Submitted:
25 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Coffee as One of the Most Appealing Beverages
1.2. Concerns of Coffee Harvest Reduction Factors and Preventive Strategies
1.3. Endophytes as a Prime Source for Improving Plant Health
1.4. Aim of the Current Article
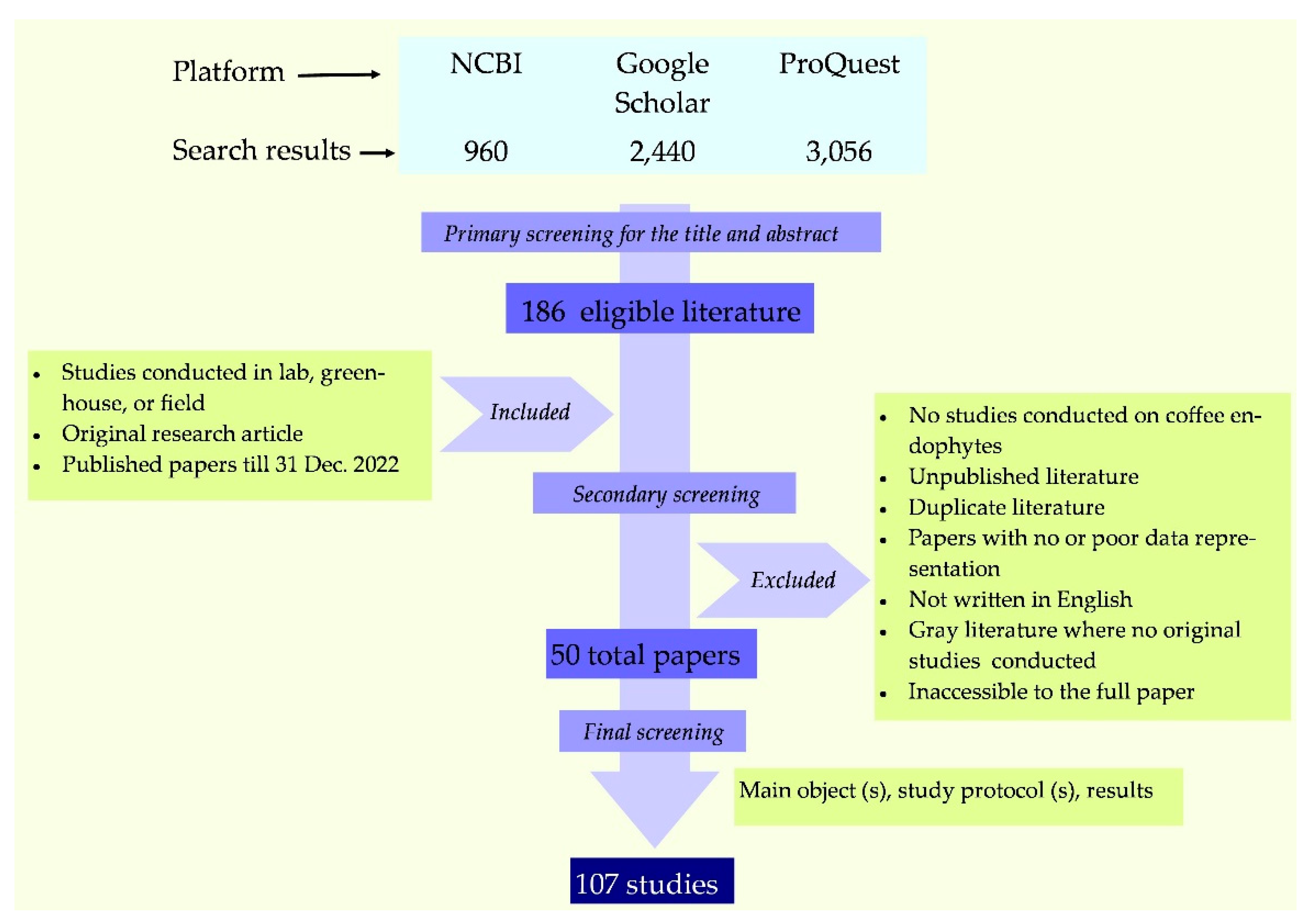
2. Data Collection and Analysis
3. General Aspects of Endophytes
3.1. Endophytic Association with Plants
3.2. Brief Classification and Important Genera/Phylogenetic Placement
4. Coffee Endophytes: Insight
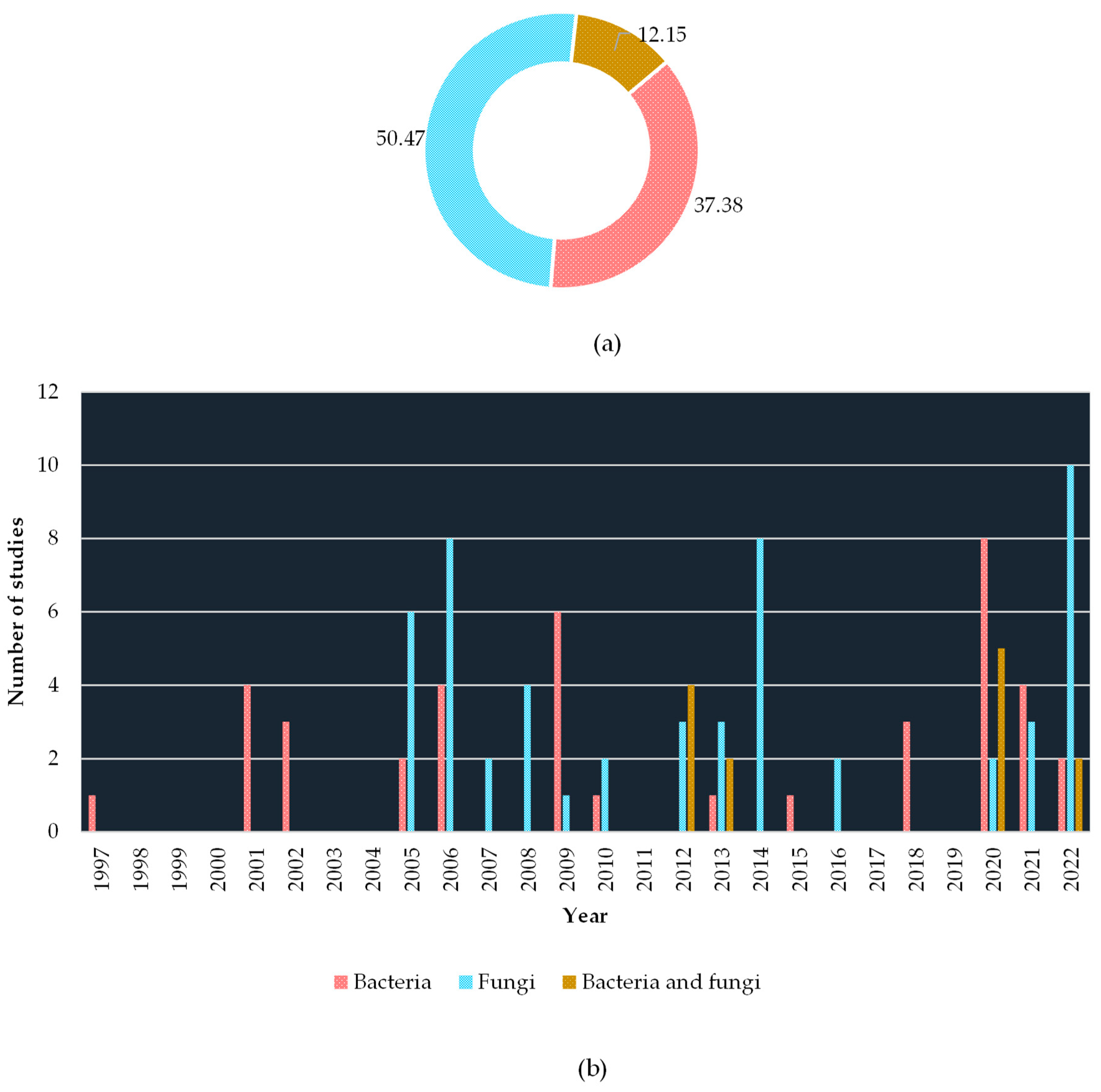
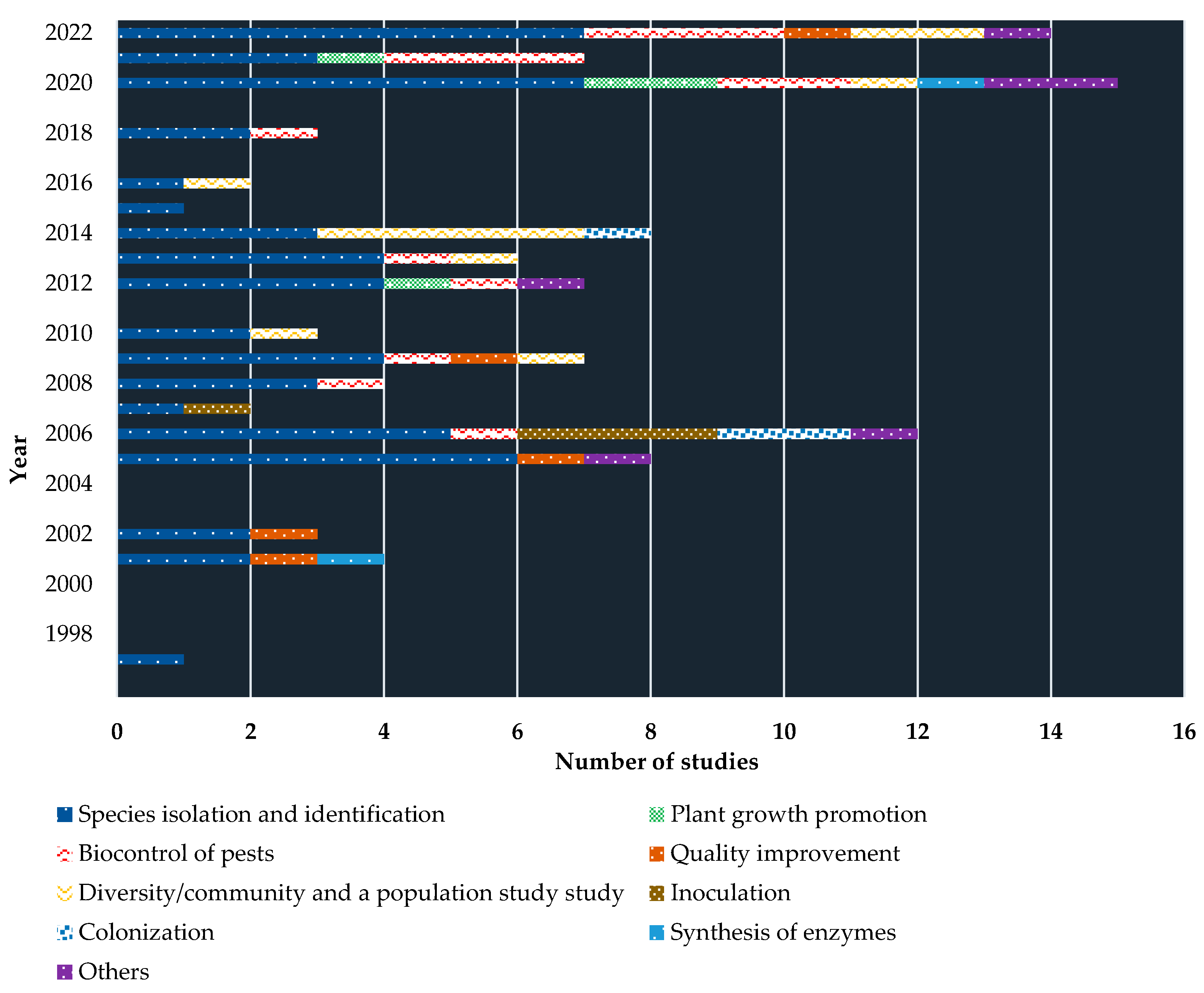
4.1. History of Studies, Current Trends, and Gaps
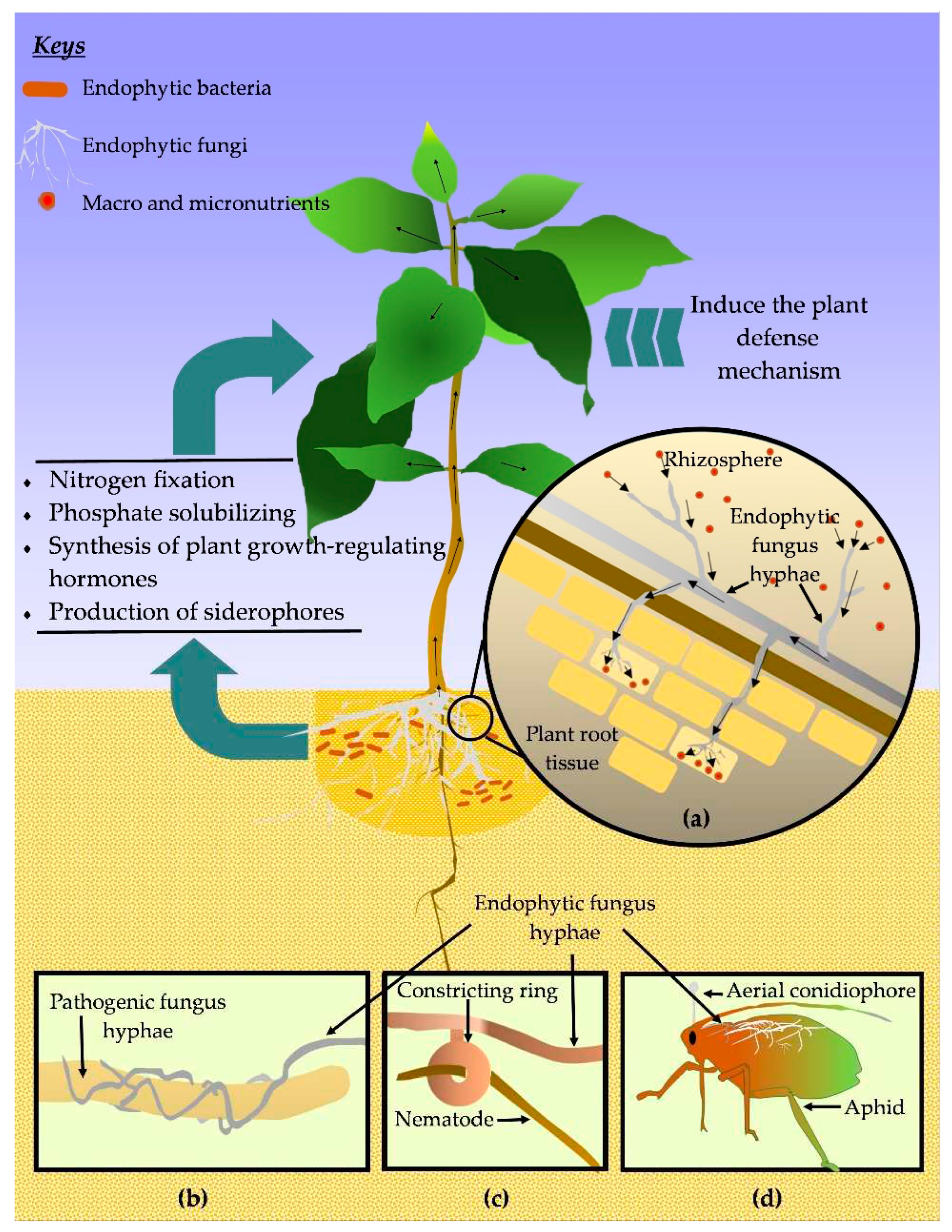
4.2. Endophytes in Coffee Plant Growth Promotion
4.2.1. Nutrient Absorption Promotion and Enhancing the Availability of Minerals
4.2.2. Secretion of Phytohormones and Other Bioactive Metabolites
4.2.3. Successful Field and Laboratory Experiments on Coffee Plant Growth Promotion.
4.3. Endophytes in Coffee Plant Protection
4.3.1. Protection against Fungi Pathogens
4.3.2. Protection against Parasitic Nematodes
4.3.3. Protection against Insect Pests
4.4. Role of Endophytes in the Improvement of Quality and Flavor of Coffee Seeds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The Impact of Climate Change and Variability on Coffee Production: A Systematic Review. Clim. Change 2019, 156, 609–630. [CrossRef]
- Bastian, F.; Hutabarat, O.S.; Dirpan, A.; Nainu, F.; Harapan, H.; Emran, T.B.; Simal-Gandara, J. From Plantation to Cup: Changes in Bioactive Compounds during Coffee Processing. Foods 2021, 10, 2827. [CrossRef]
- International Coffee Organization - What’s New Available online: https://www.ico.org/ (accessed on 16 April 2023).
- Ma, J.; Li, J.; He, H.; Jin, X.; Cesarino, I.; Zeng, W.; Li, Z. Characterization of Sensory Properties of Yunnan Coffee. Curr. Res. Food Sci. 2022, 5, 1205–1215. [CrossRef]
- Bermudez, S.; Voora, V.; Larrea, C. (the year is 2022) Coffee Prices and Sustainability Available online: https://www.iisd.org/system/files/2022-09/2022-global-market-report-coffee.pdf (accessed on 9 April 2023).
- Yebasse, M.; Shimelis, B.; Warku, H.; Ko, J.; Cheoi, K.J. Coffee Disease Visualization and Classification. Plants 2021, 10, 1257. [CrossRef]
- Noponen, M.R.A.; Haggar, J.P.; Edwards-Jones, G.; Healey, J.R. Intensification of Coffee Systems Can Increase the Effectiveness of REDD Mechanisms. Agric. Syst. 2013, 119, 1–9. [CrossRef]
- Rhiney, K.; Guido, Z.; Knudson, C.; Avelino, J.; Bacon, C.M.; Leclerc, G.; Aime, M.C.; Bebber, D.P. Epidemics and the Future of Coffee Production. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2023212118. [CrossRef]
- McCook, S.; Vandermeer, J. The Big Rust and the Red Queen: Long-Term Perspectives on Coffee Rust Research. Phytopathology 2015, 105, 1164–1173. [CrossRef]
- Gichuru, E.; Alwora, G.; Gimase, J.; Kathurima, C. Coffee Leaf Rust (Hemileia vastatrix) in Kenya—A Review. Agronomy 2021, 11, 2590. [CrossRef]
- Talhinhas, P.; Batista, D.; Diniz, I.; Vieira, A.; Silva, D.N.; Loureiro, A.; Tavares, S.; Pereira, A.P.; Azinheira, H.G.; Guerra-Guimarães, L.; et al. The Coffee Leaf Rust Pathogen Hemileia vastatrix : One and a Half Centuries around the Tropics. Mol. Plant Pathol. 2017, 18, 1039–1051. [CrossRef]
- Li, L.; Várzea, V.M.P.; Xia, Q.; Xiang, W.; Tang, T.; Zhu, M.; He, C.; Pereira, A.P.; Lavado da Silva, M. do C.M.; Wu, W.; et al. First Report of Hemileia vastatrix (Coffee Leaf Rust) Physiological Races Emergent in Coffee Germplasm Collections in the Coffee-Cropping Regions of China. Plant Dis. 2021, 105, 4162. [CrossRef]
- Motisi, N.; Ribeyre, F.; Poggi, S. Coffee Tree Architecture and Its Interactions with Microclimates Drive the Dynamics of Coffee Berry Disease in Coffee Trees. Sci. Rep. 2019, 9, 2544. [CrossRef]
- Mulatu, A.; Megersa, N.; Teferi, D.; Alemu, T.; Vetukuri, R.R. Biological Management of Coffee Wilt Disease (Fusarium xylarioides) Using Antagonistic Trichoderma Isolates. Front. Plant Sci. 2023, 14, 1113949. [CrossRef]
- Ramos, J.B.; de Resende, M.L.V.; Botelho, D.M.d.S.; Pereira, R.C.M.; Reichel, T.; Balieiro, A.A.F.; Botega, G.P.; Abrahão, J.C. d. R. Screening Coffee Genotypes for Brown Eye Spot Resistance in Brazil. PLoS One 2022, 17, e0258822. [CrossRef]
- Nair, K.P.P. Coffee. In The Agronomy and Economy of Important Tree Crops of the Developing World; Elsevier: Amsterdam, Netherlands, 2010; pp. 181–208.
- Johnson, M.A.; Ruiz-Diaz, C.P.; Manoukis, N.C.; Verle Rodrigues, J.C. Coffee Berry Borer (Hypothenemus hampei), a Global Pest of Coffee: Perspectives from Historical and Recent Invasions, and Future Priorities. Insects 2020, 11, 882. [CrossRef]
- Dantas, J.; Motta, I.O.; Vidal, L.A.; Nascimento, E.F.M.B.; Bilio, J.; Pupe, J.M.; Veiga, A.; Carvalho, C.; Lopes, R.B.; Rocha, T.L.; et al. A Comprehensive Review of the Coffee Leaf Miner Leucoptera coffeella (Lepidoptera: Lyonetiidae)-A Major Pest for the Coffee Crop in Brazil and Others Neotropical Countries. Insects 2021, 12, 1130. [CrossRef]
- Gugliuzzo, A.; Criscione, G.; Biondi, A.; Aiello, D.; Vitale, A.; Polizzi, G.; Tropea Garzia, G. Seasonal Changes in Population Structure of the Ambrosia Beetle Xylosandrus compactus and Its Associated Fungi in a Southern Mediterranean Environment. PLoS One 2020, 15, e0239011. [CrossRef]
- Hoang, H.; Tran, L.H.; Nguyen, T.H.; Nguyen, D.A.T.; Nguyen, H.H.T.; Pham, N.B.; Trinh, P.Q.; de Boer, T.; Brouwer, A.; Chu, H.H. Occurrence of Endophytic Bacteria in Vietnamese Robusta Coffee Roots and Their Effects on Plant Parasitic Nematodes. Symbiosis 2020, 80, 75–84. [CrossRef]
- Infante, F. Pest Management Strategies against the Coffee Berry Borer (Coleoptera: Curculionidae: Scolytinae). J. Agric. Food Chem. 2018, 66, 5275–5280. [CrossRef]
- Pandey, M.; Kayastha, P.; Khanal, S.; Shrestha, S.; Thakur, G.; Adhikari, K.; Shah, K.K.; Pant, D.; Khanal, D. An Overview on Possible Management Strategies for Coffee White Stem Borer Xylotrechus quadripes Chevrolat (Coleoptera: Cerambycidae) in Nepal. Heliyon 2022, 8, e10445. [CrossRef]
- Chaudhary, P.; Agri, U.; Chaudhary, A.; Kumar, A.; Kumar, G. Endophytes and Their Potential in Biotic Stress Management and Crop Production. Front. Microbiol. 2022, 13, 933017. [CrossRef]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal Endophytes and Their Role in Agricultural Plant Protection against Pests and Pathogens. Plants 2022, 11, 384. [CrossRef]
- Aamir, M.; Rai, K.K.; Zehra, A.; Kumar, S.; Yadav, M.; Shukla, V.; Upadhyay, R.S. Fungal Endophytes: Classification, Diversity, Ecological Role, and Their Relevance in Sustainable Agriculture. In Microbial Endophytes; Kumar, A., Singh, V.K., Eds.; Elsevier: Sawston, UK, 2020; pp. 291–323.
- Jia, M.; Chen, L.; Xin, H.-L.; Zheng, C.-J.; Rahman, K.; Han, T.; Qin, L.-P. A Friendly Relationship between Endophytic Fungi and Medicinal Plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [CrossRef]
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic Actinobacteria of Medicinal Plants: Diversity and Bioactivity. Antonie Van Leeuwenhoek 2015, 108, 267–289. [CrossRef]
- Mishra, V.K.; Passari, A.K.; Leo, V.V.; Singh, B.P. Molecular Diversity and Detection of Endophytic Fungi Based on Their Antimicrobial Biosynthetic Genes. In Fungal Biology, Singh, B.P., Gupta, V.K., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–35.
- Gakuubi, M.M.; Munusamy, M.; Liang, Z.-X.; Ng, S.B. Fungal Endophytes: A Promising Frontier for Discovery of Novel Bioactive Compounds. J. Fungi 2021, 7, 786. [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant Beneficial Endophytic Bacteria: Mechanisms, Diversity, Host Range and Genetic Determinants. Microbiol. Res. 2019, 221, 36–49. [CrossRef]
- Fávaro, L.C. de L.; Sebastianes, F.L. de S.; Araújo, W.L. Epicoccum nigrum P16, a Sugarcane Endophyte, Produces Antifungal Compounds and Induces Root Growth. PLoS One 2012, 7, e36826. [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities with Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [CrossRef]
- Ahlawat, O.P.; Yadav, D.; Kashyap, P.L.; Khippal, A.; Singh, G. Wheat Endophytes and Their Potential Role in Managing Abiotic Stress under Changing Climate. J. Appl. Microbiol. 2022, 132, 2501–2520. [CrossRef]
- Rosenblueth, M.; Martínez-Romero, E. Bacterial Endophytes and Their Interactions with Hosts. Mol. Plant. Microbe. Interact. 2006, 19, 827–837. [CrossRef]
- Yadav, G.; Meena, M. Bioprospecting of Endophytes in Medicinal Plants of Thar Desert: An Attractive Resource for Biopharmaceuticals. Biotechnol. Rep. 2021, 30, e00629. [CrossRef]
- Flewelling, A.J.; Currie, J.; Gray, C.A.; and Johnson. J.A. Endophytes from Marine Macroalgae: Promising Sources of Novel Natural Products. Curr. Sci. 2015, 109, 88–111.
- Deutsch, Y.; Gur, L.; Frank, I.B.; Ezra, D. Endophytes from Algae, a Potential Source for New Biologically Active Metabolites for Disease Management in Aquaculture. Front. Mar. Sci. 2021, 8. [CrossRef]
- Suryanarayanan, T.S. Endophyte Research: Going beyond Isolation and Metabolite Documentation. Fungal Ecol. 2013, 6, 561–568. [CrossRef]
- Aleynova, O.A.; Kiselev, K.V. Interaction of Plants and Endophytic Microorganisms: Molecular Aspects, Biological Functions, Community Composition, and Practical Applications. Plants 2023, 12, 714. [CrossRef]
- Redecker, D.; Kodner, R.; Graham, L.E. Glomalean Fungi from the Ordovician. Science 2000, 289, 1920–1921. [CrossRef]
- Krings, M.; Taylor, T.N.; Hass, H.; Kerp, H.; Dotzler, N.; Hermsen, E.J. Fungal Endophytes in a 400-Million-Yr-Old Land Plant: Infection Pathways, Spatial Distribution, and Host Responses. New Phytol. 2007, 174, 648–657. [CrossRef]
- Schüßler, A. Molecular phylogeny, taxonomy, and evolution of Geosiphon pyriformis and arbuscular mycorrhizal fungi. Plant Soil 2002, 244, 75–83. [CrossRef]
- Remy, W.; Taylor, T.N.; Hass, H.; Kerp, H. Four Hundred-Million-Year-Old Vesicular Arbuscular Mycorrhizae. Proc. Natl. Acad. Sci. 1994, 91, 11841–11843.
- Krings, M.; Taylor, T.N.; Dotzler, N. Fungal Endophytes as a Driving Force in Land Plant Evolution: Evidence from the Fossil Record. In Biocomplexity of Plant-Fungal Interactions; Southworth, D., Eds.; John Wiley & Sons: Oxford, UK, 2012; pp. 5–27.
- Verstraete, B.; Janssens, S.; Rønsted, N. Non-Nodulated Bacterial Leaf Symbiosis Promotes the Evolutionary Success of Its Host Plants in the Coffee Family (Rubiaceae). Mol. Phylogenet. Evol. 2017, 113, 161–168. [CrossRef]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical Ecology of Endophytic Fungi: Origins of Secondary Metabolites. Chem. Biol. 2012, 19, 792–798. [CrossRef]
- El-Bondkly, E.A.M.; El-Bondkly, A.A.M.; El-Bondkly, A.A.M. Marine Endophytic Fungal Metabolites: A Whole New World of Pharmaceutical Therapy Exploration. Heliyon 2021, 7, e06362. [CrossRef]
- Schulz, B.; Boyle, C. What Are Endophytes? In Soil Biology, Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin, Heidelberg: Germany, 2007; Volume 9, pp. 1–13.
- Tiwari, P.; Kang, S.; Bae, H. Plant-Endophyte Associations: Rich yet under-Explored Sources of Novel Bioactive Molecules and Applications. Microbiol. Res. 2023, 266, 127241. [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of Bacterial Endophytes and Their Proposed Role in Plant Growth. Trends Microbiol. 2008, 16, 463–471. [CrossRef]
- Rochín-Hernández, L.S.; Rochín-Hernández, L.J.; Flores-Cotera, L.B. Endophytes, a Potential Source of Bioactive Compounds to Curtail the Formation–Accumulation of Advanced Glycation End Products: A Review. Molecules 2022, 27, 4469. [CrossRef]
- Yarzábal, L.A.; Chica, E.J. Role of Rhizobacterial Secondary Metabolites in Crop Protection against Agricultural Pests and Diseases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds; Elsevier: Amsterdam, Netherlands, 2019; pp. 31–53.
- Oszust, K.; Cybulska, J.; Frąc, M. How Do Trichoderma Genus Fungi Win a Nutritional Competition Battle against Soft Fruit Pathogens? A Report on Niche Overlap Nutritional Potentiates. Int. J. Mol. Sci. 2020, 21, 4235. [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.d.C.; Glick, B.R. Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology 2021, 10, 475. [CrossRef]
- Hansen, M.; Kragelund, L.; Nybroe, O.; Sørensen, J. Early Colonization of Barley Roots by Pseudomonas fluorescens Studied by Immunofluorescence Technique and Confocal Laser Scanning Microscopy. FEMS Microbiol. Ecol. 1997, 23, 353–360. [CrossRef]
- Sakiyama, C.C.H; Paula, E.M.; Pereira, P.C.; Borges, A.C.; Silva, D.O. Characterization of Pectin Lyase Produced by an Endophytic Strain Isolated from Coffee Cherries. Lett. Appl. Microbiol. 2001, 33, 117–121. [CrossRef]
- Maela, P.M.; Serepa-Dlamini, M.H. Current Understanding of Bacterial Endophytes, Their Diversity, Colonization and Their Roles in Promoting Plant Growth. Appl. Microbiol. Open Access 2019, 5, 1–13.
- Huang, B.; Lv, C.; Zhuang, P.; Zhang, H.; Fan, L. Endophytic Colonisation of Bacillus subtilis in the Roots of Robinia pseudoacacia L. Plant Biol. 2011, 13, 925–931. [CrossRef]
- Patel, P.; Kumar, S.; Modi, A.; Kumar, A. Deciphering Fungal Endophytes Combating Abiotic Stresses in Crop Plants (Cereals and Vegetables). In Microbial Management of Plant Stresses; Ajay Kumar, A., Droby, S., Eds.; Elsevier: Sawston, UK, 2021; pp. 131–147.
- Malfanova, N.; Lugtenberg, B.J.J.; Berg, G. Bacterial Endophytes: Who and Where, and What Are They Doing There? In Molecular Microbial Ecology of the Rhizosphere; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 391–403.
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [CrossRef]
- Ding, L.-N.; Li, Y.-T.; Wu, Y.-Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.-L. Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. Int. J. Mol. Sci. 2022, 23, 16200. [CrossRef]
- Deng, Y.; Chen, H.; Li, C.; Xu, J.; Qi, Q.; Xu, Y.; Zhu, Y.; Zheng, J.; Peng, D.; Ruan, L.; et al. Endophyte Bacillus subtilis Evade Plant Defense by Producing Lantibiotic Subtilomycin to Mask Self-Produced Flagellin. Commun. Biol. 2019, 2, 368. [CrossRef]
- Taghavi, S.; van der Lelie, D.; Hoffman, A.; Zhang, Y.-B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome Sequence of the Plant Growth Promoting Endophytic Bacterium Enterobacter Sp. 638. PLoS Genet. 2010, 6, e1000943. [CrossRef]
- Ji, L.; Yang, X.; Qi, F. Distinct Responses to Pathogenic and Symbionic Microorganisms: The Role of Plant Immunity. Int. J. Mol. Sci. 2022, 23, 10427. [CrossRef]
- Mengistu, A. A. Endophytes: Colonization, Behaviour, and Their Role in Defense Mechanism. Int. J. Microbiol. 2020, 2020, 6927219. [CrossRef]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.-D. Harnessing Bacterial Endophytes for Promotion of Plant Growth and Biotechnological Applications: An Overview. Plants 2021, 10, 935. [CrossRef]
- Rodriguez, R.J.; White Jr, J.F.; Arnold, A.E.; Redman, R.S. Fungal Endophytes: Diversity and Functional Roles. New Phytol. 2009, 182, 314–330. [CrossRef]
- Lugtenberg, B.J.J.; Caradus, J.R.; Johnson, L.J. Fungal Endophytes for Sustainable Crop Production. FEMS Microbiol. Ecol. 2016, 92, fiw194. [CrossRef]
- Petrini, O. Fungal Endophytes of Tree Leaves. In: Andrews, J.H., Hirano, S.S. (eds) Microbial Ecology of Leaves. Brock/Springer Series in Contemporary Bioscience. Springer, New York, NY., 1991; pp. 179–197 ISBN 9781461278221.
- Sampson, K. The Presence and Absence of an Endophytic Fungus in Lolium temulentum and L. perenne. Trans. Br. Mycol. Soc. 1935, 19, 337–343. [CrossRef]
- Neill, J.C. The endophyte of ryegrass (Lolium perenne). N. Z. J. Sci. Technol., 1940, 21, 280–291.
- Carroll, G. Forest Endophytes: Pattern and Process. Can. J. Bot. 1995, 73, 1316–1324. [CrossRef]
- Petrini, O.; Fisher, P.J. Fungal Endophytes in Salicornia perennis. Trans. Br. Mycol. Soc. 1986, 87, 647–651. [CrossRef]
- Vega, F.E.; Posada, F.; Catherine Aime, M.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. Entomopathogenic Fungal Endophytes. Biol. Control 2008, 46, 72–82. [CrossRef]
- Jimenez-Salgado, T.; Fuentes-Ramirez, L.E.; Tapia-Hernandez, A.; Mascarua-Esparza, M.A.; Martinez-Romero, E.; Caballero-Mellado, J. Coffea arabica L., a New Host Plant for Acetobacter diazotrophicus, and Isolation of Other Nitrogen-Fixing Acetobacteria. Appl. Environ. Microbiol. 1997, 63, 3676–3683. [CrossRef]
- Jurburg, S.D.; Shek, K.L.; McGuire, K. Soil Microbial Composition Varies in Response to Coffee Agroecosystem Management. FEMS Microbiol. Ecol. 2020, 96. [CrossRef]
- Bongiorno, V.A.; Rhoden, S.A.; Garcia, A.; Polonio, J.C.; Azevedo, J.L.; Pereira, J.O.; Pamphile, J.A. Genetic Diversity of Endophytic Fungi from Coffea arabica Cv. IAPAR-59 in Organic Crops. Ann. Microbiol. 2016, 66, 855–865. [CrossRef]
- Vega, F.E.; Simpkins, A.; Aime, M.C.; Posada, F.; Peterson, S.W.; Rehner, S.A.; Infante, F.; Castillo, A.; Arnold, A.E. Fungal Endophyte Diversity in Coffee Plants from Colombia, Hawai’i, Mexico and Puerto Rico. Fungal Ecol. 2010, 3, 122–138. [CrossRef]
- Saucedo-García, A.; Anaya, A.L.; Espinosa-García, F.J.; González, M.C. Diversity and Communities of Foliar Endophytic Fungi from Different Agroecosystems of Coffea arabica L. in Two Regions of Veracruz, Mexico. PLoS One 2014, 9, e98454. [CrossRef]
- Oliveira, R.J.V.; Souza, R.G.; Lima, T.E.F; Cavalcanti, M.A.Q. Endophytic Fungal Diversity in Coffee Leaves (Coffea arabica) Cultivated Using Organic and Conventional Crop Management Systems. Mycosphere 2014, 5, 523–530. [CrossRef]
- Gagliardi, S.; Avelino, J.; Fulthorpe, R.; Virginio Filho, E. de M.; Isaac, M.E. No Evidence of Foliar Disease Impact on Crop Root Functional Strategies and Soil Microbial Communities: What Does This Mean for Organic Coffee? Oikos 2022, 2022, e08987. [CrossRef]
- Duong, B.; Nguyen, H.X.; Phan, H.V.; Colella, S.; Trinh, P.Q.; Hoang, G.T.; Nguyen, T.T.; Marraccini, P.; Lebrun, M.; Duponnois, R. Identification and Characterization of Vietnamese Coffee Bacterial Endophytes Displaying in Vitro Antifungal and Nematicidal Activities. Microbiol. Res. 2021, 242, 126613. [CrossRef]
- Shiomi, H.F.; Silva, H.S.A.; de Melo, I.S. de; Nunes, F.V.; Bettiol, W. Bioprospecting Endophytic Bacteria for Biological Control of Coffee Leaf Rust. Sci. Agric. (Piracicaba, Braz.) 2006, 63, 32–39. [CrossRef]
- Vega, F.E.; Pava-Ripoll, M.; Posada, F.; Buyer, J.S. Endophytic Bacteria in Coffea arabica L. J. Basic Microbiol. 2005, 45, 371–380. [CrossRef]
- Asyiah, I.N.; Soekarto; Hoesain, M; Iqbal, M.;Hindersah, R.; Narulita, E; Mudakir,I. The Endophytic Bacteria Isolation as Biological Control Agent of Pratylenchus coffeae. Asian Jr. of Microbiol. Biotech. Env. Sc. 20, 2018: 159–165.
- Baker, S.; Sahana, S.; Rakshith, D.; Kavitha, H.U.; Kavitha, K.S.; Satish, S. Biodecaffeination by Endophytic Pseudomonas sp. Isolated from Coffee arabica L. J. Pharm. Res. 2012, 5, 3654–3657.
- Mulaw, T.B.; Druzhinina, I.S.; Kubicek, C.P.; Atanasova, L. Novel Endophytic Trichoderma spp. Isolated from Healthy Coffea arabica Roots are Capable of Controlling Coffee Tracheomycosis. Diversity 2013, 5, 750-766. [CrossRef]
- Chaves, F.C.; Gianfagna, T.J.; Aneja, M.; Posada, F.; Peterson, S.W.; Vega, F.E. Aspergillus oryzae NRRL 35191 from Coffee, a Non-Toxigenic Endophyte with the Ability to Synthesize Kojic Acid. Mycol. Prog. 2012, 11, 263–267. [CrossRef]
- Prihastuti, H.; Cai, L.; Chen, H.; McKenzie, E.H.C.; Hyde, K.D. Characterization of Colletotrichum Species Associated with Coffee Berries in Northern Thailand. Fungal Divers. 2009, 89–109.
- Monteiro, M.C.P.; Tavares, D.G.; Nery, E.M.; de Queiroz, M.V.; Pereira, O.L.; Cardoso, P.G. Enzyme Production by Induratia spp. Isolated from Coffee Plants in Brazil. Braz. Arch. Biol. Technol. 2020, 63. [CrossRef]
- Monteiro, M.C.; Alves, N.M.; Queiroz, M.V.; Pinho, D.B.; Pereira, O.L.; Souza, S.M.; Cardoso, P.G. Antimicrobial activity of endophytic fungi from coffee plants. Biosci. j. 2017, 381–389.
- Peterson, S.W.; Vega, F.E.; Posada, F.; Nagai, C. Penicillium Coffeae,a New Endophytic Species Isolated from a Coffee Plant and Its Phylogenetic Relationship to P. fellutanum, P. thiersiiand P. brocae Based on Parsimony Analysis of Multilocus DNA Sequences. Mycologia 2005, 97, 659–666. [CrossRef]
- Mamani-Huayhua, G.; Leon-Ttacca, B; Palao-Iturregui, L.A.; Borja-Loza, Y.R. Biocontrol of coffee yellow rust (Hemileia vastatrix Berk. & Br.) with Trichoderma sp. endophyte strains. Cultivos Tropicales. 2021, 42, e01.
- de Lima, T.E.F.; de Oliveira, R.J.V.; Neves, R.P.; Bezerra, J.L.; de Queiroz Cavalcanti, M.A. Endophytic Yeasts of Coffea arabica and Vitis labrusca Cv. Isabel from Pernambuco, Brazil. Nova Hedwigia 2013, 96, 463–469. [CrossRef]
- Cun, H.; Munir, S.; He, P.; Wu, Y.; He, P.; Ahmed, A.; Che, H.; Li, J.; He, Y. Diversity of Root Endophytic Bacteria from Maize Seedling Involved in Biocontrol and Plant Growth Promotion. Egypt. J. Biol. Pest Contr. 2022, 32. [CrossRef]
- Sessitsch, A.; Reiter, B.; Berg, G. Endophytic Bacterial Communities of Field-Grown Potato Plants and Their Plant-Growth-Promoting and Antagonistic Abilities. Can. J. Microbiol. 2004, 50, 239–249. [CrossRef]
- Walitang, D.I.; Kim, K.; Madhaiyan, M.; Kim, Y.K.; Kang, Y.; Sa, T. Characterizing Endophytic Competence and Plant Growth Promotion of Bacterial Endophytes Inhabiting the Seed Endosphere of Rice. BMC Microbiol. 2017, 17, 209. [CrossRef]
- Dhungana, S.A; Adachi, F.; Hayashi, S.; Puri, R.R; Itoh, K. Plant Growth Promoting Effects of Nepalese Sweet Potato Endophytes. Horticulturae 2018, 4, 53. [CrossRef]
- Yan, X.; Wang, Z.; Mei, Y.; Wang, L.; Wang, X.; Xu, Q.; Peng, S.; Zhou, Y.; Wei, C. Isolation, Diversity, and Growth-Promoting Activities of Endophytic Bacteria from Tea Cultivars of Zijuan and Yunkang-10. Front. Microbiol. 2018, 9, 1848. [CrossRef]
- Morsy, M.; Cleckler, B.; Armuelles-Millican, H. Fungal Endophytes Promote Tomato Growth and Enhance Drought and Salt Tolerance. Plants 2020, 9, 877. [CrossRef]
- Fouda, A.; Eid, A.M.; Elsaied, A.; El-Belely, E.F.; Barghoth, M.G.; Azab, E.; Gobouri, A.A.; Hassan, S.E.-D. Plant Growth-Promoting Endophytic Bacterial Community Inhabiting the Leaves of Pulicaria incisa (Lam.) DC Inherent to Arid Regions. Plants 2021, 10, 76. [CrossRef]
- Hassan, S.E.-D. Plant Growth-Promoting Activities for Bacterial and Fungal Endophytes Isolated from Medicinal Plant of Teucrium polium L. J. Adv. Res. 2017, 8, 687–695. [CrossRef]
- Lin, H.; Liu, C.; Peng, Z.; Tan, B.; Wang, K.; Liu, Z. Distribution Pattern of Endophytic Bacteria and Fungi in Tea Plants. Front. Microbiol. 2022, 13, 872034. [CrossRef]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte Roles in Nutrient Acquisition, Root System Architecture Development and Oxidative Stress Tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [CrossRef]
- Chaudhary, R.; Kumar, V.; Gupta, S.; Naik, B.; Prasad, R.; Mishra, S.; Saris, P.E.J.; Kumar, V. Finger Millet (Eleusine coracana) Plant–Endophyte Dynamics: Plant Growth, Nutrient Uptake, and Zinc Biofortification. Microorganisms 2023, 11, 973. [CrossRef]
- Pratiwi, E.R.; Ardyati, T.; Suharjono, S. Plant Growth Promoting Endophytic Bacteria of Coffea canephora and Coffea arabica L. in UB Forest. J. Exp. Life Sci. 2020, 10, 119–126. [CrossRef]
- Jha, C.K.; Patel, B.; Saraf, M. Stimulation of the Growth of Jatropha curcas by the Plant Growth Promoting Bacterium Enterobacter cancerogenus MSA2. World J. Microbiol. Biotechnol. 2012, 28, 891–899. [CrossRef]
- Muleta, D.; Assefa, F.; Börjesson, E.; Granhall, U. Phosphate-Solubilising Rhizobacteria Associated with Coffea arabica L. in Natural Coffee Forests of Southwestern Ethiopia. J. Saudi Soc. Agric. Sci. 2013, 12, 73–84. [CrossRef]
- Teshome, B.; Wassie, M.; Abatneh, E. Isolation, Screening and Biochemical Characterization of Phosphate-Solubilizing Rhizobacteria Associated with Coffea arabica L. J Fertil Pestic 2017, 8. [CrossRef]
- García-Latorre, C.; Rodrigo, S.; Santamaria, O. Effect of Fungal Endophytes on Plant Growth and Nutrient Uptake in Trifolium subterraneum and Poa pratensis as Affected by Plant Host Specificity. Mycol. Prog. 2021, 20, 1217–1231. [CrossRef]
- Silva, H.S.A.; Tozzi, J.P.L.; Terrasan, C.R.F.; Bettiol, W. Endophytic Microorganisms from Coffee Tissues as Plant Growth Promoters and Biocontrol Agents of Coffee Leaf Rust. Biol. Control 2012, 63, 62–67. [CrossRef]
- Asyiah, I.N.; Mudakir, I.; Hoesain, M.; Pradana, A.P.; Djunaidy, A.; Sari, R.F. Consortium of Endophytic Bacteria and Rhizobacteria Effectively Suppresses the Population of Pratylenchus coffeae and Promotes the Growth of Robusta Coffee. Biodiversitas 2020, 21, 4702–4708. [CrossRef]
- Clay, K. The Ecology and Evolution of Endophytes. Agric. Ecosyst. Environ. 1993, 44, 39–64. [CrossRef]
- Ali, S.; Hameed, S.; Imran, A.; Iqbal, M.; Lazarovits, G. Genetic, Physiological and Biochemical Characterization of Bacillus sp. Strain RMB7 Exhibiting Plant Growth Promoting and Broad Spectrum Antifungal Activities. Microb. Cell Fact. 2014, 13, 144. [CrossRef]
- Kejela, T.; Thakkar, V.R.; Thakor, P. Bacillus Species (BT42) Isolated from Coffea arabica L. Rhizosphere Antagonizes Colletotrichum gloeosporioides and Fusarium oxysporum and Also Exhibits Multiple Plant Growth Promoting Activity. BMC Microbiol. 2016, 16, 277. [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [CrossRef]
- Trutmann, P.; Keane, P.J. Trichoderma koningii as a Biological Control Agent for Sclerotinia sclerotiorum in Southern Australia. Soil Biol. Biochem. 1990, 22, 43–50. [CrossRef]
- Morandi, M.A.B.; Sutton, J.C.; Maffia, L.A. Effects of Host and Microbial Factors on Development of Clonostachys rosea and Control of Botrytis cinerea in Rose. Eur. J. Plant Pathol. 2000, 106, 439–448. [CrossRef]
- Mousa, W.K.; Raizada, M.N. The Diversity of Anti-Microbial Secondary Metabolites Produced by Fungal Endophytes: An Interdisciplinary Perspective. Front. Microbiol. 2013, 4, 65. [CrossRef]
- Lo Presti, L.; Lanver, D.; Schweizer, G.; Tanaka, S.; Liang, L.; Tollot, M.; Zuccaro, A.; Reissmann, S.; Kahmann, R. Fungal Effectors and Plant Susceptibility. Annu. Rev. Plant Biol. 2015, 66, 513–545. [CrossRef]
- Baiyee, B.; Ito, S.-I.; Sunpapao, A. Trichoderma asperellum T1 Mediated Antifungal Activity and Induced Defense Response against Leaf Spot Fungi in Lettuce (Lactuca sativa L.). Physiol. Mol. Plant Pathol. 2019, 106, 96–101. [CrossRef]
- Li, Z.; Wen, W.; Qin, M.; He, Y.; Xu, D.; Li, L. Biosynthetic Mechanisms of Secondary Metabolites Promoted by the Interaction between Endophytes and Plant Hosts. Front. Microbiol. 2022, 13, 928967. [CrossRef]
- Cacefo, V.; De Araújo, F. F.; Pacheco, A. C. Biological control of Hemileia vastatrix berk. & broome with bacillus subtilis cohn and biochemical changes in the coffee. Coffee Sci. 2017, 11, 567–574.
- Gomes, A.A.M.; Paes, S.A.; Ferreira, A.P.S.; Pinho, D.B.; de Lourdes Cardeal, Z.; Menezes, H.C.; Cardoso, P.G.; Pereira, O.L. Endophytic Species of Induratia from Coffee and Carqueja Plants from Brazil and Its Potential for the Biological Control of Toxicogenic Fungi on Coffee Beans by Means of Antimicrobial Volatiles. Braz. J. Microbiol. 2023, 54, 349–360. [CrossRef]
- Badel, J.L.; Zambolim, L. Coffee Bacterial Diseases: A Plethora of Scientific Opportunities. Plant Pathol. 2019, 68, 411–425. [CrossRef]
- Tian, B.; Yang, J.; Zhang, K.-Q. Bacteria Used in the Biological Control of Plant-Parasitic Nematodes: Populations, Mechanisms of Action, and Future Prospects: Nematophagous Bacteria. FEMS Microbiol. Ecol. 2007, 61, 197–213. [CrossRef]
- Moosavi, M.R.; Zare, R. Fungi as Biological Control Agents of Plant-Parasitic Nematodes. In Plant Defence: Biological Control; Mérillon, J., Ramawat, K., Eds.; Springer: Dordrecht, Netherlands, 2012; Volume 12, pp. 67–107.
- Usta, C. Microorganisms in Biological Pest Control—A Review (Bacterial Toxin Application and Effect of Environmental Factors). In Current Progress in Biological Research; Silva-Opps, M., Eds.; InTech: London, UK, 2013, pp. 287–317.
- Chattopadhyay, P.; Banerjee, G.; Mukherjee, S. Recent Trends of Modern Bacterial Insecticides for Pest Control Practice in Integrated Crop Management System. 3 Biotech 2017, 7. [CrossRef]
- Kumar, K.K.; Dara, S.K. Fungal and Bacterial Endophytes as Microbial Control Agents for Plant-Parasitic Nematodes. Int. J. Environ. Res. Public Health 2021, 18, 4269. [CrossRef]
- Mekete, T.; Hallmann, J.; Kiewnick, S.; Sikora, R. Endophytic Bacteria from Ethiopian Coffee Plants and Their Potential to Antagonise Meloidogyne incognita. Nematology 2009, 11, 117–127. [CrossRef]
- Ambele, C.F.; Ekesi, S.; Bisseleua, H.D.B.; Babalola, O.O.; Khamis, F.M.; Djuideu, C.T.L.; Akutse, K.S. Entomopathogenic Fungi as Endophytes for Biological Control of Subterranean Termite Pests Attacking Cocoa Seedlings. J. Fungi 2020, 6, 126. [CrossRef]
- Deka, B.; Babu, A.; Peter, A.J.; Kumhar, K.C.; Sarkar, S.; Rajbongshi, H.; Dey, P.;Amalraj, E.L.D.; Talluri, V.R. Potential of the Entomopathogenic Fungus, Metarhizium anisopliae s.l. in Controlling Live-Wood Eating Termite, Microtermes obesi (Holmgren) (Blattodea: Termitidae) Infesting Tea Crop. Egypt. J. Biol. Pest Contr. 2021, 31. [CrossRef]
- Viswakethu, V.; Balakrishanan, P.; Murugan, L.; Narayana swamy, B.; Subbaraya, U. Entomopathogenic Fungi as a Promising Biological Control Agent against Banana Fruit Scarring Beetle, Basilepta subcostata (Jac.) (Chrysomelidae: Coleoptera). Egypt. J. Biol. Pest Contr. 2021, 31. [CrossRef]
- Donzelli, B. G.G.; Krasnoff, S. B. Molecular Genetics of Secondary Chemistry in Metarhizium Fungi. Adv. Genet., 2016, 94, 365–436. [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-Fungal-Interactions: A Detailed Review on Entomopathogenic Fungi Pathogenicity to Combat Insect Pests. Microb. Pathog. 2021, 159, 105122. [CrossRef]
- Zhou, Q.; Yu, L.; Ying, S.-H., Feng, M.-G. Comparative Roles of Three Adhesin Genes (adh1-3) in Insect-Pathogenic Lifecycle of Beauveria bassiana. Appl. Micro;iol. Biotechnol. 2021, 105, 5491–5502. [CrossRef]
- Rehner, S.A.; Posada, F.; Buckley, E.P.; Infante, F.; Castillo, A.; Vega, F.E. Phylogenetic Origins of African and Neotropical Beauveria Bassiana s.l. Pathogens of the Coffee Berry Borer, Hypothenemus hampei. J. Invertebr. Pathol. 2006, 93, 11–21. [CrossRef]
- Posada, F.; Vega, F.E. Inoculation and Colonization of Coffee Seedlings (Coffea arabica L.) with the Fungal Entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycoscience 2006, 47, 284–289. [CrossRef]
- Gasmi, L.; Baek, S.; Kim, J.C.; Kim, S.; Lee, M.R.; Park, S.E.; Shin, T.Y.; Lee, S.J.; Parker, B.L.; Kim, J.S. Gene Diversity Explains Variation in Biological Features of Insect Killing Fungus, Beauveria bassiana. Sci. Rep. 2021, 11, 91. [CrossRef]
- Posada, F.; Aime, M.C.; Peterson, S.W.; Rehner, S.A.; Vega, F.E. Inoculation of Coffee Plants with the Fungal Entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycol. Res. 2007, 111, 748–757. [CrossRef]
- Bayman, P.; Mariño, Y.A.; García-Rodríguez, N.M.; Oduardo-Sierra, O.F.; Rehner, S.A. Local Isolates of Beauveria bassiana for Control of the Coffee Berry Borer Hypothenemus hampei in Puerto Rico: Virulence, Efficacy and Persistence. Biol. Control 2021, 155, 104533. [CrossRef]
- Samuels, R.I.; Pereira, R.C.; Gava, C.A.T. Infection of the Coffee Berry Borer Hypothenemus hampei (Coleoptera: Scolytidae) by Brazilian Isolates of the Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae (Deuteromycotina: Hyphomycetes). Biocontrol Sci. Technol. 2002, 12, 631–635. [CrossRef]
- Jia-ning, W.; Rong-ping, K. Biological Control of Coffee Stem Borers, Xylotrechus quardrlpes and Acalolepla cervznus, By Beaweha bassiana Preparation. Insect Sci. 2002, 9, 43–50. [CrossRef]
- Batista, L.R.; Chalfoun, S.M.; Prado, G.; Schwan, R.F.; Wheals, A.E. Toxigenic Fungi Associated with Processed (Green) Coffee Beans (Coffea arabica L.). Int. J. Food Microbiol. 2003, 85, 293–300. [CrossRef]
- Oliveira, M.N.V.; Santos, T.M.A.; Vale, H.M.M.; Delvaux, J.C.; Cordero, A.P.; Ferreira, A.B.; Miguel, P.S.B.; Tótola, M.R.; Costa, M.D.; Moraes, C.A.; et al. Endophytic Microbial Diversity in Coffee Cherries of Coffea arabica from Southeastern Brazil. Can. J. Microbiol. 2013, 59, 221–230. [CrossRef]
- Haile, M.; Kang, W.H. The Role of Microbes in Coffee Fermentation and Their Impact on Coffee Quality. J. Food Qual. 2019, 4836709. [CrossRef]
- Liu, F.; Song, Z.; Zhang, T.; Tong, X.; Chen, M.Y.; Gao, D.; Chen, J.; Ho, C.L. Characterization of the Therapeutic Properties and Flavor Profile of Coffee via Monoculture Fermentation with Endophytic Microbial Isolates. ACS Food Sci. Technol. 2022, 2, 1039–1049. [CrossRef]
- Abrahão, M.R.E.; Molina, G.; Pastore, G.M. Endophytes: Recent Developments in Biotechnology and the Potential for Flavor Production. Food Res. Int. 2013, 52, 367–372. [CrossRef]
- Nunes, F.V.; de Melo, I.S. Isolation and Characterization of Endophytic Bacteria of Coffee Plants and Their Potential in Caffeine Degradation. In Proceedings of the Environmental Toxicology, Kungolos, A.G., Brebbia, C.A., Samaras, C.P., Eds.; WIT Press: Southampton, UK, 2006, pp. 293–297.
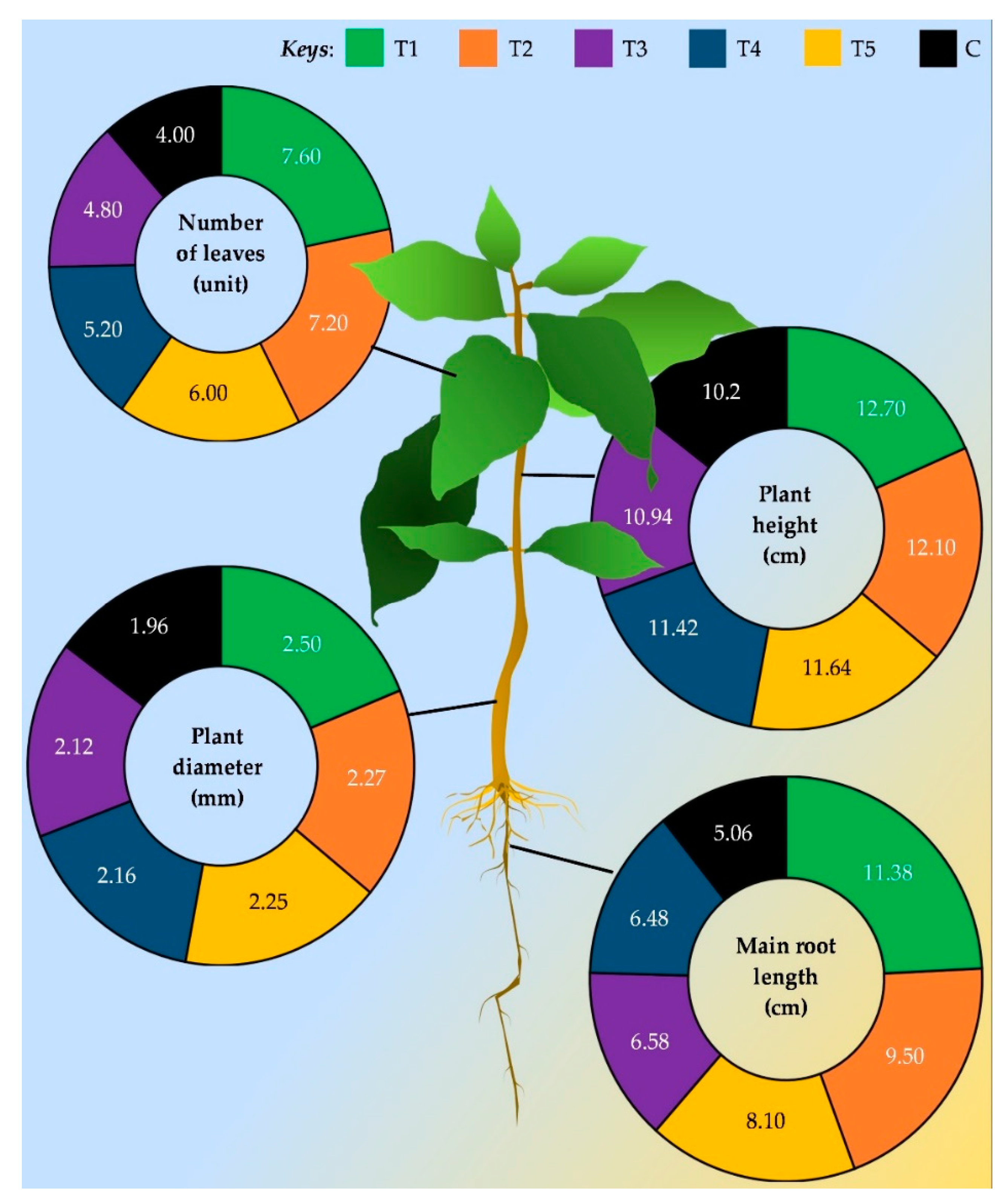
| Phylum/Division | Species | Associated coffee species | Isolated part of the coffee plant | Locality | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
|
Actinobacteria |
Arthrobacter sp. | C.c and C.l | SE and RO | Buon Ma Thuot, Bao Loc, Vietnam |
[84] |
| Clavibacter michiganensis |
C.c |
LE | State of São Paulo, Brazil |
[85] | |
| SE | Cacahoatán, Chiapas, Mexico | [86] | |||
| Curtobacterium sp. | C.a | ST | Centro Nacional de Investigaciones de café, Cenicafé, Chinchin, Colombia |
[86] | |
| Curtobacterium sp. | C.c | RO | Buon Ma Thuot, Bao Loc | [84] | |
| Curtobacterium flaccumfaciens | C.a | SE and LE | Centro Nacional deInvestigaciones de café, Cenicafé, Chinchin, Colombia | [86] | |
| Gordona sp. | C.a | CH | Hawaii | [86] | |
| Kitasatospora sp. | C.c | RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Kocuria sp. | C.c | RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Kocuria kristinae | C.a | LE | State of São Paulo, Brazil |
[85] | |
| Lechevalieria sp. | C.a and C.l | SE and RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Sinomonas sp. | C.c | RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Streptomyces sp. | C.c and C.l | SE and RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Firmicutes |
Bacillus sp. |
C.a | LE | State of São Paulo, Brazil |
[85] |
| C.c and C.l | SE and RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | ||
| Bacillus cereus | C.c | LE | State of São Paulo, Brazil |
[85] | |
| C.a | LE | Hawaii | [86] | ||
| Bacillus lentimorbus | C.c | ST | State of São Paulo, Brazil |
[85] | |
| Bacillus megaterium | C.a | LE | Hawaii | [86] | |
| Bacillus subtilis | C.a | CR | Hawaii | [86] | |
| C.a | RO | East Java Province, Indonesia |
[87] | ||
| Lysinibacillus sp. | C.c and C.l | SE and RO | Buon Ma Thuot, Bao Loc | [84] | |
| Paenibacillus sp. | C.c and C.l | SE and RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Paenibacillus amylolyticus. | C.a | CH | Minas Gerais, Brazil. | [57] | |
| Staphylococcus sp. | C.C and C.l | SE and RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
|
Proteobacteria |
Acinetobacter calcoaceticus | C.a | ST | State of São Paulo, Brazil |
[85] |
| Acetobacter diazotrophicus | C.a | ST and RO | Guerrero and Puebla, Mexico | [77] | |
| Burkholderia sp. | C.c | SE | Buon Ma Thuot, Vietnam | [84] | |
| Cedecea davisae | C.a | LE | State of São Paulo, Brazil |
[85] | |
| Enterobacter sp. | C.c | RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Herbaspirillum sp. | C.c and C.l | SE and RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Klebsiella pneumonia | Cc | LE | State of São Paulo, Brazil |
[85] | |
| Luteibacter sp. | C.c and C.l | SE and RO | Buon Ma Thuot, Bao Loc | [84] | |
| Methylobacterium sp. | C.c and C.l | SE and RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Methylobacterium radiotolerans | C.a | CH | Centro Nacional deInvestigaciones de café, Cenicafé, Chinchin, Colombia | [86] | |
| Pandoraea pnomenusa | C.a | LE | State of São Paulo, Brazil |
[85] | |
| Paracoccus sp. | C.c and C.l | SE and RO | Buon Ma Thuot, Bao Loc, Vietnam | [84] | |
| Pseudomonas sp. | C.a | ST, RO, and LE (NS) | Southern parts of India. | [88] | |
| Fungi | |||||
| Ascomycota | Acremonium alternatum | C.a | EP | Caldas, Colombia | [76] |
| Aspergillus sp. | C.a | RO | Ethiopia | [89] | |
| Aspergillus oryzae | C.a | LE | Caldas, Colombia | [89] | |
| Aureobasidium pullulans | C.a | LE | Pernambuco, Brazil | [82] | |
| Beauveria bassiana | C.a | SE, EP, EP, and CR | Caldas, Colombia | [76] | |
| Cercospora iranica | C.a | LE | Paraná, Brazil. | [79] | |
| Cercospora tezpurensis | C.a | LE | Paraná, Brazil. | [79] | |
| Cladosporium sp. | C.a | RO | Ethiopia | [89] | |
| Cladosporium cladosporioides | C.a | LE | Adjuntas, Puerto Rico and Kona, Hawaii, USA | [76] | |
|
Cladosporium pini-ponderosae |
C.a | LE | Paraná, Brazil. | [79] | |
| Cladosporium sphaerospermum | C.a | LE | Beltsville, Maryland, USA | [76] | |
| Cladosporium tenuissimum | C.a | LE | Pernambuco, Brazil | [82] | |
| Clonostachys rosea | C.a | LE | Chinchina, Caldas, Colombia | [76] | |
| Cladosporium cladosporioides | C.a | LE | Pernambuco, Brazil | [82] | |
| Colletotrichum asianum | C.a | CH | Chiang Mai, Thailand | [91] | |
| Colletotrichum brassicicola | C.a | LE | Veracruz, Mexico | [81] | |
| Colletotrichum coffeanum | C.a | LE | Pernambuco, Brazil | [82] | |
| Colletotrichum endophytica | C.a | LE | Paraná, Brazil. | [79] | |
| Colletotrichum fructicola | C.a | CH | Chiang Mai, Thailand | [91] | |
| Colletotrichum gloeosporioides | C.a | LE | Veracruz, Mexico | [81] | |
| Colletotrichum gloeosporioides | C.a | LE | Pernambuco, Brazil | [82] | |
| Colletotrichum musae | C.a | LE | Veracruz, Mexico | [81] | |
| Colletotrichum queenslandicum | C.a | LE | Paraná, Brazil. | [79] | |
| Colletotrichum siamense | C.a | CH | Chiang Mai, Thailand | [91] | |
|
Cryptosporiopsis corticola |
C.a | LE | Veracruz, Mexico | [81] | |
| Diaporthe liquidambaris | C.a | LE | Pernambuco, Brazil | [82] | |
|
Dipodascaceae sp. |
C.a | RO | Ethiopia | [89] | |
| Drechslera biseptata | C.a | LE | Pernambuco, Brazil | [82] | |
| Fusarium equiseti | C.a | RO | Ethiopia | [89] | |
| Fusarium oxysporum | C.a | RO | Ethiopia | [89] | |
| Fusarium solani | C.a | RO | Ethiopia | [89] | |
| Glomerella cingulata | C.a | LE | Veracruz, Mexico | [81] | |
| Guignardia mangiferae | C.a | LE | Veracruz, Mexico | [81] | |
| Induratia coffeana | C.a | NS | Minas Gerais, Brazil | [92] | |
| Induratia yucatanensis | C.a | NS | Minas Gerais, Brazil | [92] | |
| Khuskia oryzae | C.a | LE | Pernambuco, Brazil | [82] | |
| Lasiodiplodia pseudotheobromae | C.a | LE | Pernambuco, Brazil | [82] | |
| Macrophomina sp. | C.a | RO | Ethiopia | [89] | |
| Muscodor coffeanum* | C.a | LE | Minas Gerais, Brazil | [93] | |
| Muscodor vitigenus* | C.a | ST | Minas Gerais, Brazil | [93] | |
| Muscodor yucatanensis* | C.a | LE | Minas Gerais, Brazil | [93] | |
| Mycosphaerella pseudovespa | C.a | LE | Paraná, Brazil. | [79] | |
| Nodulisporium gregarium | C.a | LE | Pernambuco, Brazil | [82] | |
|
Ophioceras leptosporum |
C.a | LE | Paraná, Brazil. | [79] | |
| Ophiognomonia sp. | C.a | LE | Paraná, Brazil. | [79] | |
| Paecilomyces fumosoroseus | C.a | CR | Adjuntas, Puerto Rico | [76] | |
| Paecilomyces javanicus | C.a | PE | Chinchina, caldas, Colombia | [76] | |
| Paenibacillus amylolyticus | C.a | CH | Minas Gerais, Brazil. | [57] | |
| Penicillium sp. | C.a | RO | Ethiopia | [89] | |
| Penicillium coffeae | C.a | NS | Kunia, Hawaii |
[94] | |
| Pestalotiopsis maculans | C.a | LE | Pernambuco, Brazil | [82] | |
| Phoma eupyrena | C.a | LE | Pernambuco, Brazil | [82] | |
| Phomopsis sp. | C.a | RO | Ethiopia | [89] | |
| Phomopsis arnoldiae | C.a | LE | Veracruz, Mexico | [81] | |
| Phyllosticta sp. | C.a | LE | Pernambuco, Brazil | [82] | |
|
Phyllosticta capitalensis (=Guignardia mangiferae) |
C.a | LE | Pernambuco, Brazil | [82] | |
|
Pleosporales sp. |
C.a | RO | Ethiopia | [89] | |
| Sarocladium bacillisporum | C.a | LE | Pernambuco, Brazil | [82] | |
|
Simplicillium sp. |
C.a | ST | Minas Gerais, Brazil |
[93] | |
| Trichoderma sp. | C.a | LE and ST | San Juan del Oro, Peru |
[95] | |
| Trichoderma flagellatum | C.a | RO | Ethiopia | [89] | |
| Trichoderma hamatum | C.a | RO | Ethiopia | [89] | |
| Trichoderma neokoningii | C.a | LE | Paraná, Brazil. | [79] | |
| Xylaria spp. | C.a | LE | Veracruz, Mexico | [81] | |
| Xylaria sp. | C.a | LE | Pernambuco, Brazil | [82] | |
| Basidiomycota | |||||
| Rhodotorula acheniorum | C.a | LE | Pernambuco, Brazil | [96] | |
| Rhodotorula aurantiaca | C.a | LE | Pernambuco, Brazil | [96] | |
| Rhodotorula aurantiaca | C.a | LE | Pernambuco, Brazil | [82] | |
| Rhodotorula glutinis | C.a | LE | Pernambuco, Brazil | [96] | |
| Rhodotorula mucilaginosa | C.a | LE | Pernambuco, Brazil | [96] | |
| Schizophyllum commune | C.a | LE | Paraná, Brazil. | [79] | |
| Trametes sp. | C.a | RO | Ethiopia | [89] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





