Submitted:
22 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
Statistical Analysis
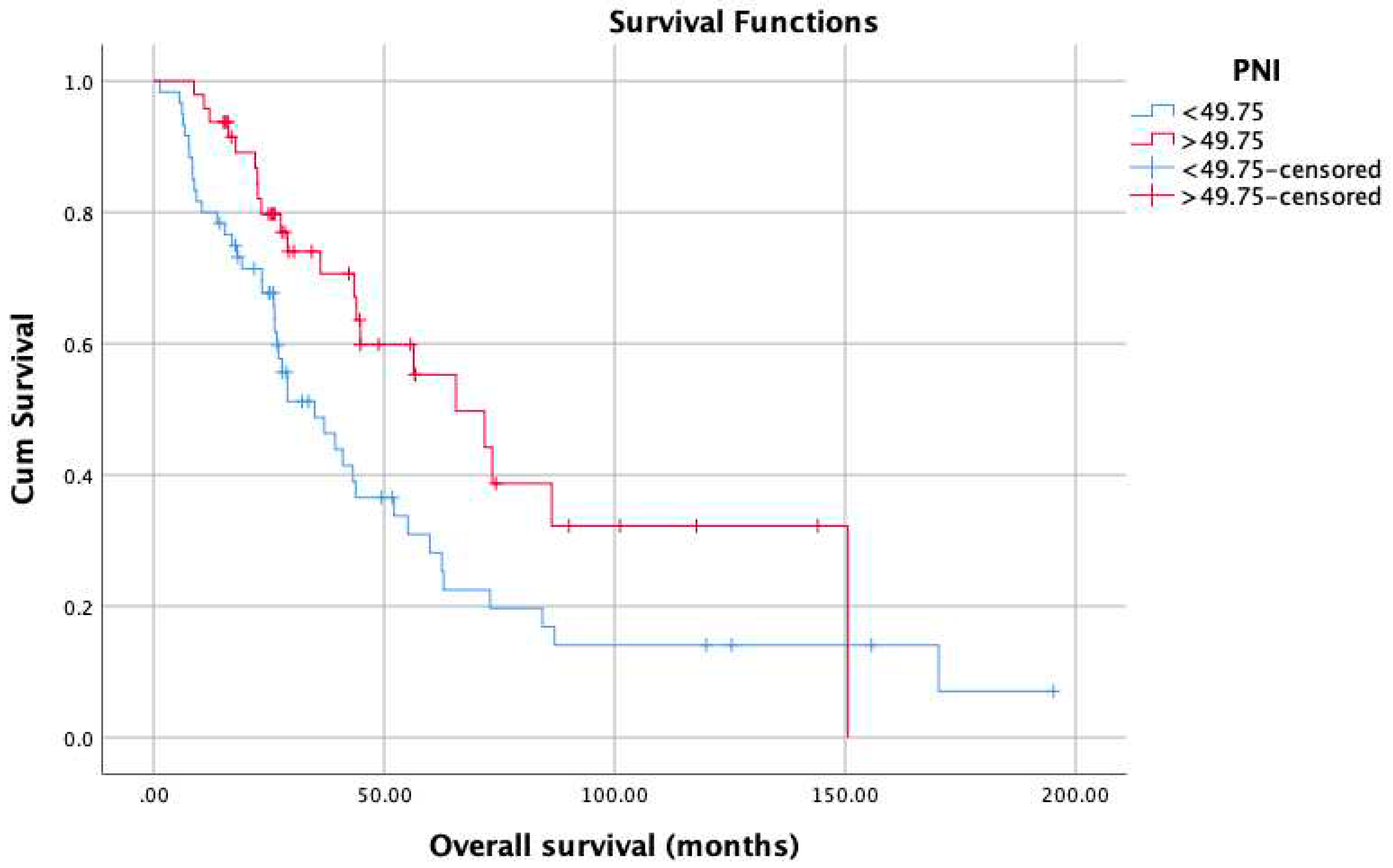
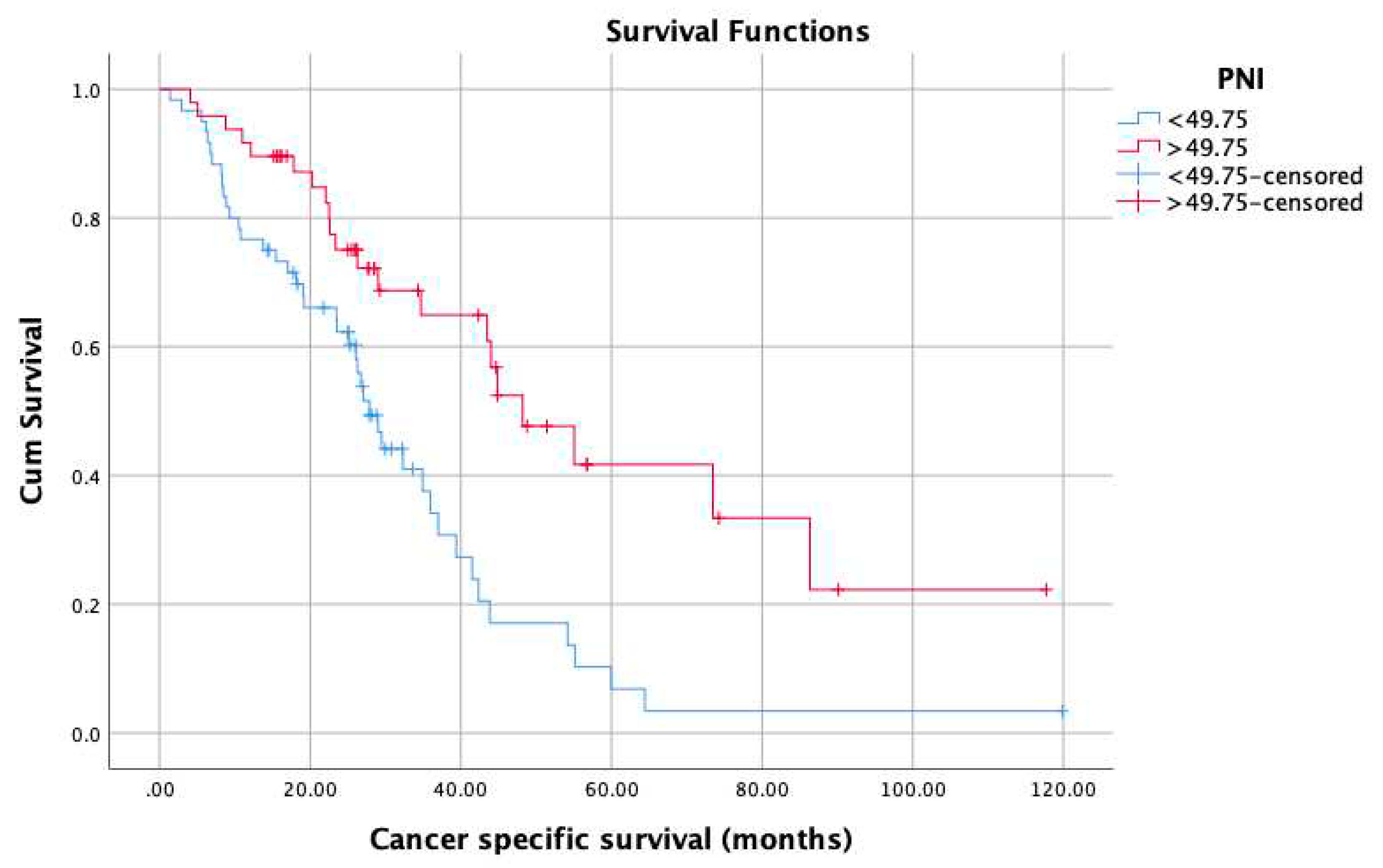
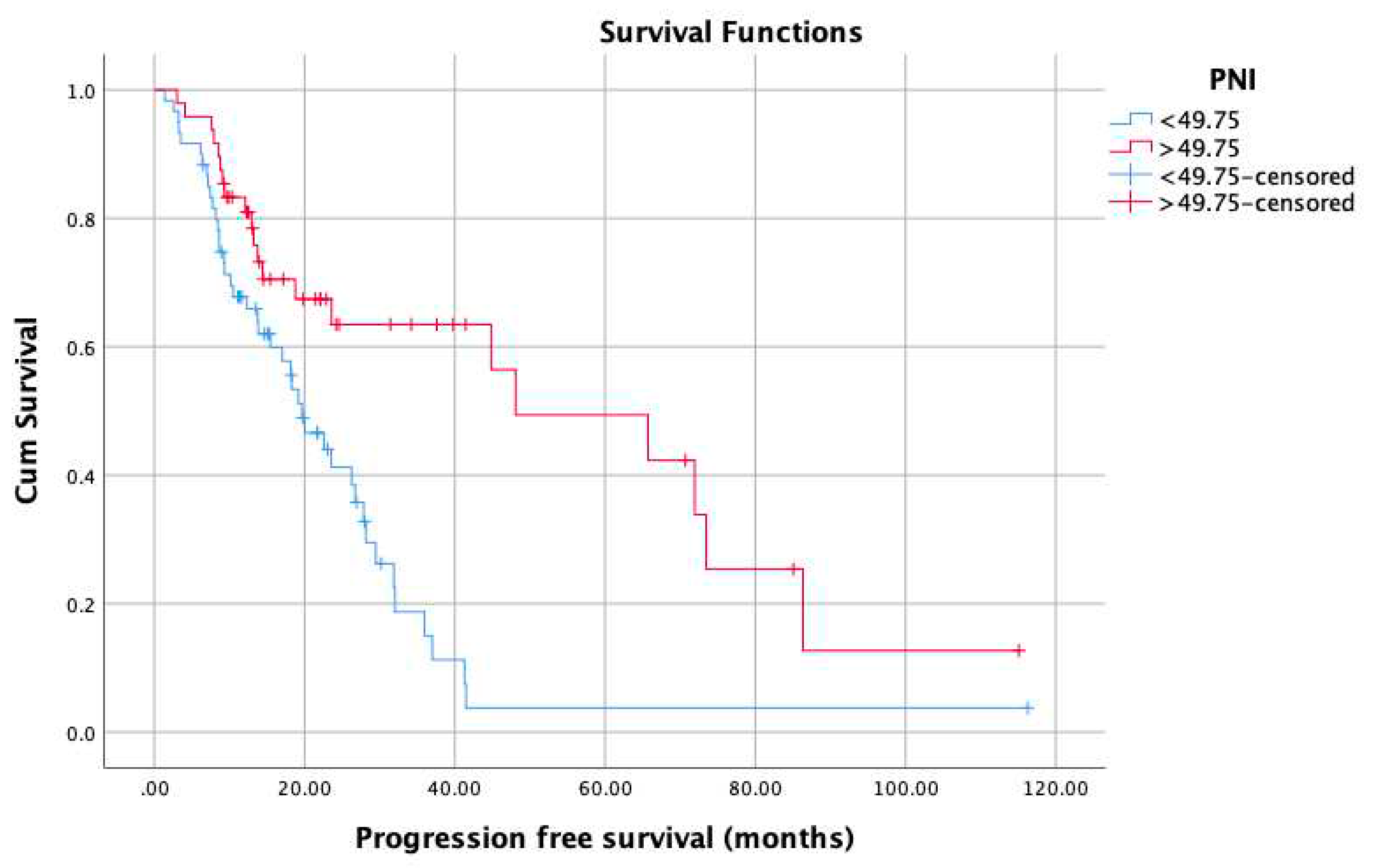
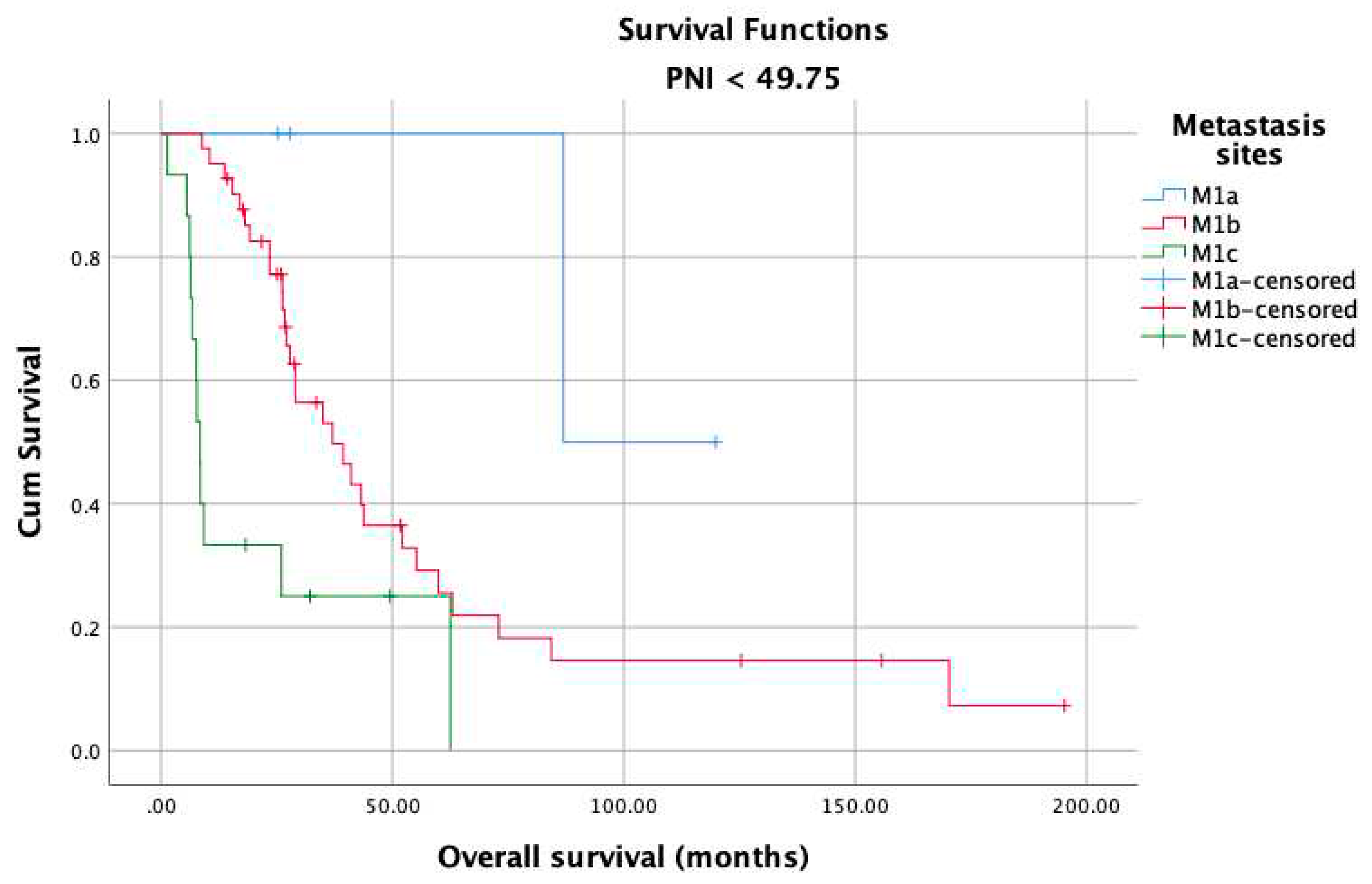
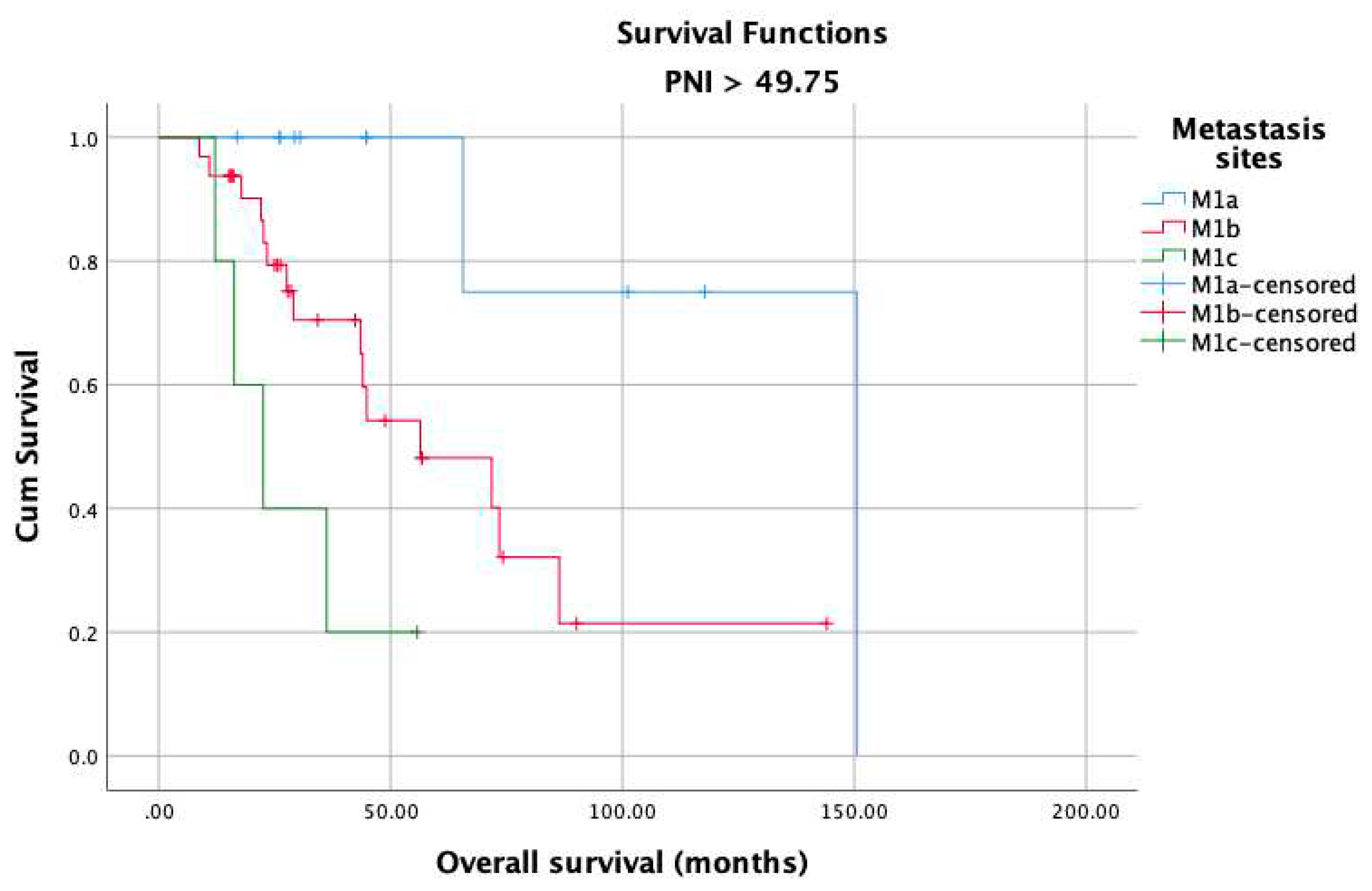
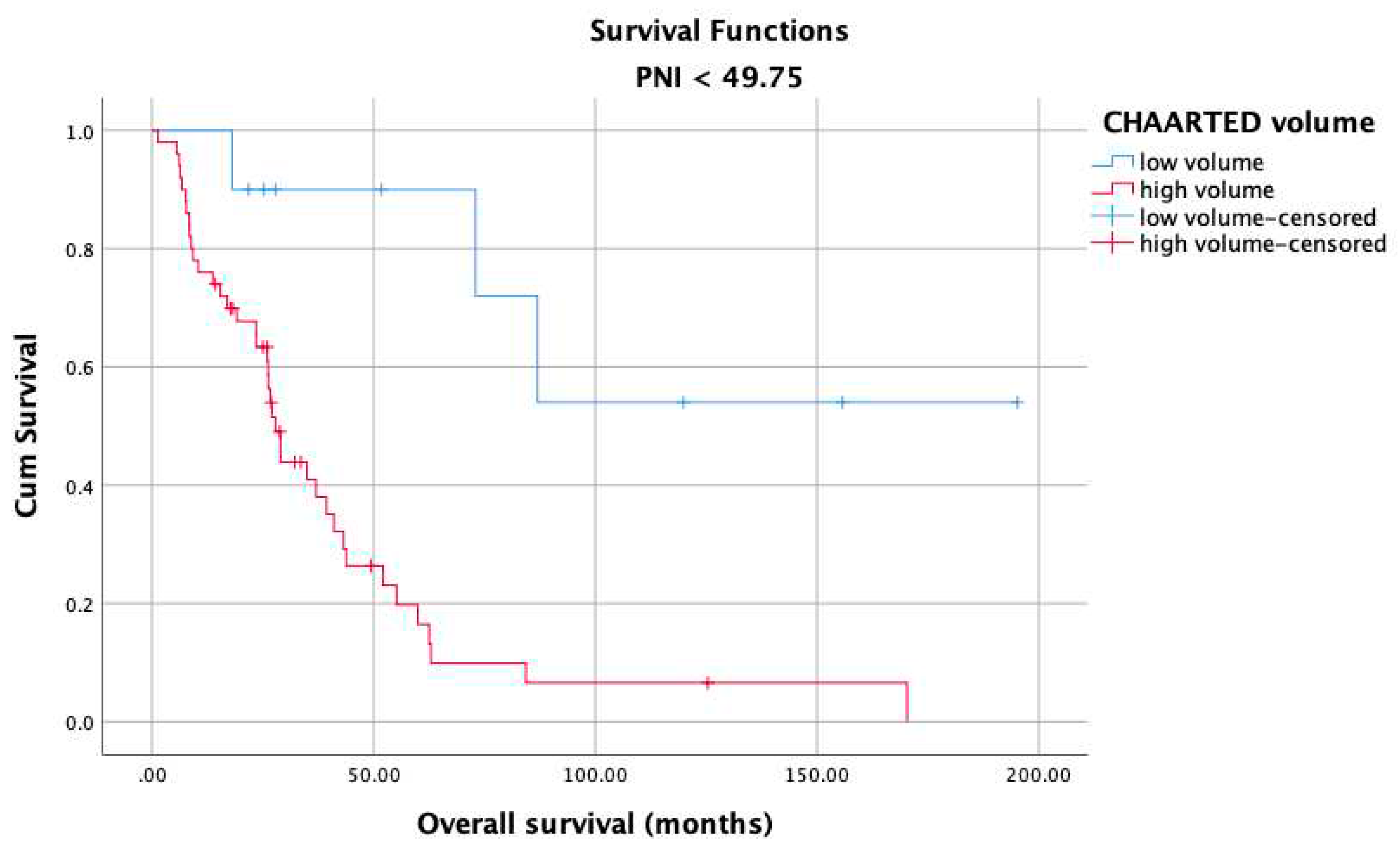
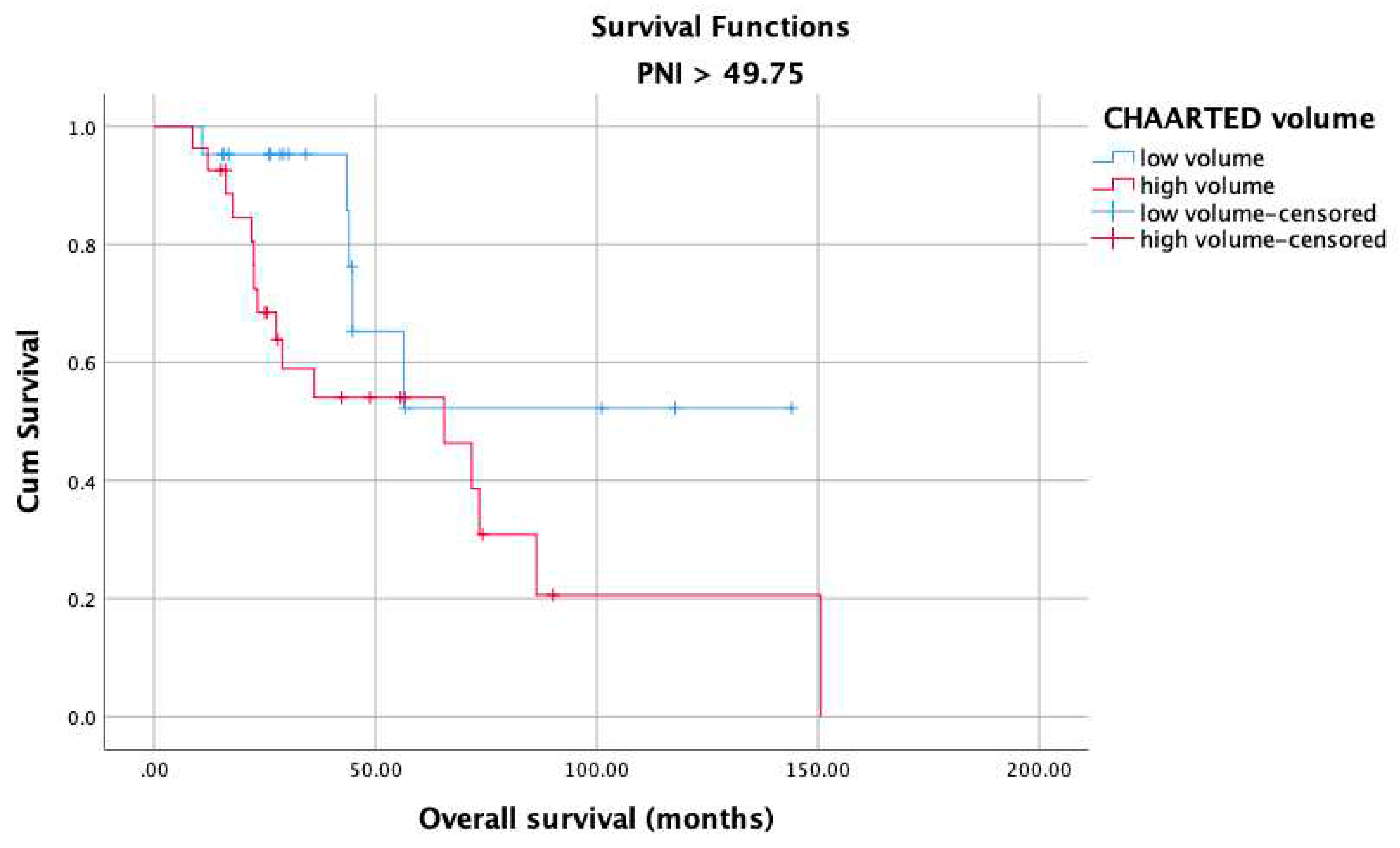
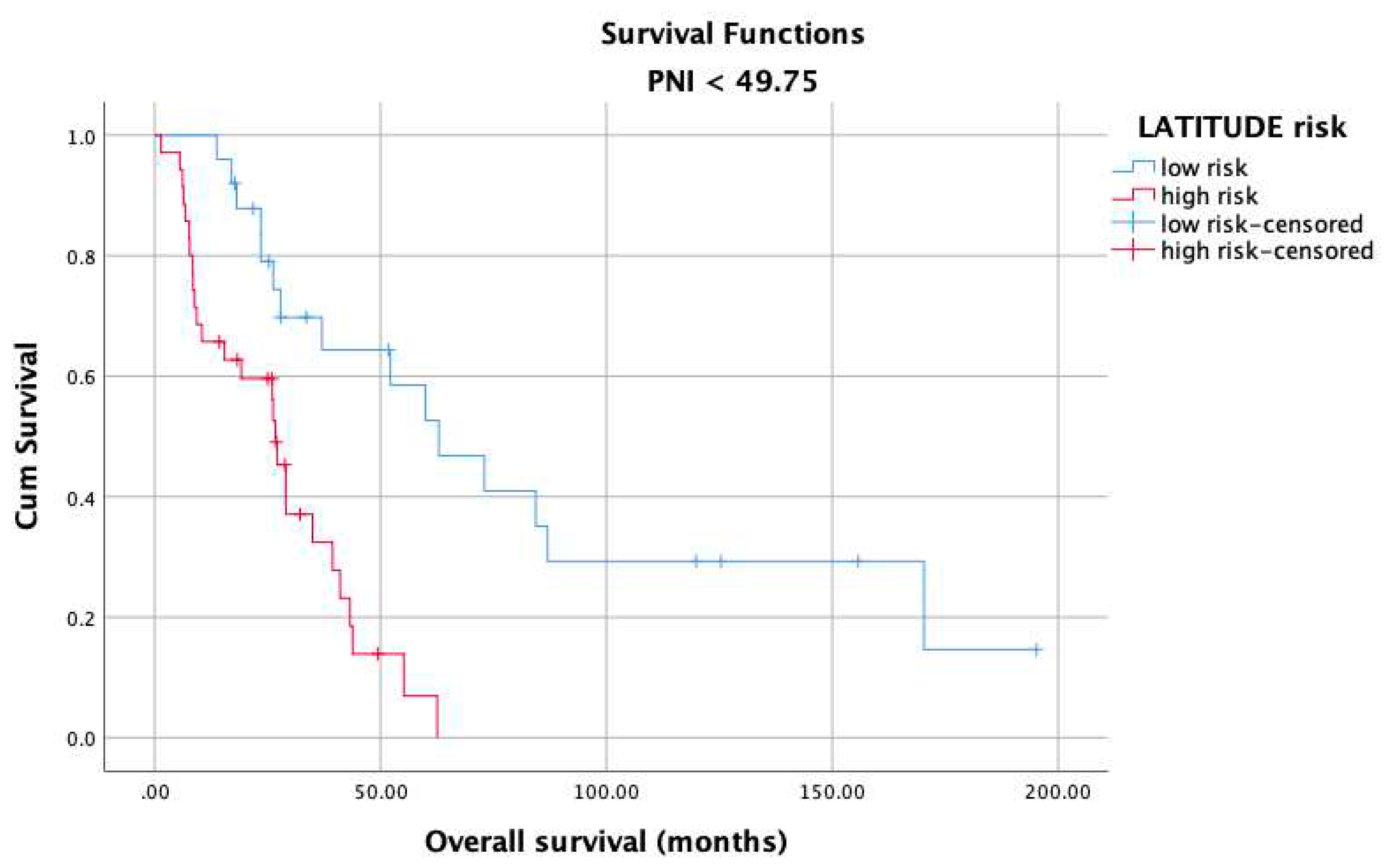
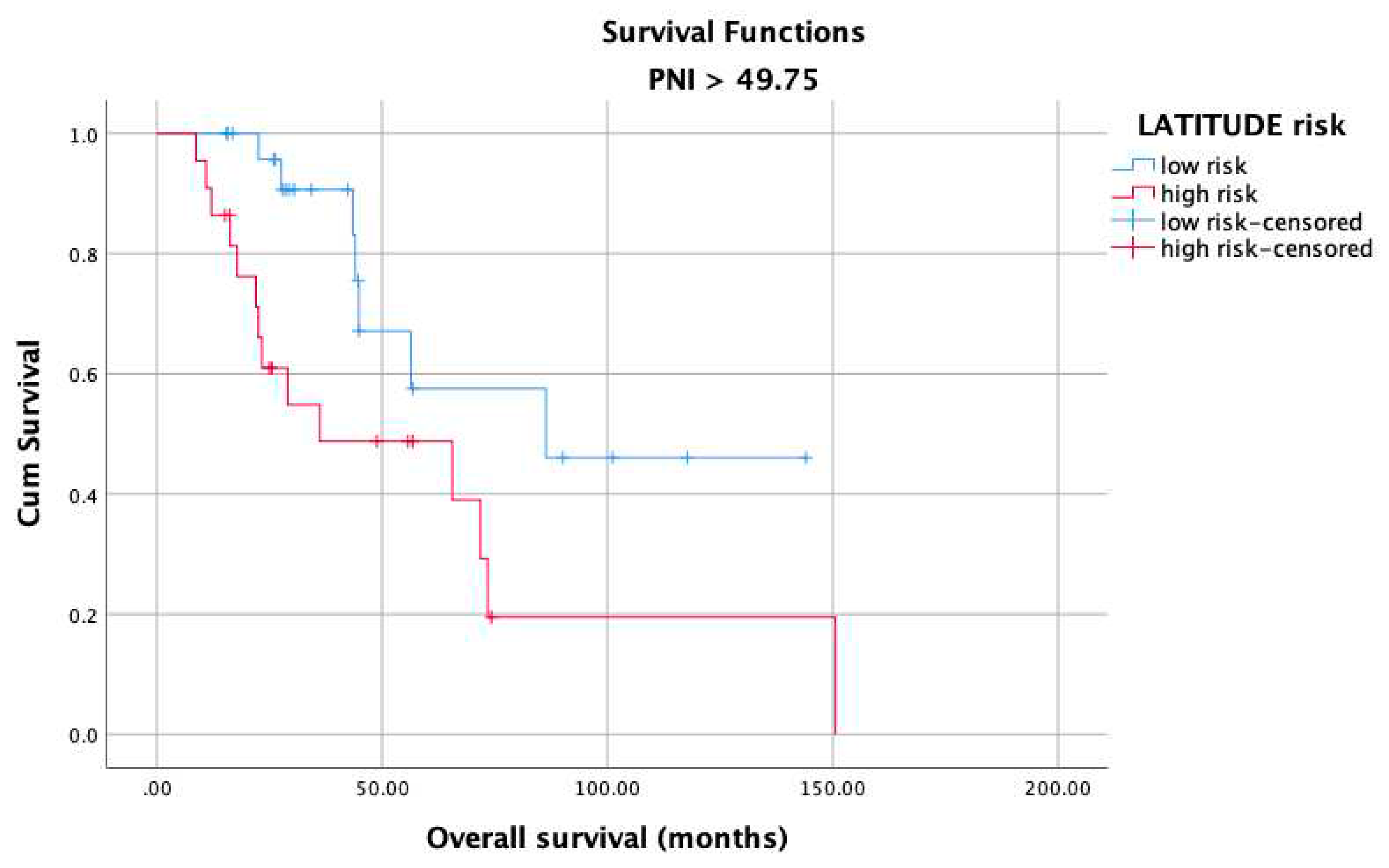
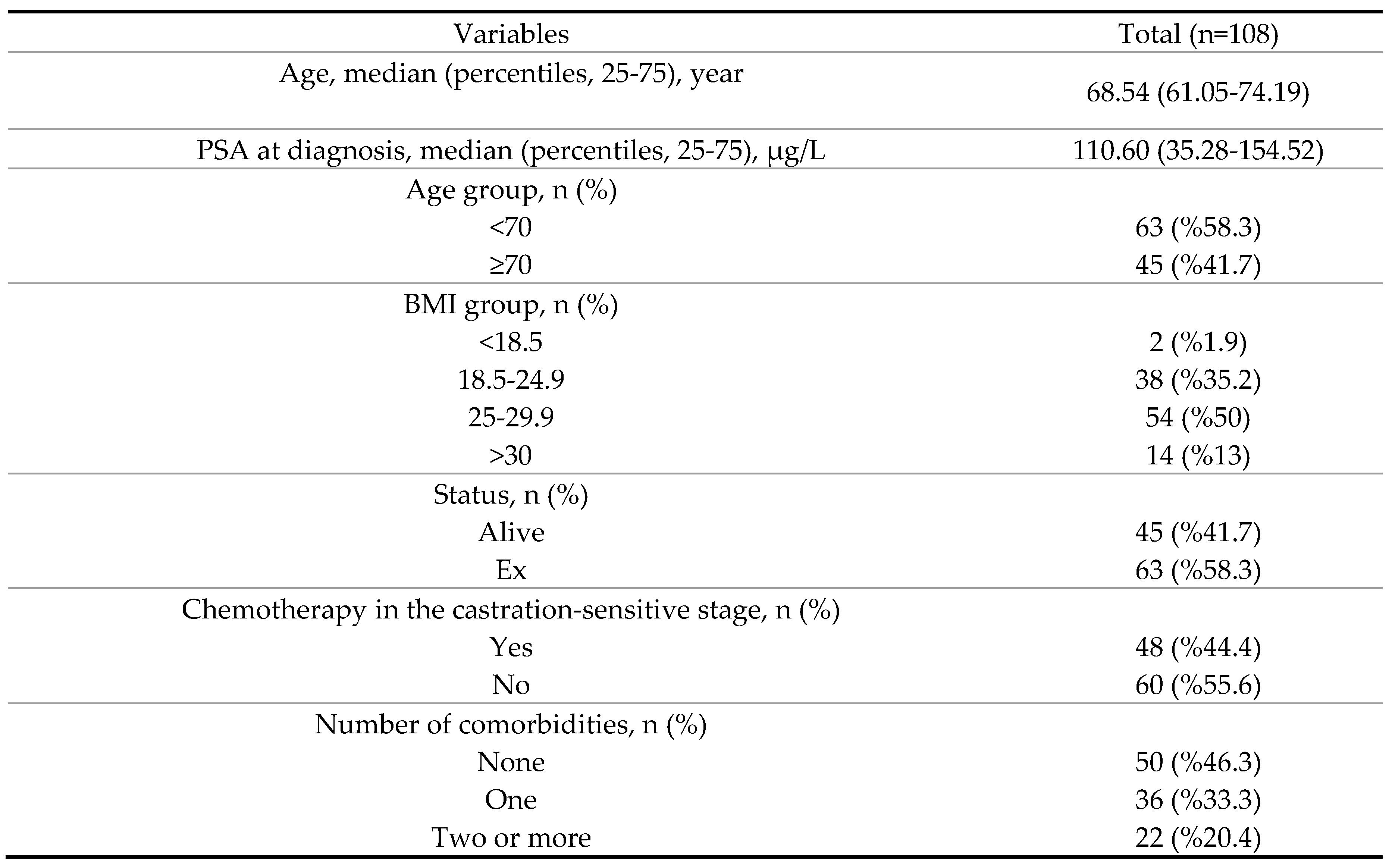
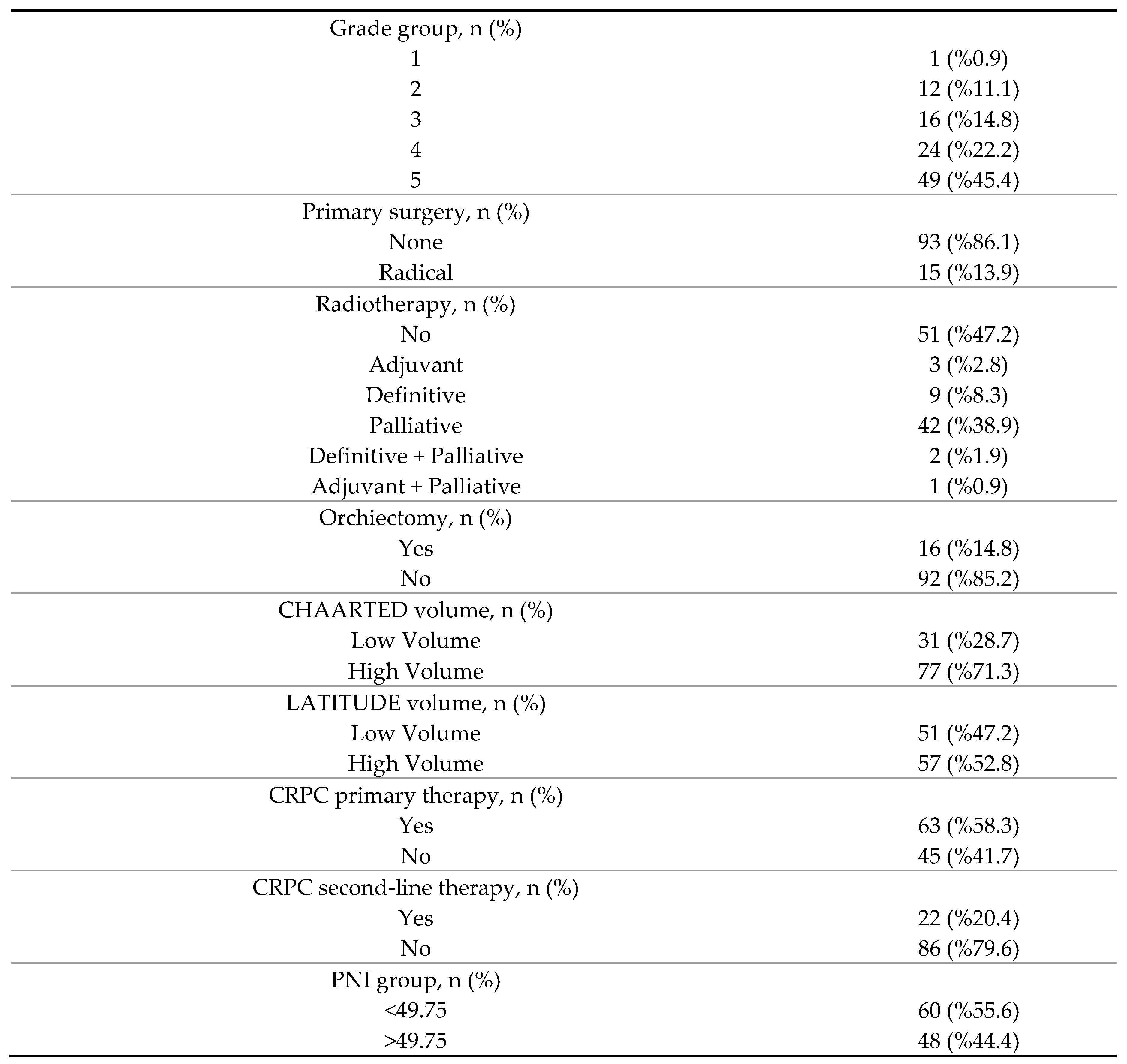
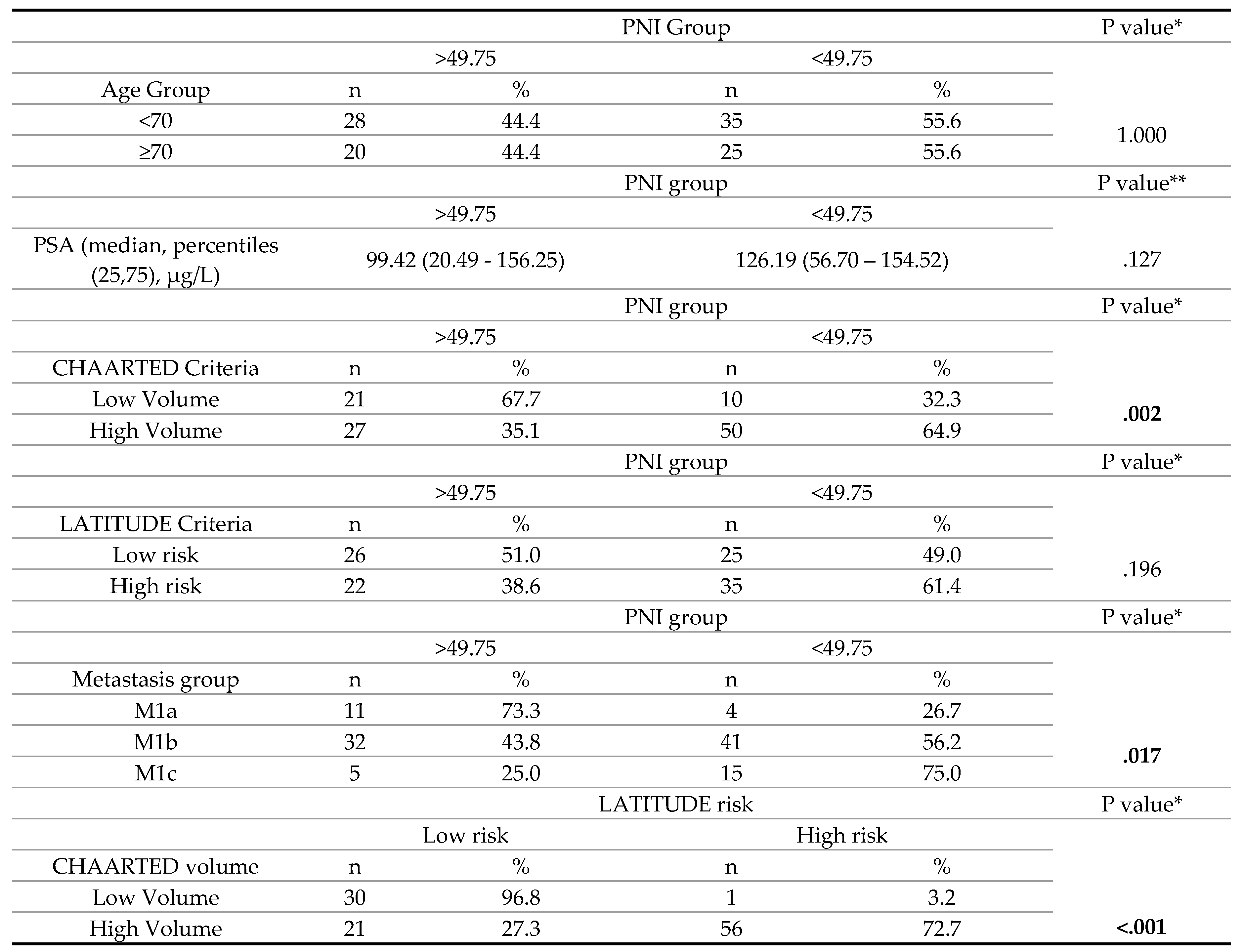
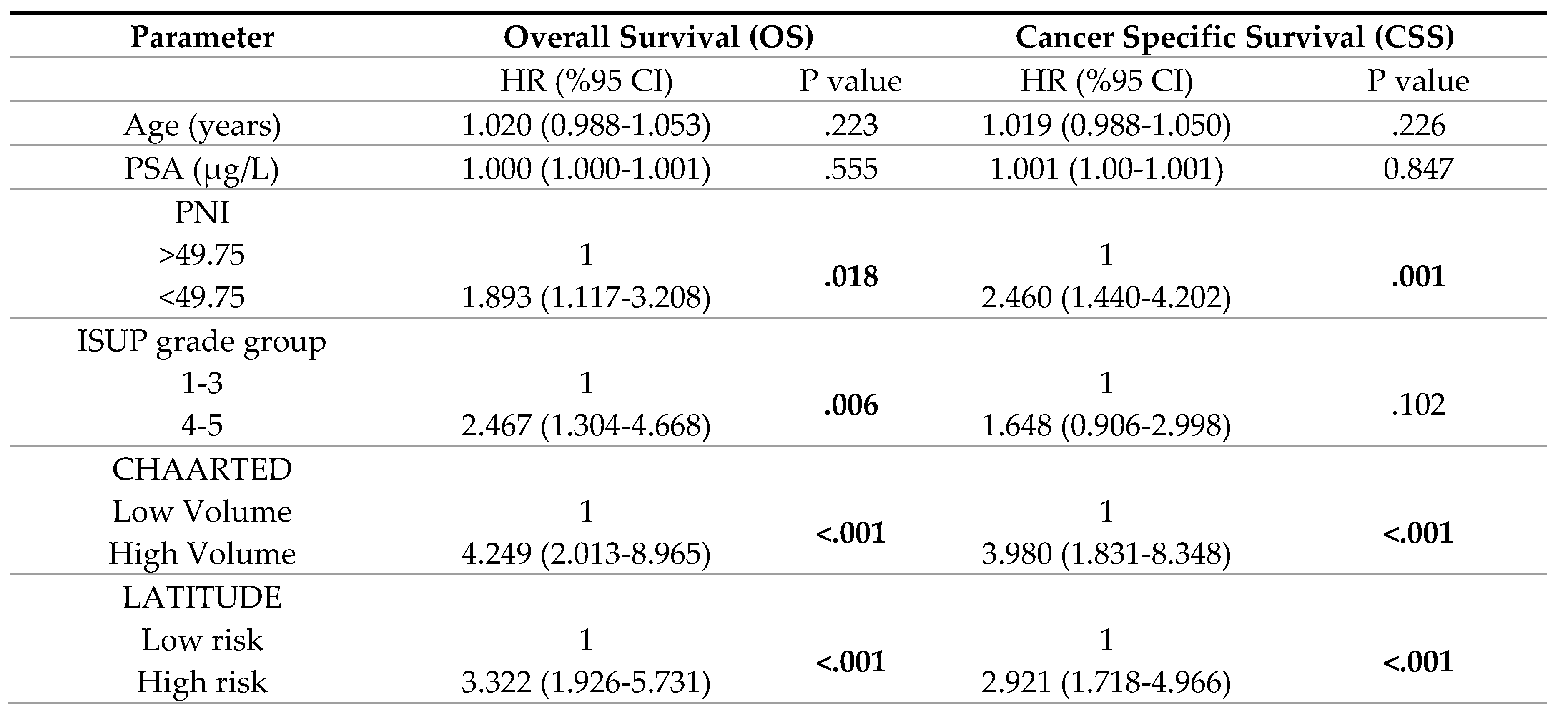
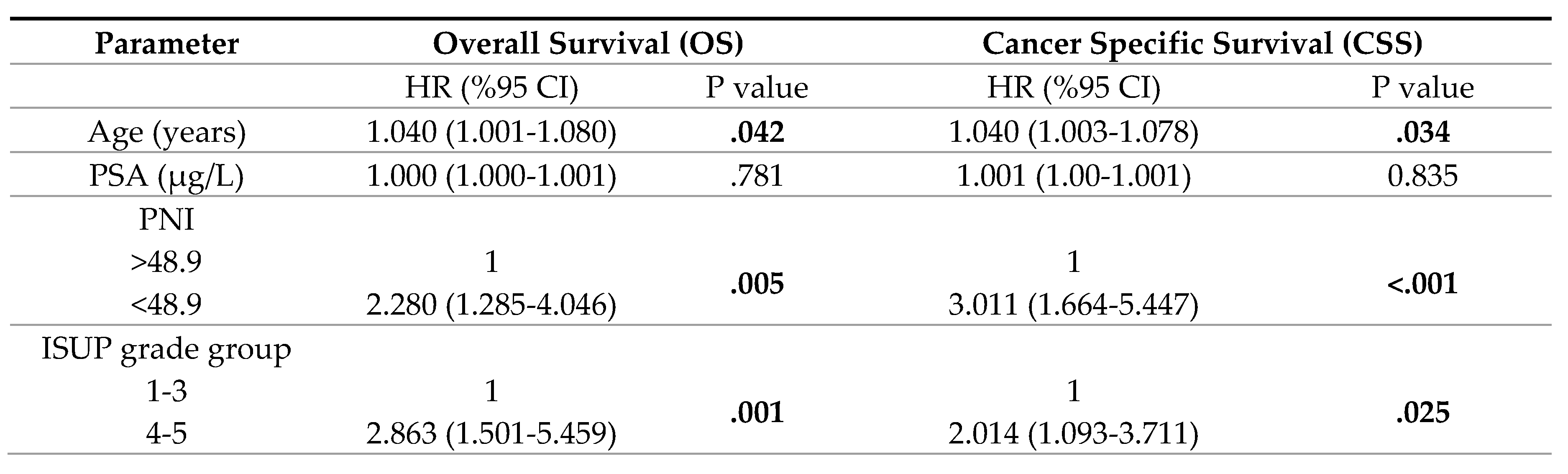
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data availability statement
Conflicts of Interest
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-249.
- Shore ND, Antonarakis ES, Cookson MS, et al. Optimizing the role of androgen deprivation therapy in advanced prostate cancer: Challenges beyond the guidelines. Prostate 2020;80:527-544.
- Ferro M, Lucarelli G, Crocetto F, et al. First-line systemic therapy for metastatic castration-sensitive prostate cancer: An updated systematic review with novel findings. Crit Rev Oncol Hematol 2021;157:103198.
- Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur Urol 2021;79:263-282.
- Aly M, Leval A, Schain F, et al. Survival in patients diagnosed with castration-resistant prostate cancer: a population-based observational study in Sweden. Scand J Urol 2020;54:115-121.
- Francini E, Gray KP, Xie W, et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate 2018;78:889-895.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015;373:737-746.
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019;20:686-700.
- Semiz HS, Keskinkılıç M, Ellez HI, Arayıcı ME, Karaoglu A. Approach to the Therapy of Metastatic Castration-Sensitive Prostate Carcinoma: A Single Center Experience. JBACHS 2022;6:296-304.
- Tan CS, Read JA, Phan VH, Beale PJ, Peat JK, Clarke SJ. The relationship between nutritional status, inflammatory markers and survival in patients with advanced cancer: a prospective cohort study. Support Care Cancer 2015;23:385-91.
- Yapar Taskoylu B, Avci E, Gokcen Demiray A, et al. Relationship between neutrophil/lymphocyte, platelet/lymphocyte, CRP/Albumin ratio and survival in ovarian cancer. Pam Med J 2021;14:666-674.
- Shu W, Tao W, Chunyan H, et al. Preoperative nutritional evaluation of prostate cancer patients undergoing laparoscopic radical prostatectomy. PLoS One 2022;17:e0262630.
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon GekaGakkaishi Zasshi 1984;85:1001–5.
- Yan L, Nakamura T, Casadei-Gardini A, Bruixola G, Huang YL, Hu ZD. Long-term and short-term prognostic value of the prognostic nutritional index in cancer: a narrative review. Ann Transl Med 2021;9:1630.
- Fan L, Wang X, Chi C, et al. Prognostic nutritional index predicts initial response to treatment and prognosis in metastatic castration-resistant prostate cancer patients treated with abiraterone. Prostate 2017;77:1233-1241.
- Küçükarda A, Gökyer A, Gökmen İ, et al. Prognostic nutritional index is an independent prognostic factor for therapy response, survival and drug choice in metastatic castration-resistant prostate cancer treated with abiraterone acetate or enzalutamide [published online ahead of print, 2022 March 4]. Actas Urol Esp (Engl Ed) 2022;S:2173-5786(21)00158-X.
- Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022;399:1695-1707.
- Velho PI, Bastos DA, Antonarakis ES. New approaches to targeting the androgen receptor pathway in prostate cancer. Clin Adv Hematol Oncol 2021;19:228-240.
- Li B, Lu Z, Wang S, et al. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer 2020;20:361.
- Kobayashi H, Shiota M, Sato N, et al. Differential prognostic impact of complete blood count-related parameters by prior use of novel androgen receptor pathway inhibitors in docetaxel-treated castration-resistant prostate cancer patients. Anticancer Drugs 2022;33:e541-e547.
- Yalav O, Topal U, Unal AG, Eray IC. Prognostic significance of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients undergoing curative resection for colorectal cancer. Ann Ital Chir 2021;92:283-292.
- Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target 2021;6:263.
- Kawahara T, Yoneyama S, Ohno Y, et al. Prognostic Value of the LATITUDE and CHAARTED Risk Criteria for Predicting the Survival of Men with Bone Metastatic Hormone-Naïve Prostate Cancer Treated with Combined Androgen Blockade Therapy: Real-World Data from a Japanese Multi-Institutional Study. Biomed Res Int 2020;2020:7804932.
- de Bono JS, Smith MR, Saad F, et al. Subsequent Chemotherapy and Treatment Patterns After Abiraterone Acetate in Patients with Metastatic Castration-resistant Prostate Cancer: Post Hoc Analysis of COU-AA-302. Eur Urol 2017;71:656-664.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





