producing strains
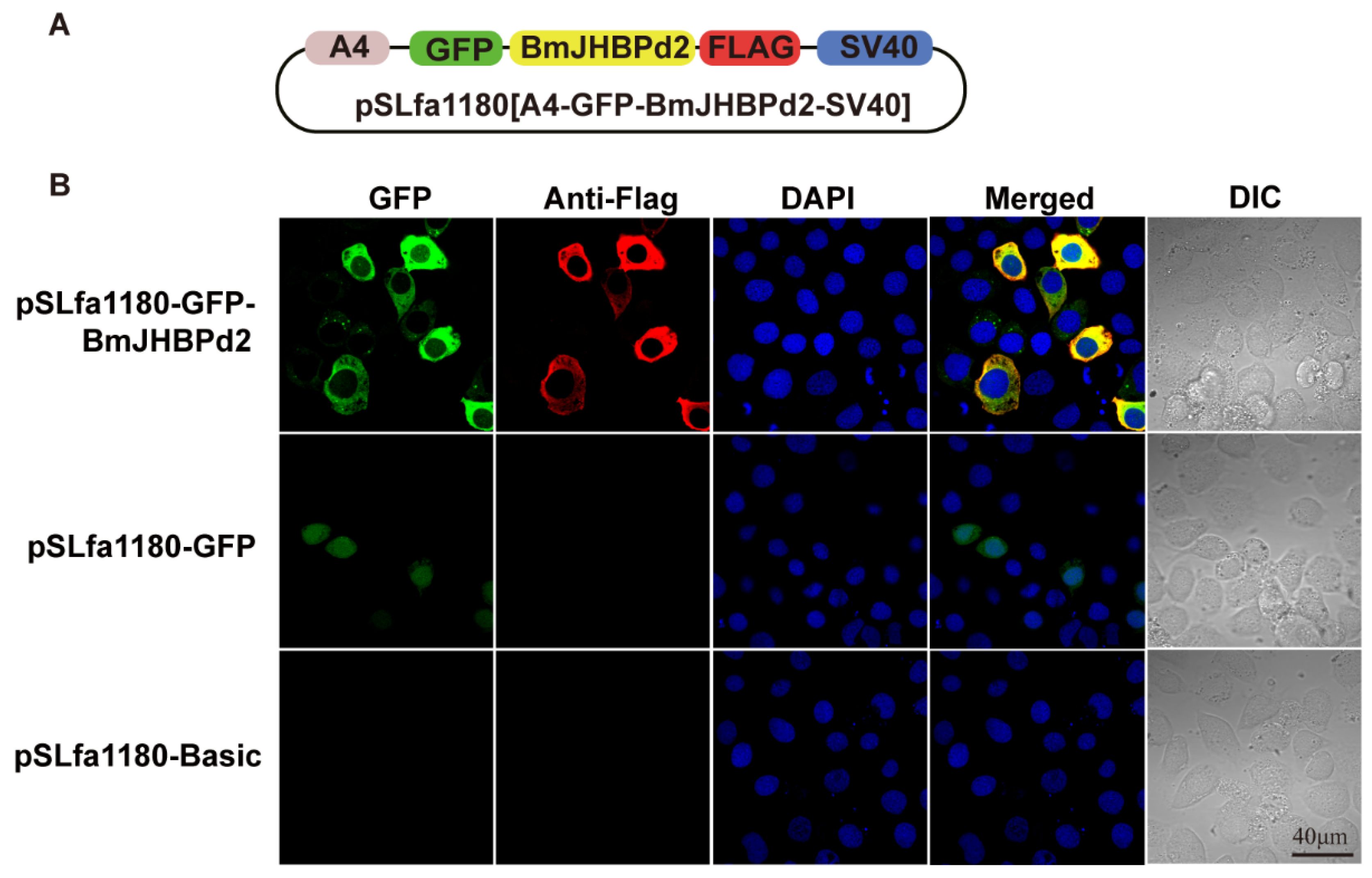
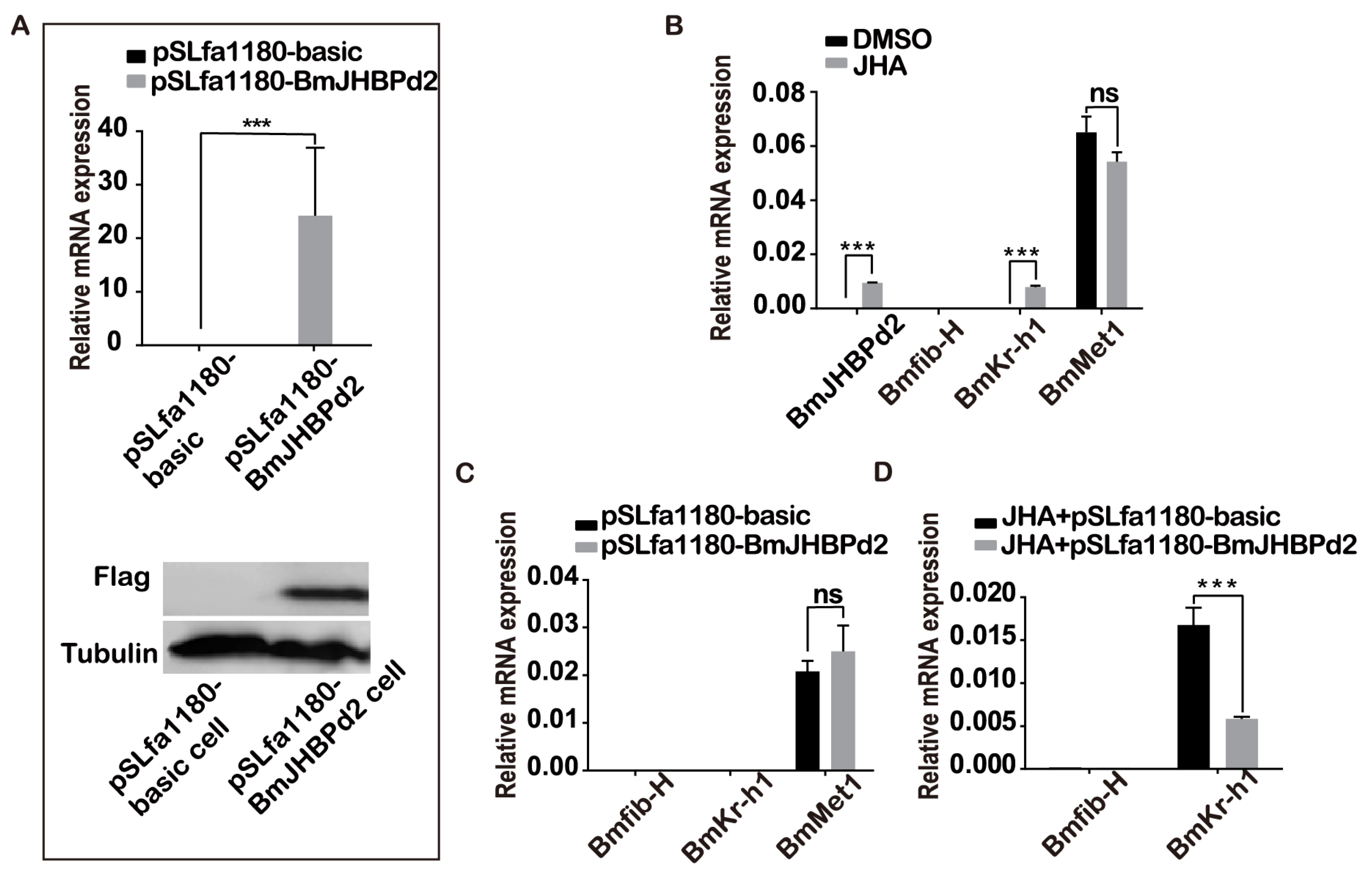
2.2. Overexpression of BmJHBPd2 at the cellular level
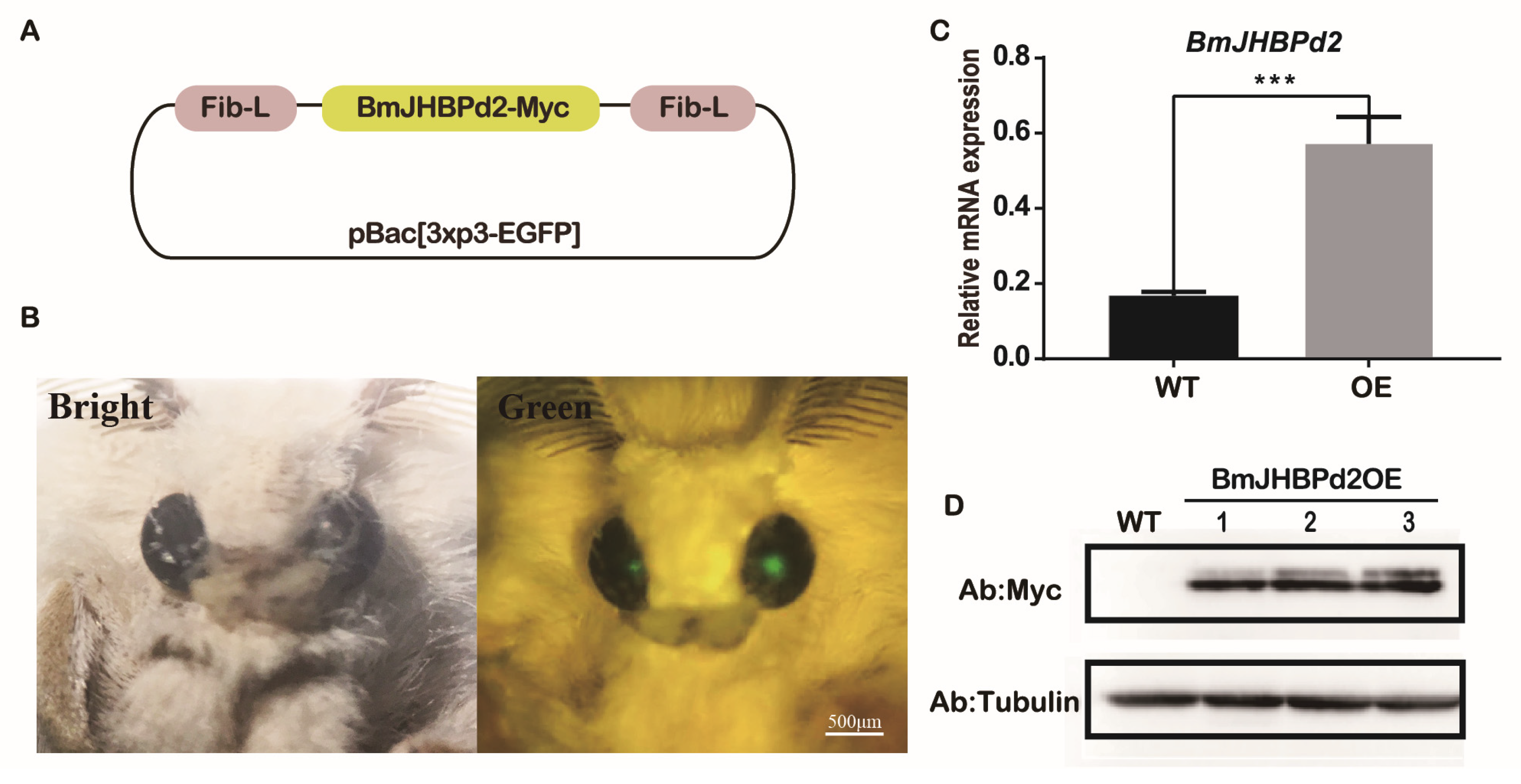
2.3. Transgenic overexpression of BmJHBPd2 in the silk gland
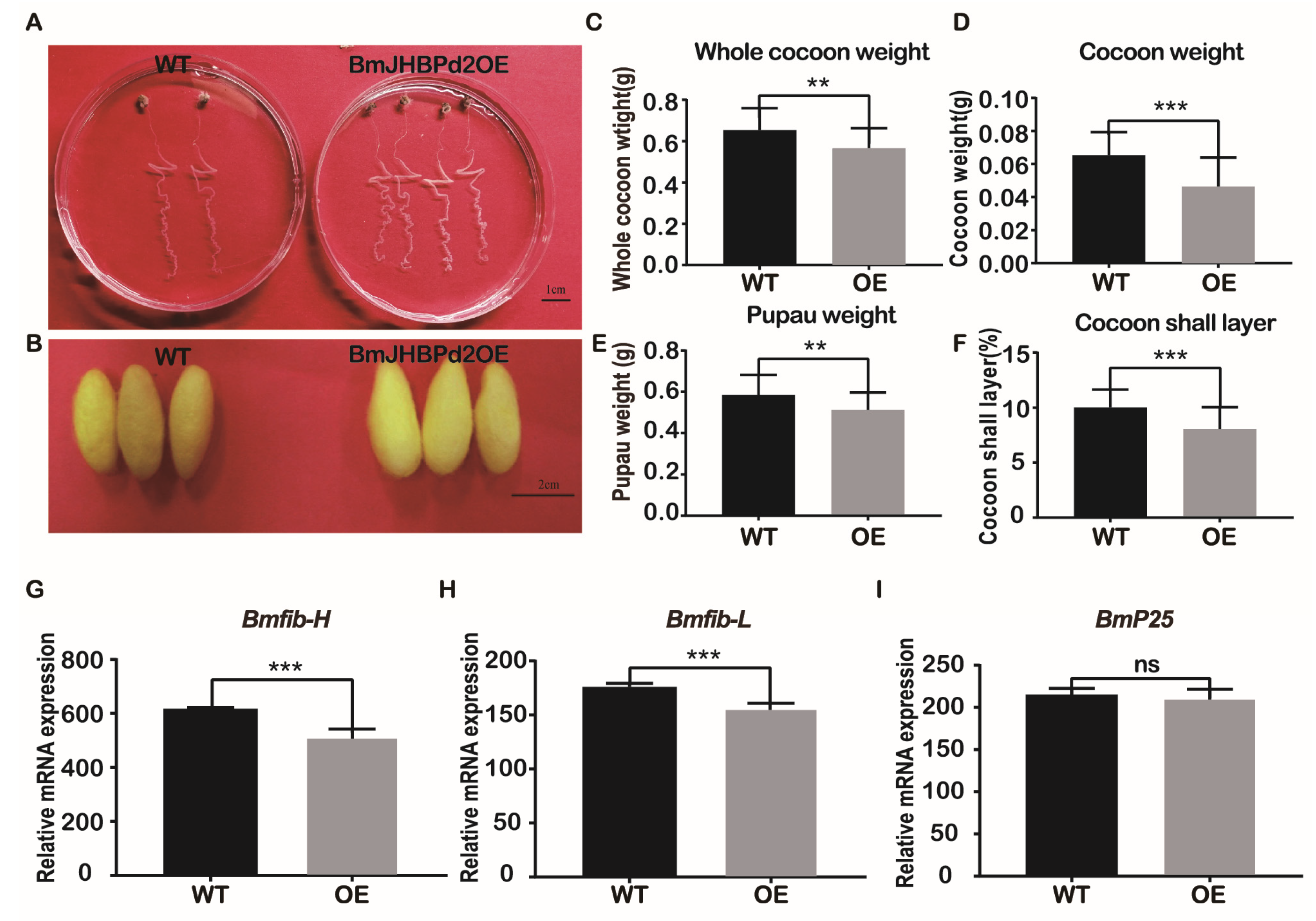
2.4. Overexpression of BmJHBPd2 affects silk synthesis and silk yield
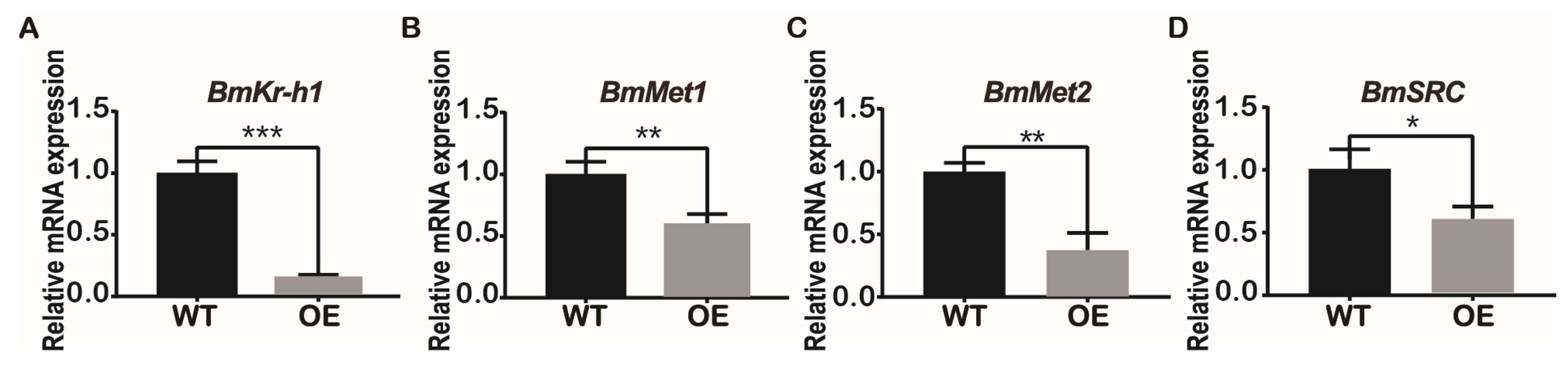
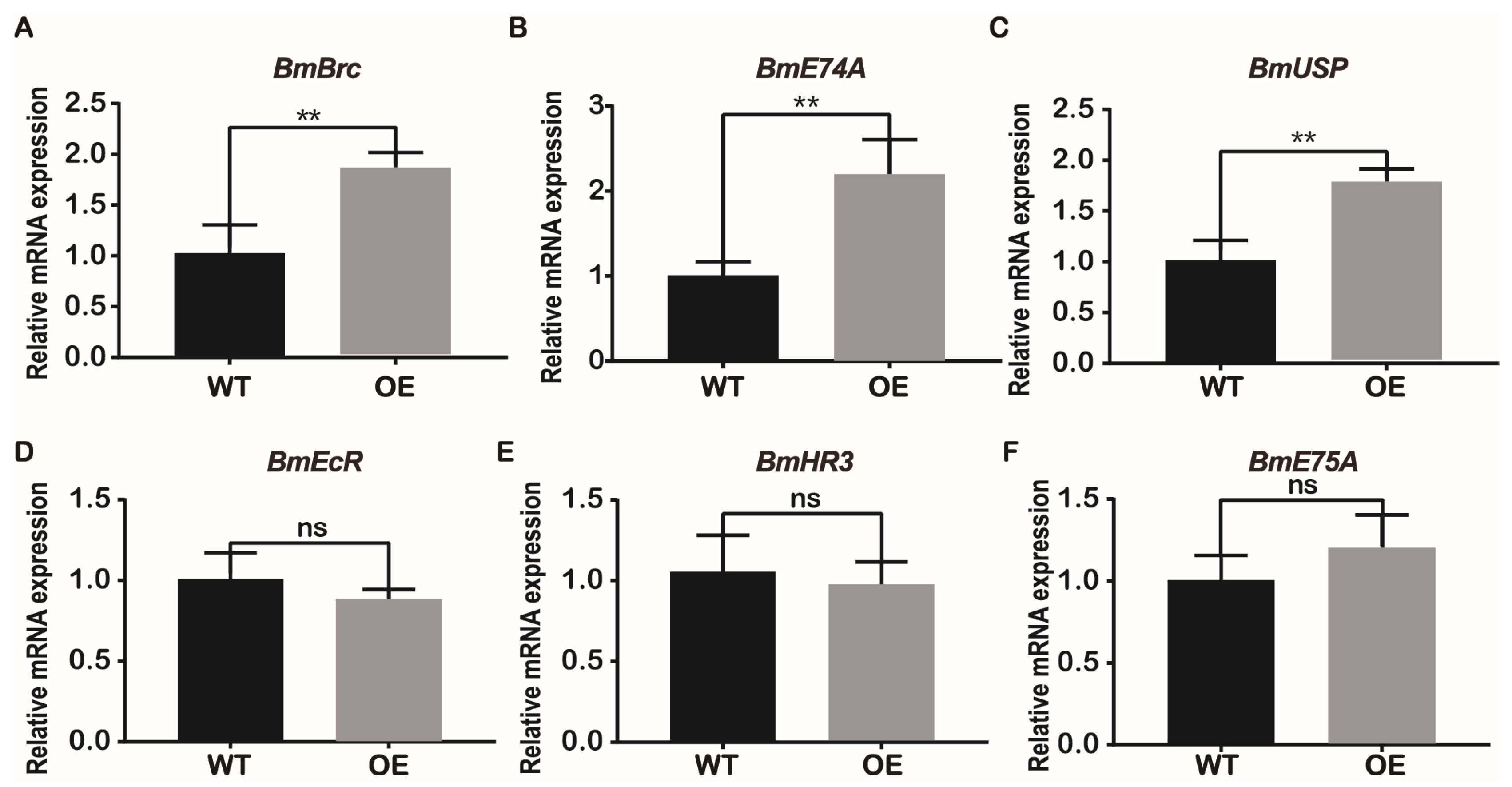
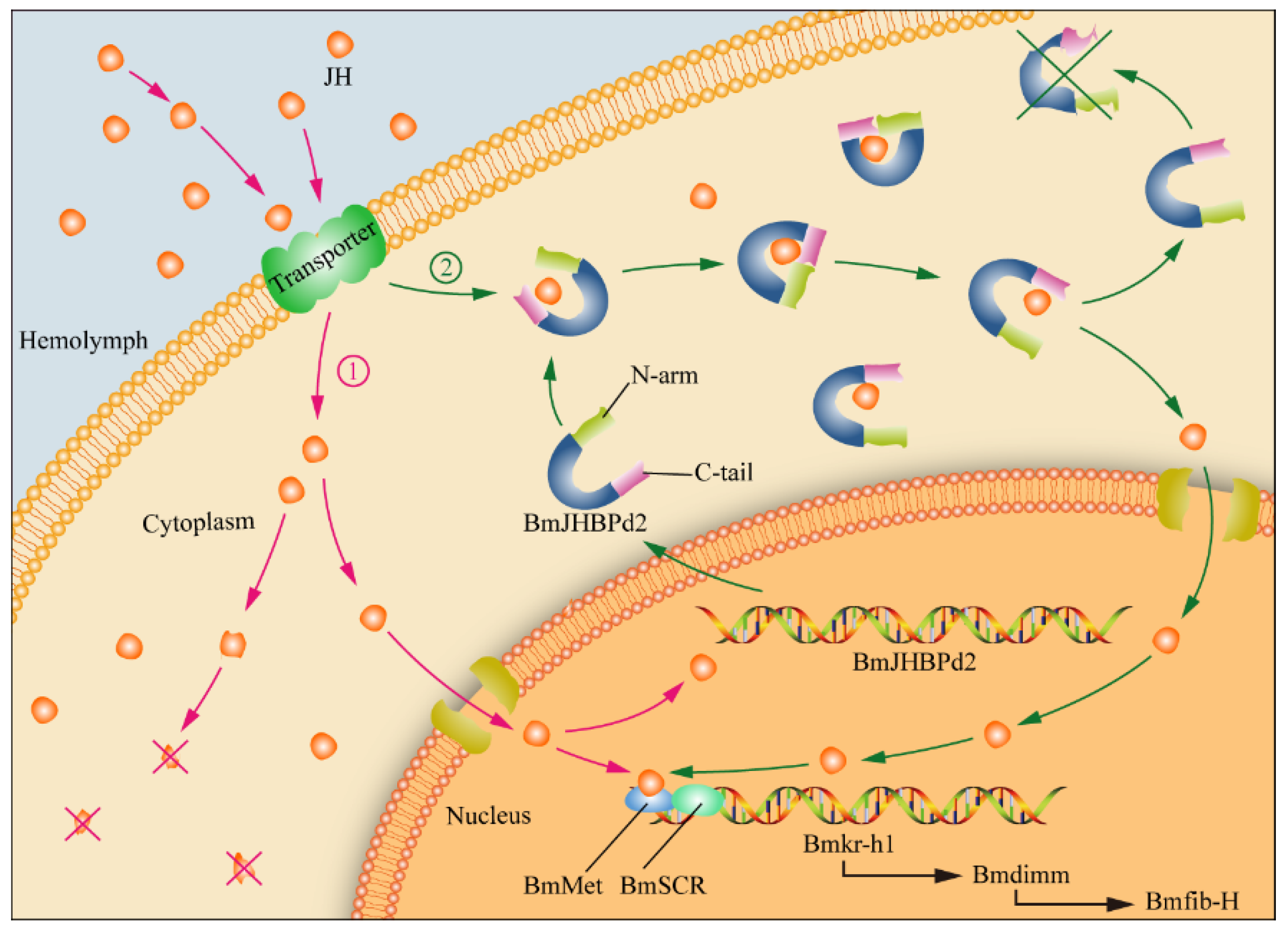
2.5. Overexpression of BmJHBPd2 led to repressed the silk synthesis by inhibiting Bmkr-h1 expression in the silk glands
3. Discussion
4. Materials and Methods
4.1. Silkworm strain and cell culture
4.2. RNA preparation and quantitative real time-PCR (qRT-PCR)
4.3. Subcellular localization
4.4. Western blot
4.5. Statistical method for cocoon layer rate
4.6. Plasmid Construction
4.7. Silkworm germline transformation
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Truman, J. W.; Riddiford, L. M., The origins of insect metamorphosis. Nature 1999, 401, 447-52. [CrossRef]
- Shin, S. W.; Zou, Z.; Saha, T. T.; Raikhel, A. S., bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 16576-81. [CrossRef]
- Wiśniewski, J. R.; Muszyńska-Pytel, M.; Grzelak, K.; Kochman, M., Biosynthesis and degradation of juvenile hormone in corpora allata and imaginal wing discs of Galleria mellonella (L.). Insect Biochemistry 1987, 17, 249-254. [CrossRef]
- Sok, A. J.; Andruszewska, G.; Niewiadomska-Cimicka, A.; Grad, I.; Rymarczyk, G.; Pajdzik, D.; Orłowski, M.; Schmidt, M. T.; Grajek, W.; Ozyhar, A.; Kochman, M., Regulatory elements in the juvenile hormone binding protein gene from Galleria mellonella--topography of binding sites for Usp and EcRDBD. Biochimica et biophysica acta 2008, 1779, 390-401. [CrossRef]
- Röller, D. H.; Dahm, K. H.; Sweeley, C. C.; Trost, B. M., The structure of the juvenile hormone. Angewandte Chemie 2010, 79, 190-191. [CrossRef]
- Gilbert, L. I.; Granger, N. A.; Roe, R. M., The juvenile hormones: Historical facts and speculations on future research directions. Insect biochemistry and molecular biology 2000, 30, 617-44. [CrossRef]
- Hammock, B.; Nowock, J.; Goodman, W.; Stamoudis, V.; Gilbert, L. I., The influence of hemolymph-binding protein on juvenile hormone stability and distribution in Manduca sexta fat body and imaginal discs in vitro. Molecular and cellular endocrinology 1975, 3, 167-84. [CrossRef]
- Sanburg, L. L.; Kramer, K. J.; Kezdy, F. J.; Law, J. H.; Oberlander, H., Role of juvenile hormone esterases and carrier proteins in insect development. Nature 1975, 253, 266-7. [CrossRef]
- Tauchman, S. J.; Lorch, J. M.; Orth, A. P.; Goodman, W. G., Effects of stress on the hemolymph juvenile hormone binding protein titers of Manduca sexta. Insect biochemistry and molecular biology 2007, 37, 847-54. [CrossRef]
- Chang, E. S.; Bruce, M. J.; Prestwich, G. D., Further characterization of the juvenile hormone binding protein from the cytosol of a Drosophila cell line: Use of a photoaffinity label. Insect Biochemistry 1985, 15, 197-204. [CrossRef]
- Shemshedini, L.; Lanoue, M.; Wilson, T. G., Evidence for a juvenile hormone receptor involved in protein synthesis in Drosophila melanogaster. The Journal of biological chemistry 1990, 265, 1913-8. [CrossRef]
- Ritdachyeng, E.; Manaboon, M.; Tobe, S. S.; Singtripop, T., Molecular characterization and gene expression of juvenile hormone binding protein in the bamboo borer, Omphisa fuscidentalis. Journal of insect physiology 2012, 58, 1493-501. [CrossRef]
- Rebijith, K. B.; Asokan, R.; Ranjitha, H. H.; Rajendra Prasad, B. S.; Krishna, V.; Krishna Kumar, N. K., Diet-Delivered dsRNAs for Juvenile Hormone-Binding Protein and Vacuolar ATPase-H Implied Their Potential in the Management of the Melon Aphid (Hemiptera: Aphididae). Environmental entomology 2016, 45, 268-75. [CrossRef]
- Wei, D.; Li, H. M.; Tian, C. B.; Smagghe, G.; Jia, F. X.; Jiang, H. B.; Dou, W.; Wang, J. J., Proteome analysis of male accessory gland secretions in oriental fruit flies reveals juvenile hormone-binding protein, suggesting impact on female reproduction. Scientific reports 2015, 5, 16845. [CrossRef]
- Saito, K.; Su, Z. H.; Emi, A.; Mita, K.; Takeda, M.; Fujiwara, Y., Cloning and expression analysis of takeout/JHBP family genes of silkworm, Bombyx mori. Insect molecular biology 2006, 15, 245-51. [CrossRef]
- Whitmore, E.; Gilbert, L. I., Haemolymph lipoprotein transport of juvenile hormone. Journal of insect physiology 1972, 18, 1153-67. [CrossRef]
- Kramer, K. J.; Sanburg, L. L.; Kézdy, F. J.; Law, J. H., The Juvenile Hormone Binding Protein in the Hemolymph of Manduca sexta Johannson (Lepidoptera: Sphingidae). Proceedings of the National Academy of Sciences of the United States of America 1974, 71, 493-7. [CrossRef]
- Lerro, K. A.; Prestwich, G. D., Cloning and sequencing of a cDNA for the hemolymph juvenile hormone binding protein of larval Manduca sexta. The Journal of biological chemistry 1990, 265, 19800-6. [CrossRef]
- Wojtasek, H.; Prestwich, G. D., Key disulfide bonds in an insect hormone binding protein: cDNA cloning of a juvenile hormone binding protein of Heliothis virescens and ligand binding by native and mutant forms. Biochemistry 1995, 34, 5234-41. [CrossRef]
- Rodriguez Parkitna, J. M.; Ozyhar, A.; Wiśniewski, J. R.; Kochman, M., Cloning and sequence analysis of Galleria mellonella juvenile hormone binding protein--a search for ancestors and relatives. Biological chemistry 2002, 383, 1343-55. [CrossRef]
- Vermunt, A. M.; Kamimura, M.; Hirai, M.; Kiuchi, M.; Shiotsuki, T., The juvenile hormone binding protein of silkworm haemolymph: Gene and functional analysis. Insect molecular biology 2001, 10, 147-54. [CrossRef]
- Li, W.; Cheng, T.; Hu, W.; Peng, Z.; Liu, C.; Xia, Q., Genome-wide identification and analysis of JHBP-domain family members in the silkworm Bombyx mori. Molecular genetics and genomics : MGG 2016, 291, 2159-2171. [CrossRef]
- Xia, Q.; Li, S.; Feng, Q., Advances in silkworm studies accelerated by the genome sequencing of Bombyx mori. Annu Rev Entomol 2014, 59, 513-36. [CrossRef]
- Gamo, T.; Inokuchi, T.; Laufer, H., Polypeptides of fibroin and sericin secreted from the different sections of the silk gland in Bombyx mori. Insect Biochemistry 1977, 7, 285-295. [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S., Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. The Journal of biological chemistry 2000, 275, 40517-28. [CrossRef]
- Akai, H.; Kiguchi, K.; Mori, K., Increased Accumulation of Silk Protein Accompanying JH-Induced Prolongation of Larval Life in Bombyx mori L.(Lepidoptera : Bombycidae). Applied Entomology & Zoology 1971, 6, 218-220. [CrossRef]
- Akai, H.; Kobayashi, M., Induction of Prolonged Larval Instar by the Juvenile Hormone in Bombyx mori L. : Lepidoptera : Bombycidae. Applied Entomology & Zoology 1971, 6, 138-139. [CrossRef]
- Kurata, S.; Koga, K.; Sakaguchi, B., Nucleolar size in parallel with ribosomal RNA synthesis at diapause termination in the eggs of Bombyx mori. Chromosoma 1978, 68, 313-7. [CrossRef]
- Kurata, K., Effect of a juvenile hormon analogue given at various ages of 5th instar larvae on RNA synthesis in the posterior silk gland of the silkworm, Bombyx mori. Journal of Insect Biotechnology & Sericology 2010, 53, 421-426.
- Kayukawa, T.; Murata, M.; Kobayashi, I.; Muramatsu, D.; Okada, C.; Uchino, K.; Sezutsu, H.; Kiuchi, M.; Tamura, T.; Hiruma, K.; Ishikawa, Y.; Shinoda, T., Hormonal regulation and developmental role of Krüppel homolog 1, a repressor of metamorphosis, in the silkworm Bombyx mori. Developmental biology 2014, 388, 48-56. [CrossRef]
- Zhao, X. M.; Liu, C.; Jiang, L. J.; Li, Q. Y.; Zhou, M. T.; Cheng, T. C.; Mita, K.; Xia, Q. Y., A juvenile hormone transcription factor Bmdimm-fibroin H chain pathway is involved in the synthesis of silk protein in silkworm, Bombyx mori. The Journal of biological chemistry 2015, 290, 972-86. [CrossRef]
- Zhang, T.; Song, W.; Li, Z.; Qian, W.; Wei, L.; Yang, Y.; Wang, W.; Zhou, X.; Meng, M.; Peng, J.; Xia, Q.; Perrimon, N.; Cheng, D., Krüppel homolog 1 represses insect ecdysone biosynthesis by directly inhibiting the transcription of steroidogenic enzymes. Proceedings of the National Academy of Sciences of the United States of America 2018, 115, 3960-3965. [CrossRef]
- Ma, L.; Xu, H.; Zhu, J.; Ma, S.; Liu, Y.; Jiang, R. J.; Xia, Q.; Li, S., Ras1(CA) overexpression in the posterior silk gland improves silk yield. Cell research 2011, 21, 934-43. [CrossRef]
- Furuta, K.; Ichikawa, A.; Murata, M.; Kuwano, E.; Shinoda, T.; Shiotsuki, T., Determination by LC-MS of juvenile hormone titers in hemolymph of the silkworm, Bombyx mori. Bioscience, biotechnology, and biochemistry 2013, 77, 988-91. [CrossRef]
- Kayukawa, T.; Nagamine, K.; Ito, Y.; Nishita, Y.; Ishikawa, Y.; Shinoda, T., Krüppel Homolog 1 Inhibits Insect Metamorphosis via Direct Transcriptional Repression of Broad-Complex, a Pupal Specifier Gene. The Journal of biological chemistry 2016, 291, 1751-1762. [CrossRef]
- Kayukawa, T.; Jouraku, A.; Ito, Y.; Shinoda, T., Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval-adult metamorphosis. Proceedings of the National Academy of Sciences of the United States of America 2017, 114, 1057-1062. [CrossRef]
- Li, K.; Tian, L.; Guo, Z.; Guo, S.; Zhang, J.; Gu, S. H.; Palli, S. R.; Cao, Y.; Li, S., 20-Hydroxyecdysone (20E) Primary Response Gene E75 Isoforms Mediate Steroidogenesis Autoregulation and Regulate Developmental Timing in Bombyx. The Journal of biological chemistry 2016, 291, 18163-75. [CrossRef]
- Liu, X.; Dai, F.; Guo, E.; Li, K.; Ma, L.; Tian, L.; Cao, Y.; Zhang, G.; Palli, S. R.; Li, S., 20-Hydroxyecdysone (20E) Primary Response Gene E93 Modulates 20E Signaling to Promote Bombyx Larval-Pupal Metamorphosis. The Journal of biological chemistry 2015, 290, 27370-27383. [CrossRef]
- Li, J. Y.; Yang, H. J.; Lan, T. Y.; Wei, H.; Zhang, H. R.; Chen, M.; Fan, W.; Ma, Y. Y.; Zhong, B. X., Expression profiling and regulation of genes related to silkworm posterior silk gland development and fibroin synthesis. Journal of proteome research 2011, 10, 3551-64. [CrossRef]
- Zeng, B.; Huang, Y.; Xu, J.; Shiotsuki, T.; Bai, H.; Palli, S. R.; Huang, Y.; Tan, A., The FOXO transcription factor controls insect growth and development by regulating juvenile hormone degradation in the silkworm, Bombyx mori. The Journal of biological chemistry 2017, 292, 11659-11669. [CrossRef]
- Zhang, Z.; Liu, X.; Shiotsuki, T.; Wang, Z.; Xu, X.; Huang, Y.; Li, M.; Li, K.; Tan, A., Depletion of juvenile hormone esterase extends larval growth in Bombyx mori. Insect biochemistry and molecular biology 2017, 81, 72-79. [CrossRef]
- Kayukawa, T.; Minakuchi, C.; Namiki, T.; Togawa, T.; Yoshiyama, M.; Kamimura, M.; Mita, K.; Imanishi, S.; Kiuchi, M.; Ishikawa, Y., Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 11729-11734. [CrossRef]
- Pan, M. H.; Xiao, S. Q.; Chen, M.; Hong, X. J.; Lu, C., Establishment and characterization of two embryonic cell lines of Bombyx mori. In vitro cellular & developmental biology. Animal 2007, 43, 101-4. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




