Submitted:
19 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction:
2. Material and Methods
2.1. Protocol and registration:
2.2. Evidence Acquisition:
- Population: Male patients, prostatectomy for prostate cancer,
- Intervention: Pharmacological/ Combined Prophylaxis (PP) for VTE
- Comparator/Control: No Prophylaxis or Mechanical Prophylaxis for VTE
- Population: Prostatectomy for non-prostate cancer or part of other surgery like cystoprostatectomy.
- Intervention: If the interventions are ill-defined or structural methods are inadequate.
- Comparator/ Control: Studies that lacked proper grouping into control, and intervention.
- Study Design: Studies that did not fulfill the above criterion and lacked any defined outcomes.
2.3. Outcome measures:
-
Primary Outcomes- VTE occurrence with
- Overall Incidence of VTE in post-RP patients
- Surgical approach. (Open, Minimally Invasive)
- Pelvic Lymph node dissection (PLND)
- Prophylaxis (No prophylaxis, Mechanical only, Pharmacological only, Combined).
- Secondary Outcomes-
2.4. Search Methods:
2.5. Study Selection:
2.6. Data Extraction:
2.7. Quality assessment:
2.8. Statistical analysis:
- a.
- Statistical evaluation of overall VTE occurrence
- b.
- Statistical evaluation of VTE occurrence depending on the type of surgical procedure (Open/ Minimally Invasive Surgery (MIS)) and whether PLND was performed or not
- MIS procedures vs. Open procedures
- Procedures using PLND vs. procedures without PLND
- c.
- Statistical Evaluation of VTE occurrence depending on the method of prophylaxis used (mechanical or combined).
3. Results
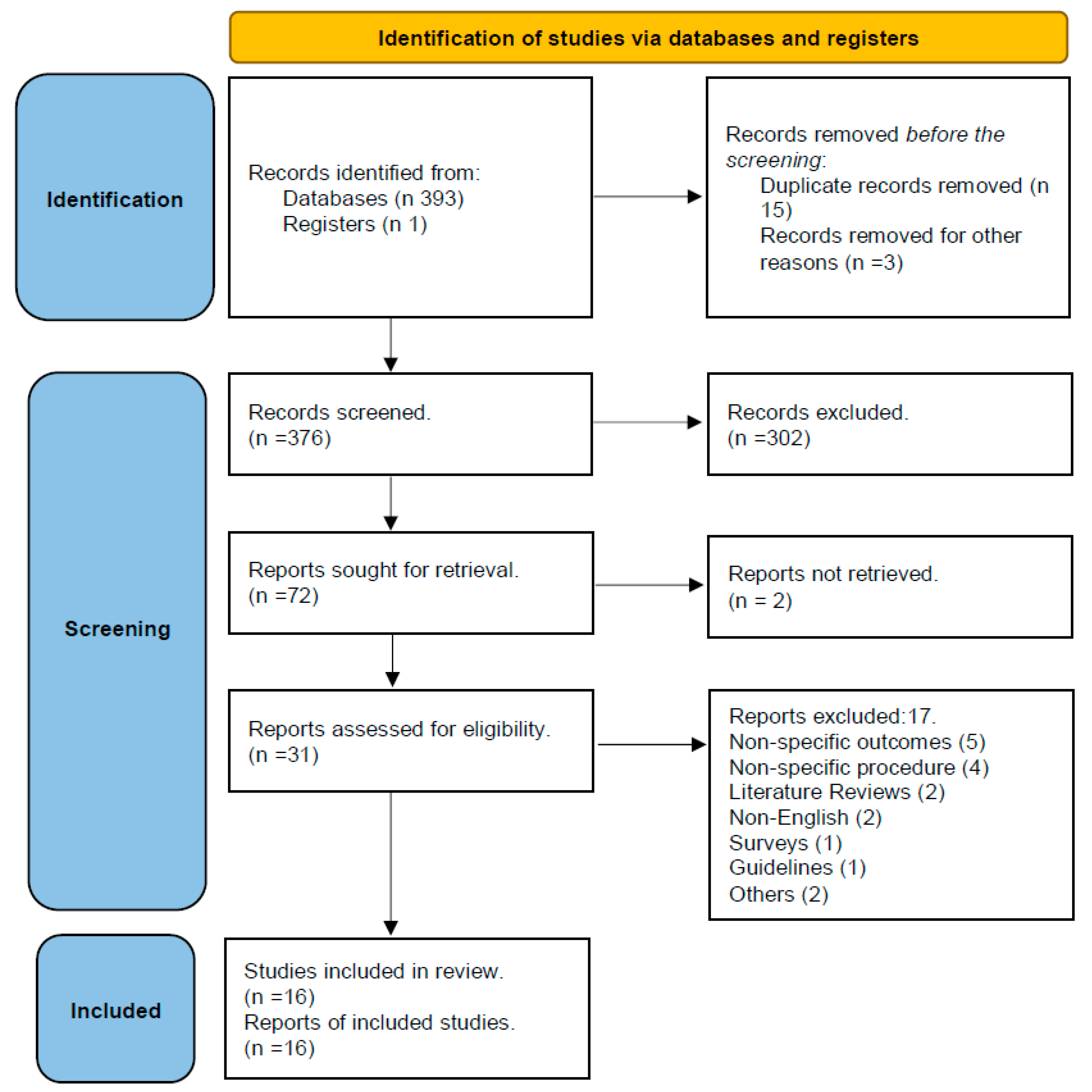
3.1. Study selection results:
3.2. Quality assessment results:
3.3. Study characteristics:
3.4. Clinic-pathological results:
3.5. Demographics and VTE risk factors
3.6. Surgical Procedure results:
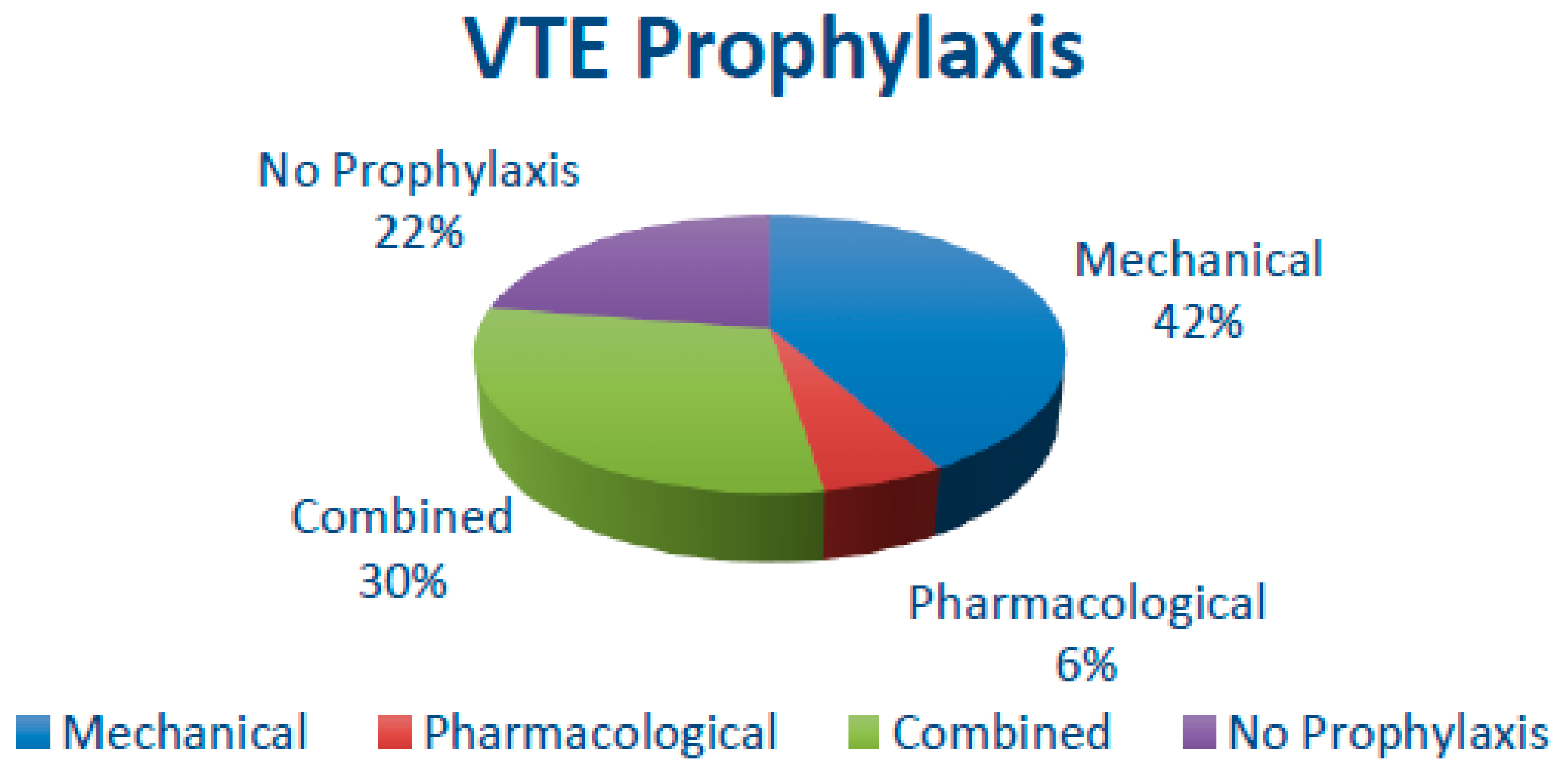
3.7. Thromboprophylaxis and VTE episodes
3.8. Surgical approaches and VTE episodes
3.9. Duration and timing of Thromboprophylaxis
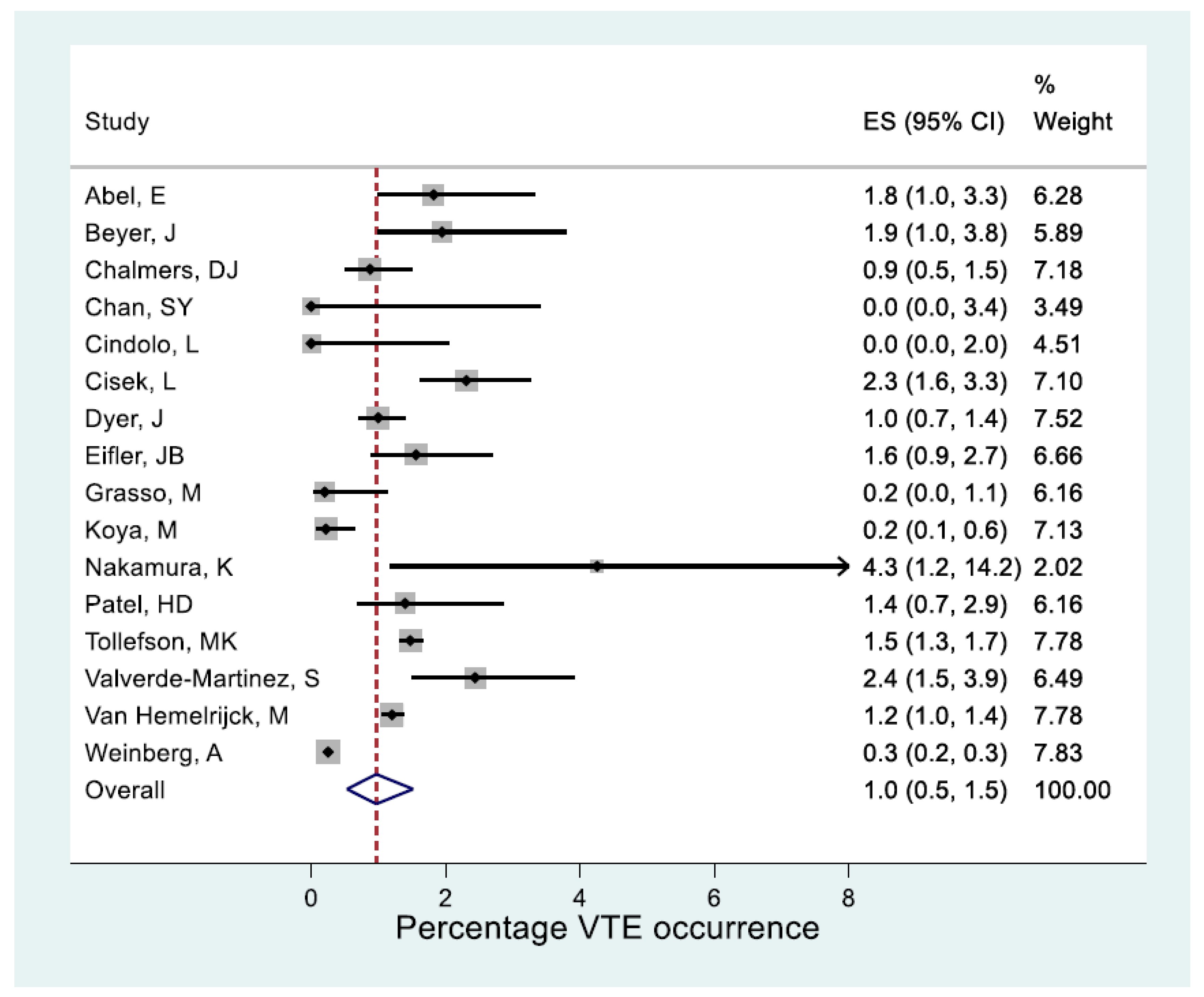
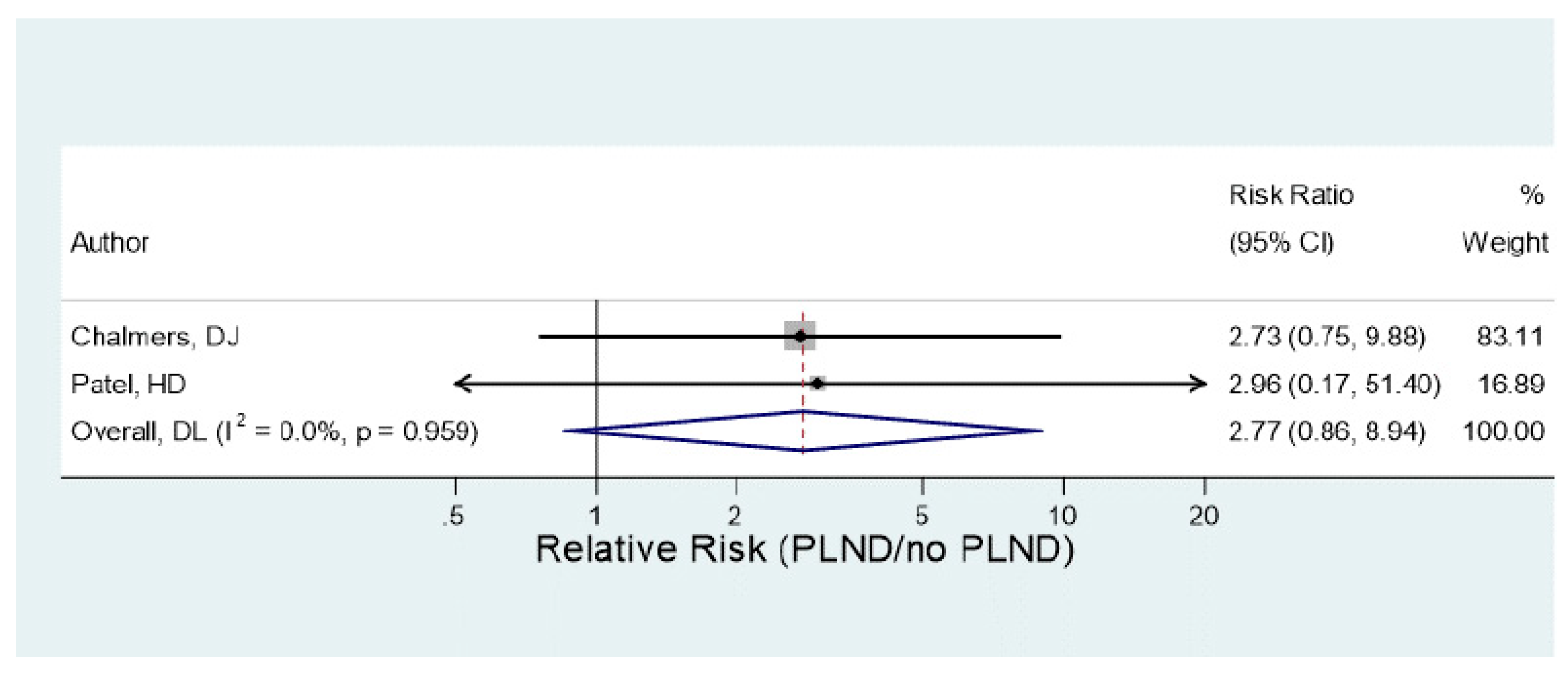
3.10. Statistical Results
- a.
- Statistical outcome of overall VTE occurrence
- b.
- Statistical outcome of VTE occurrence depending on the type of surgical procedure (Open/MIS) and whether PLND was performed or not.
- c.
- Statistical outcome of VTE occurrence depending on the method of prophylaxis used (mechanical or combined).
4. Discussion
5. Conclusion
Author Contributions
Acknowledgments
References
- Guyatt, G. H.; Eikelboom, J. W.; Gould, M. K.; Garcia, D. A.; Crowther, M.; Murad, M. H.; Kahn, S. R.; Falck-Ytter, Y.; Francis, C. W.; Lansberg, M. G.; Akl, E. A.; Hirsh, J. Approach to Outcome Measurement in the Prevention of Thrombosis in Surgical and Medical Patients. Chest 2012, 141, e185Se194S. [Google Scholar] [CrossRef] [PubMed]
- Kibel, A. S.; Loughlin, K. R. Pathogenesis and Prophylaxis of Postoperative Thromboembolic Disease in Urological Pelvic Surgery. The Journal of Urology 1995, 1763–1774. [Google Scholar] [CrossRef]
- Geerts, W. H.; Heit, J. A.; Clagett, G. P.; Pineo, G. F.; Colwell, C. W.; Anderson, F. A.; Wheeler, H. B. Prevention of Venous Thromboembolism. Chest 2001, 119. [Google Scholar] [CrossRef] [PubMed]
- Geerts, W. H.; Pineo, G. F.; Heit, J. A.; Bergqvist, D.; Lassen, M. R.; Colwell, C. W.; Ray, J. G. Prevention of Venous Thromboembolism. Chest 2004, 126. [Google Scholar] [CrossRef]
- Zhan, C. Excess Length of Stay, Charges, and Mortality Attributable to Medical Injuries during Hospitalization. JAMA 2003, 290, 1868. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, G.; Léandri, P.; Gautier, J. R.; Quintens, H.; Gabay-Torbiero, L.; Tap, G. Radical Retropubic Prostatectomy: Complications and Quality of Life (429 Cases, 1983-1989). European Urology 1991, 19, 186–191. [Google Scholar] [CrossRef]
- Brenner, D. W.; Fogle, M. A.; Schellhammer, P. F. Venous Thromboembolism. Journal of Urology 1989, 142, 1403–1411. [Google Scholar] [CrossRef]
- De Martino, R. R.; Goodney, P. P.; Spangler, E. L.; Wallaert, J. B.; Corriere, M. A.; Rzucidlo, E. M.; Walsh, D. B.; Stone, D. H. Variation in Thromboembolic Complications among Patients Undergoing Commonly Performed Cancer Operations. Journal of Vascular Surgery 2012, 55, 1035–1040. [Google Scholar] [CrossRef]
- Beyer, J.; Wessela, S.; Hakenberg, O.; Wirth, M.; Schellong, S. Incidence and Disease Pattern of Venous Thrombembolism after Radical Prostatectomy. Blood 2005, 106, 1627–1627. [Google Scholar] [CrossRef]
- O’Donnell, M.; Weitz, J. I. Thromboprophylaxis in Surgical Patients. Canadian journal of surgery. Journal canadien de chirurgie 2003, 46, 129–135. [Google Scholar]
- Pridgeon, S.; Allchorne, P.; Turner, B.; Peters, J.; Green, J. Venous Thromboembolism (VTE) Prophylaxis and Urological Pelvic Cancer Surgery: A UK National Audit. BJU International 2014, 115, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tikkinen, K. A. O.; Craigie, S.; Agarwal, A.; Violette, P. D.; Novara, G.; Cartwright, R.; Naspro, R.; Siemieniuk, R. A. C.; Ali, B.; Eryuzlu, L.; Geraci, J.; Winkup, J.; Yoo, D.; Gould, M. K.; Sandset, P. M.; Guyatt, G. H. Procedure-Specific Risks of Thrombosis and Bleeding in Urological Cancer Surgery: Systematic Review and Meta-Analysis. European Urology 2018, 73, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Violette, P. D.; Cartwright, R.; Briel, M.; Tikkinen, K. A. O.; Guyatt, G. H. Guideline of Guidelines: Thromboprophylaxis for Urological Surgery. BJU International 2016, 118, 351–358. [Google Scholar] [CrossRef]
- Weinberg, A.; Wright, J.; Deibert, C.; Lu, Y.-S.; Hershman, D.; Neugut, A.; Spencer, B. Nationwide Practice Patterns for the Use of Venous Thromboembolism Prophylaxis among Men Undergoing Radical Prostatectomy. World Journal of Urology 2013, 32, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Page, M. J.; McKenzie, J. E.; Bossuyt, P. M.; Boutron, I.; Hoffmann, T. C.; Mulrow, C. D.; Shamseer, L.; Tetzlaff, J. M.; Akl, E. A.; Brennan, S. E.; Chou, R.; Glanville, J.; Grimshaw, J. M.; Hróbjartsson, A.; Lalu, M. M.; Li, T.; Loder, E. W.; Mayo-Wilson, E.; McDonald, S.; McGuinness, L. A. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. British Medical Journal 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Patel, H. D.; Faisal, F. A.; Trock, B. J.; Joice, G. A.; Schwen, Z. R.; Pierorazio, P. M.; Johnson, M. H.; Bivalacqua, T. J.; Han, M.; Gorin, M. A.; Carter, H. B.; Partin, A. W.; Pavlovich, C. P.; Allaf, M. E. Effect of Pharmacologic Prophylaxis on Venous Thromboembolism after Radical Prostatectomy: The PREVENTER Randomized Clinical Trial. European Urology 2020, 78, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Martinez, S.; Gonzalez-Rayo, L.-A.; Padilla-Fernandez, B.; Pereira-Bruno, J.; Coelho, H.; Montesino-Semper, M.; Müller-Arteaga, C.; Alvarez-Ossorio-Fernandez, J.-L.; Migliorini, F.; Garcia-Cenador, M.-B.; Lorenzo-Gomez, M.-F. Profilaxis Farmacológica de La Tromboembolia Venosa En La Prostatectomía Radical. Medicina Clínica 2020, 154, 113–118. [Google Scholar] [CrossRef]
- Weinberg, A.; Wright, J.; Deibert, C.; Lu, Y.-S.; Hershman, D.; Neugut, A.; Spencer, B. Nationwide Practice Patterns for the Use of Venous Thromboembolism Prophylaxis among Men Undergoing Radical Prostatectomy. World Journal of Urology 2013, 32, 1313–1321. [Google Scholar] [CrossRef]
- Tollefson, M. K.; Karnes, R. J.; Rangel, L.; Carlson, R.; Boorjian, S. A. Blood Type, Lymphadenectomy and Blood Transfusion Predict Venous Thromboembolic Events Following Radical Prostatectomy with Pelvic Lymphadenectomy. Journal of Urology 2014, 191, 646–651. [Google Scholar] [CrossRef]
- Chan, S. Y. S.; Leung, V. F. Y.; Yee, C. H.; Chan, E. S. Y.; Hou, S. S. M.; Chu, W.; Ng, C. F. Incidence of Postoperative Deep Vein Thrombosis after Robotic-Assisted Laparoscopic Prostatectomy: A Prospective Study in Chinese Patients. International Urology and Nephrology 2014, 46, 2139–2142. [Google Scholar] [CrossRef]
- Chalmers, D. J.; Scarpato, K. R.; Staff, I.; Champagne, A.; Tortora, J.; Wagner, J. R.; Kesler, S. S. Does Heparin Prophylaxis Reduce the Risk of Venous Thromboembolism in Patients Undergoing Robot-Assisted Prostatectomy? Journal of Endourology 2013, 27, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Abel, E. J.; Wong, K.; Sado, M.; Leverson, G. E.; Patel, S. R.; Downs, T. M.; Jarrard, D. F. Surgical Operative Time Increases the Risk of Deep Venous Thrombosis and Pulmonary Embolism in Robotic Prostatectomy. JSLS : Journal of the Society of Laparoendoscopic Surgeons 2014, 18, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.; Wyke, S.; Lynch, C. Hospital Episode Statistics Data Analysis of Postoperative Venous Thromboembolus in Patients Undergoing Urological Surgery: A Review of 126,891 Cases. The Annals of The Royal College of Surgeons of England 2013, 95, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Bill-Axelson, A.; Carlsson, S.; Akre, O.; Stattin, P.; Adolfsson, J. Thromboembolic Events Following Surgery for Prostate Cancer. European Urology 2013, 63, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Eifler, J. B.; Levinson, A. W.; Hyndman, M. E.; Trock, B. J.; Pavlovich, C. P. Pelvic Lymph Node Dissection Is Associated with Symptomatic Venous Thromboembolism Risk during Laparoscopic Radical Prostatectomy. Journal of Urology 2011, 185, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- BEYER, J.; WESSELA, S.; HAKENBERG, O. W.; KUHLISCH, E.; HALBRITTER, K.; FROEHNER, M.; WIRTH, M. P.; SCHELLONG, S. M. Incidence, Risk Profile and Morphological Pattern of Venous Thromboembolism after Prostate Cancer Surgery. Journal of Thrombosis and Haemostasis 2009, 7, 597–604. [Google Scholar] [CrossRef]
- Grasso, M.; Confalonieri, S.; Blanco, S.; Grasso, A.; Angelo, S. Preoperative Blood Donation Program and Postoperative Low Molecular Weight Heparine (LMWH) Prophylaxis in Patients Undergoing Radical Prostatectomy. Archivos Españoles de Urología (Ed. impresa) 2009, 62. [Google Scholar] [CrossRef] [PubMed]
- Cindolo, L.; Salzano, L.; Mirone, V.; Imbimbo, C.; Longo, N.; Kakkos, S. K.; Reddy, D. J. Thromboprophylaxis in Radical Retropubic Prostatectomy: Efficacy and Patient Compliance of a Dual Modality. Urologia Internationalis 2009, 83, 12–18. [Google Scholar] [CrossRef]
- Nakamura, K.; Kasraeian, A.; Yacoub, S.; Pendleton, J.; Anai, S.; Rosser, C. J. The Use of Enoxaparin to Prevent Venous Thromboembolism in Patients Undergoing Radical Retropubic Prostatectomy: Feasibility and Utility. International braz j urol 2007, 33, 347–354. [Google Scholar] [CrossRef]
- Koya, M. P.; Manoharan, M.; Kim, S. S.; Soloway, M. S. Venous Thromboembolism in Radical Prostatectomy: Is Heparinoid Prophylaxis Warranted? BJU International 2005, 96, 1019–1021. [Google Scholar] [CrossRef]
- Cisek, L. J.; Walsh, P. C. Thromboembolic Complications Following Radical Retropubic Prostatectomy Influence of External Sequential Pneumatic Compression Devices. Urology 1993, 42, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Salous, A. K.; Reyad, A.; Sweeney, K.; Mavanur, A. A Significant Proportion of Venous Thromboembolism Events in General Surgical Patients Occurs after Discharge: Analysis of the ACS-NSQIP Essentials Database. Perioperative Medicine 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, A.; Piotr Skrudlik; Kowalski, F.; Lipowski, P.; Ostrowska, M.; Adamczyk, P.; Adamowicz, J.; Drewa, T.; Juszczak, K. Current Thromboprophylaxis in Urological Cancer Patients during COVID Pandemic. Central European Journal of Urology 2022. [CrossRef]
- Violette, P. D.; Cartwright, R.; Briel, M.; Tikkinen, K. A. O.; Guyatt, G. H. Guideline of Guidelines: Thromboprophylaxis for Urological Surgery. BJU International 2016, 118, 351–358. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam 2022. ISBN 978-94-92671-16-5.
- NICE. Recommendations | Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism | Guidance | NICE. Nice.org.uk. https://www.nice.org.uk/guidance/ng89/chapter/Recommendations#interventions-for-people-having-abdominal-thoracic-or-head-and-neck-surgery.
- Forrest, J. B.; Clemens, J. Q.; Finamore, P.; Leveillee, R.; Lippert, M.; Pisters, L.; Touijer, K.; Whitmore, K. AUA Best Practice Statement for the Prevention of Deep Vein Thrombosis in Patients Undergoing Urologic Surgery. Journal of Urology 2009, 181, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Barber, E. L.; Gehrig, P. A.; Clarke-Pearson, D. L. Venous Thromboembolism in Minimally Invasive Compared with Open Hysterectomy for Endometrial Cancer. Obstetrics & Gynecology 2016, 128, 121–126. [Google Scholar] [CrossRef]
- Eifler, J. B.; Levinson, A. W.; Hyndman, M. E.; Trock, B. J.; Pavlovich, C. P. Pelvic Lymph Node Dissection Is Associated with Symptomatic Venous Thromboembolism Risk during Laparoscopic Radical Prostatectomy. Journal of Urology 2011, 185, 1661–1666. [Google Scholar] [CrossRef]
- Muntz, J. Duration of Deep Vein Thrombosis Prophylaxis in the Surgical Patient and Its Relation to Quality Issues. The American Journal of Surgery 2010, 200, 413–421. [Google Scholar] [CrossRef]

| Author | Number of awarded stars in each domain | ||
|---|---|---|---|
| Selection | Comparability | Outcome | |
| (1) Patel, H. D [17] | **** | ** | ** |
| (2) Valverde-Martinez, S. [18] | *** | * | ** |
| (3) Weinberg, A. [19] | *** | ** | |
| (4) Tollefson, M. K. [20] | *** | ** | |
| (5) Chan, S. [21] | ** | ** | |
| (6) Chalmers, D. J. [22] | *** | ** | |
| (7) Abel ,E.[23] | *** | ** | |
| (8) Dyer, J [24] | ** | * | |
| (9) Van Hemelrijck, M.[25] | ** | ** | |
| (10) Eifler, J. B .[26] | ** | ** | |
| (11) Beyer, J. [27] | *** | ** | |
| (12) Grasso, M. [28] | ** | * | |
| (13) Cindolo, L.[29] | ** | * | |
| (14) Nakamura, K. [30] | ** | * | |
| (15)Koya, M. [31] | *** | * | |
| (16) Cisek, L.[32] | ** | * | |
| Study | Study Type/ Time |
Study Characteristics | Conclusion |
|---|---|---|---|
|
(1) Patel, H. D. [17] |
RCT (2017-18) |
|
|
|
(2) Valverde-Martinez, S. [18] |
Retrospective (2013-14) |
|
|
|
(3) Weinberg, A. [19] |
Observational (2000-10) |
|
|
|
(4) Tollefson, M. K [20] |
Retrospective (1987-2010) |
|
|
|
(5) Chan, S.Y. [21] |
Prospective (2007-2010) |
|
|
|
(6) Chalmers, D. J. [22] |
Prospective (2007-2011) |
|
|
|
(7) Abel, .E [23] |
Retrospective (2007-2011) |
|
|
|
(8) Dyer, J. [24] |
Retrospective (2009-2010) |
|
|
|
(9) Van Hemelrijck, M. [25] |
Retrospective (2002-2010) |
|
|
|
(10) Eifler, J. B. [26] |
Retrospective (2001-2009) |
|
|
|
(11) Beyer, J. [27] |
Prospective (2001-2003) |
|
|
|
(12) Grasso, M. [28] |
Retrospective (1999-2006) |
|
|
|
(13) Cindolo, L. [29] |
Prospective (2004-2006) |
|
|
|
(14) Nakamura, K . [30] |
Prospective (2003-2005) |
|
|
|
(15) Koya, M . [31] |
Prospective (1992-2004) |
|
|
|
(16) Cisek, L . [32] |
Prospective (1982-1993) |
|
|
| BMI-Body Mass Index; DVT-Deep Vein Thrombosis; HES- Hospital Episode Statistics; LMHW-Low Molecular Weight heparin; IPC- Intermittent pneumatic compression; ORP-Open Radical Prostatectomy; PCa- Prostate Cancer; PCS- Pneumatic Compression Stocking; PLND- Pelvic Lymph Node dissection; PP-Pharmacological Prophylaxis; RARP-Robot-assisted radical prostatectomy; SCD- Sequential Compression Device; VTE-Venous thromboembolism; | |||
| Study | Total patients | Mean Age | Mean BMI kg/m2 |
Family history (%) |
VTE Back- ground (%) |
Smoking | Overall risk assessment (in %) |
Caprini Score | Remarks in relation to VTE risk factors/ Scores | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Int | High | |||||||||
| (1) Patel, H. D. [17] | 501 | 62 | 27.4 | - | - | - | - | - | - | 6 | The study concluded that most patients with prostate cancer undergoing RP are relatively healthy, our study suggests that PP may be deferred based on surgeon preference up to a Caprini score of 7; PP may be justified for higher-risk patients with scores of 8. |
| (2) Valverde-Martinez, S. [18] | 610 | 64.1 | 28.03 | - | - | - | 94.8 | 4.1 | 1.1 | - | This study concluded that with respect to the PP used in different thromboembolic risk groups, there were differences in the low-risk group but not in the intermediate and high-risk groups; this was probably due to the fact that this group covered 95% of the cases in the series |
| (3) Weinberg, A. [19] | 94,709 | - | - | - | - | - | - | - | - | - | - |
| (4) Tollefson, M. K [20] | 18,472 | 63 | 27.7 | - | - | - | - | - | - | - | They concluded that patients with VTE were significantly older than those not diagnosed with VTE (median age 65 vs 63 years, p <0.001). |
| (5) Chan, S.Y [21] | 109 | 65.7 | <23 (33) >23 (67) |
- | - | 18.1 | - | - | - | - | This study concluded that there was no difference in the incidence of DVT between patients with a history of smoking or diabetes or a high body mass (BMI) index and those without. |
| (6) Chalmers, D. J . [22] | 1486 | 59.9 | 28.1 | - | - | - | - | - | - | - | In this study, BMI was not found to be associated with VTE. |
| (7) Abel, E. [23] | 549 | 59.8 | - | - | 1.6 | 43.8 | - | A 5-point increase in body mass index was associated with an increased risk of VTEs (odds ratios of 2.0). | |||
|
(8)Dyer, J. [24] |
3,213 | 72.5 | - | - | - | - | - | - | - | - | - |
| (9) Van Hemelrijck, M [25] | 16,304 | - | - | - | 0.6 | - | - | - | - | - | A previous history of VTE is a risk factor in patients undergoing RP. |
| (10)Eifler, J. B [26] | 773 | 57.8 | 27.3 | - | - | - | - | - | - | - | A high incidence of VTE was found in patients with BMI in the top quartile who concomitantly underwent PLND. |
| (11) Beyer, J [27] | 411 | 65.0 | 27.0 | 4.0 | 4.8 |
- | - | - | - | A statistically higher risk was found in patients with a personal history of VTE, however, family history was not found with increased risk. | |
| (12) Grasso, M. [28] | 500 | 65.0 | - | - | - | - | - | - | - | - | - |
| (13) Cindolo, L.[29] | 184 | 69.0 | >25 (30%) | - | - | 28 | - | - | - | - | - |
| (14) Nakamura, K . [30] | 47 | 64.0 | - | - | - | - | - | - | - | - | - |
|
(15)Koya, M . [31] |
1364 | 60.8 | - | - | - | - | - | - | - | - | - |
|
(16) Cisek, L. [32] |
1300 | - | - | - | - | - | - | - | - | - | - |
| Study | Total Procedures | Open | Laparoscopic | Robotic | Unknown | PLND (%) |
|---|---|---|---|---|---|---|
| (1) Patel, H. D [17] | 501 | 124 | - | 377 | - | 83.5 (419) |
| (2) Valverde-Martinez, S. [18] | 610 | 268 | 311 | 31 | - | - |
| (3) Weinberg, A. [19] | 94,709 | 68,244 | - | 26,465 | - | - |
| (4) Tollefson, M. K. [20] | 18,472 | 16,374 | - | 2,098 | - | 100 |
| (5) Chan, S. [21] | 109 | - | - | 109 | - | 33.94 (37) |
| (6) Chalmers, D. J. [22] | 1486 | - | - | 1486 | - | 55 |
| (7) Abel ,E.[23] | 549 | - | - | 549 | - | 12.9 (71/549) |
| (8) Dyer, J [24] | 3,213 | - | - | - | 3213 | - |
| (9) Van Hemelrijck, M.[25] | 16304 | 11137 | - | 5167 | - | 21.6 (3258/16304) |
| (10) Eifler, J. B .[26] | 770 | - | 770 | - | - | 60.8 (468/770) |
| (11) Beyer, J. [27] | 411 | 411 | - | - | - | 100 |
| (12) Grasso, M. [28] | 500 | 500 | - | - | - | - |
| (13) Cindolo, L.[29] | 184 | 184 | - | - | - | 100 |
| (14) Nakamura, K. [30] | 47 | 47 | - | - | - | 87 (41/47) |
| (15)Koya, M. [31] | 1373 | 1373 | - | - | - | 67 (920/1373) |
| (16) Cisek, L.[32] | 1300 | 1300 | - | - | - | - |
| Total | 140,541 | 100,088 (71.21%) |
1084 (0.77%) |
36,156 (25.72%) |
3213 (2.28%) |
33.82% (6229/18417) |
| Thromboprophylaxis | VTE Symptomatic Episodes (in %) |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | M | P | C | N | M | P | C | |
| (1) Patel, H. D [17] | - | 250 | - | 251 | - | 2.0 | - | 0.8 |
| (2) Valverde-Martinez, S. [18] | 94 | 25 | 516 | 21 | 2.5 | |||
| (3) Weinberg, A. [19] | 20438 | 35591 | 4945 | 7720 | 0.25 | |||
| (4) Tollefson, M. K. [20] | - | - | - | 18472 | 1.47 | |||
| (5) Chan, S. [21] | - | 109 | - | - | 0.09 | |||
| (6) Chalmers, D. J. [22] | - | 564 | - | 922 | - | 1.0 | - | 0.7 |
| (7) Abel ,E.[23] | - | 540 | - | 9 | 1.8 | |||
| (8) Dyer, J [24] | - | - | - | - | 1.0 | |||
| (9) Van Hemelrijck, M.[25] | - | - | - | - | 1.2 | |||
| (10) Eifler, J. B .[26] | - | 770 | - | - | 1.5 | |||
| (11) Beyer, J. [27] | - | - | - | 411 | 1.9 | |||
| (12) Grasso, M. [28] | - | - | - | 500 | 0.2 | |||
| (13) Cindolo, L.[29] | - | - | - | 184 | 0 | |||
| (14) Nakamura, K. [30] | - | - | - | 47 | 4 | |||
| (15)Koya, M. [31] | - | 1373 | - | - | 0.21 | |||
| (16) Cisek, L.[32] | 784 | 516 | - | - | 2.3 | |||
| Study | VTE Incidence procedure specific |
DVT Incidence (in %) |
PE Incidence (in %) |
PLND (VTE) |
Post Op Bleeding Episodes (in %) |
|||
|---|---|---|---|---|---|---|---|---|
| O | MIS | O | MIS | O | MIS | |||
| (1) Patel, H. D. [17] | 2.4 | 1.1 | - | - | 1.7 | 1.1 | ||
| (2) Valverde-Martinez, S [18] | 2.5 | - | 1.4 | - | - | |||
| (3) Weinberg, A. [19] | 0.3 | 0.2 | - | 0.1 | 0.1 | - | - | |
| (4) Tollefson, M. K. [20] | 1.5 | 1.0 | - | 1.8 | 1.47 | - | ||
| (5) Chan, S.Y [21] | - | 0.9 | - | 0.0 | - | - | ||
| (6) Chalmers, D. J .[22] | - | 0.9 | - | - | 1.2 | - | ||
| (7) Abel, E. [23] | - | 1.8 | - | - | 0.5 | - | - | |
| (8) Dyer, J. [24] | 1.0 | - | - | - | - | |||
| (9) Van Hemelrijck, M. [25] | 1.5 | 0.8 | 0.9 | 0.6 | 0.6 | 0.2 | - | - |
| (10)Eifler, J. B. [26] | - | - | 0 | 1.5 | - | - | ||
| (11) Beyer, J. [27] | 1.9 | - | 0.9 | 0.9 | - | - | ||
| (12) Grasso, M. [28] | 0.2 | - | 0 | 0.2 | - | - | ||
| (13) Cindolo, L. [29] | 0 | - | 0 | 0 | - | - | ||
| (14) Nakamura, K. [30] | 4 | - | 0 | 4 | - | 2.1 | ||
| (15)Koya, M .[31] | 0.21 | - | 0.21 | 0 | - | - | ||
| (16) Cisek, L .[32] | 2.3 | - | 0.45 | 1.3 | - | - | ||
| Outcome | Method | Number | Heterogeneity | Outcome occurrence | |
|---|---|---|---|---|---|
| Studies | p-value | I2 | % (95% CI) | ||
| VTE | All combined | 16 | <0.001 | 97% | 1.0 (0.5, 1.5) |
| Comparison | Number | Heterogeneity | Group difference | ||
|---|---|---|---|---|---|
| Studies | p-value | I2 | RR (95% CI) (*) | P-value | |
| MIS / Open | 5 | 0.55 | 0% | 0.63 (0.52, 0.77) | <0.001 |
| PLND / no PLND | 2 | 0.96 | 0% | 2.79 (0.86, 8.94) | 0.09 |
| Prophylaxis | Number | Heterogeneity | VTE occurrence | Method diff. | |
| method | Studies | p-value | I2 | % (95% CI) | P-value |
| Mechanical | 5 | 0.002 | 76% | 0.7 (0.1, 1.6) | 0.42 |
| Combined | 6 | 0.07 | 51% | 1.0 (0.5, 1.6) | |
| Open Radical Prostatectomy (+/- PLND) | |||
|---|---|---|---|
| Pharmacological# | Low Risk | Suggests | weak, moderate-quality evidence |
| Medium / High Risk | Recommends | strong, moderate- or high-quality evidence | |
| Mechanical* | All patients | Suggested | weak, low-quality evidence |
| Open radical prostatectomy with extended PLND | |||
| Pharmacological# | All patients | Recommends | strong, moderate, or high-quality evidence |
| Mechanical* | All patients | Suggests | weak, low-quality evidence |
| Laparoscopic Radical prostatectomy (Without PLND) | |||
| Pharmacological# | Low Risk | Recommends (Against) | strong, moderate-quality evidence |
| Medium and high risk | Suggests (Against) | weak, moderate- or high-quality evidence | |
| Mechanical* | Low risk | Suggests (Against) | weak, low-quality evidence |
| Medium and high risk | Suggests | weak, low-quality evidence | |
| Laparoscopic Radical prostatectomy (With Standard PLND) | |||
| Pharmacological# | Low Risk | Recommends (Against) | strong, moderate-quality evidence |
| Medium Risk | Suggests (Against) | weak, moderate- or high-quality evidence | |
| High Risk | Recommends | strong, high-quality evidence | |
| Mechanical* | All patients | Suggests | weak, low-quality evidence |
| Laparoscopic Radical prostatectomy (With Extended PLND) | |||
| Pharmacological# | Low Risk | Suggests (Against) | weak, moderate-quality evidence |
| Medium Risk | Suggests | weak, high-quality evidence | |
| High Risk | Recommends | strong, high-quality evidence | |
| Mechanical* | All patients | Suggested | weak, low-quality evidence |
| Robotic Radical prostatectomy (Without PLND) | |||
| Pharmacological# | Low Risk | Recommends (Against) | strong, moderate-quality evidence |
| Medium and High Risk | Suggests (Against) | weak, moderate-quality evidence | |
| Mechanical* | Low Risk | Suggests (Against) | weak, low-quality evidence |
| Medium and High Risk | Suggests | weak, low-quality evidence | |
| Robotic Radical prostatectomy (With Standard PLND) | |||
| Pharmacological# | Low Risk | Recommends (Against) | strong, moderate-quality evidence |
| Medium Risk | Suggests | weak, moderate-quality evidence | |
| High Risk | Suggests | weak, moderate-quality evidence | |
| Mechanical* | All patients | Suggests | weak, low-quality evidence |
| Robotic Radical prostatectomy (With Extended PLND) | |||
| Pharmacological# | Low Risk | Suggests (Against) | weak, moderate-quality evidence |
| Medium Risk | Suggests | weak, moderate-quality evidence | |
| High Risk | Recommends | strong, moderate-quality evidence | |
| Mechanical* | All patients | Suggests | weak, low-quality evidence |
| Pharmacological#- For 4 weeks post-operatively Mechanical*- Until ambulation | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





