Submitted:
18 May 2023
Posted:
19 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Structure characterization of CAL-TsOH
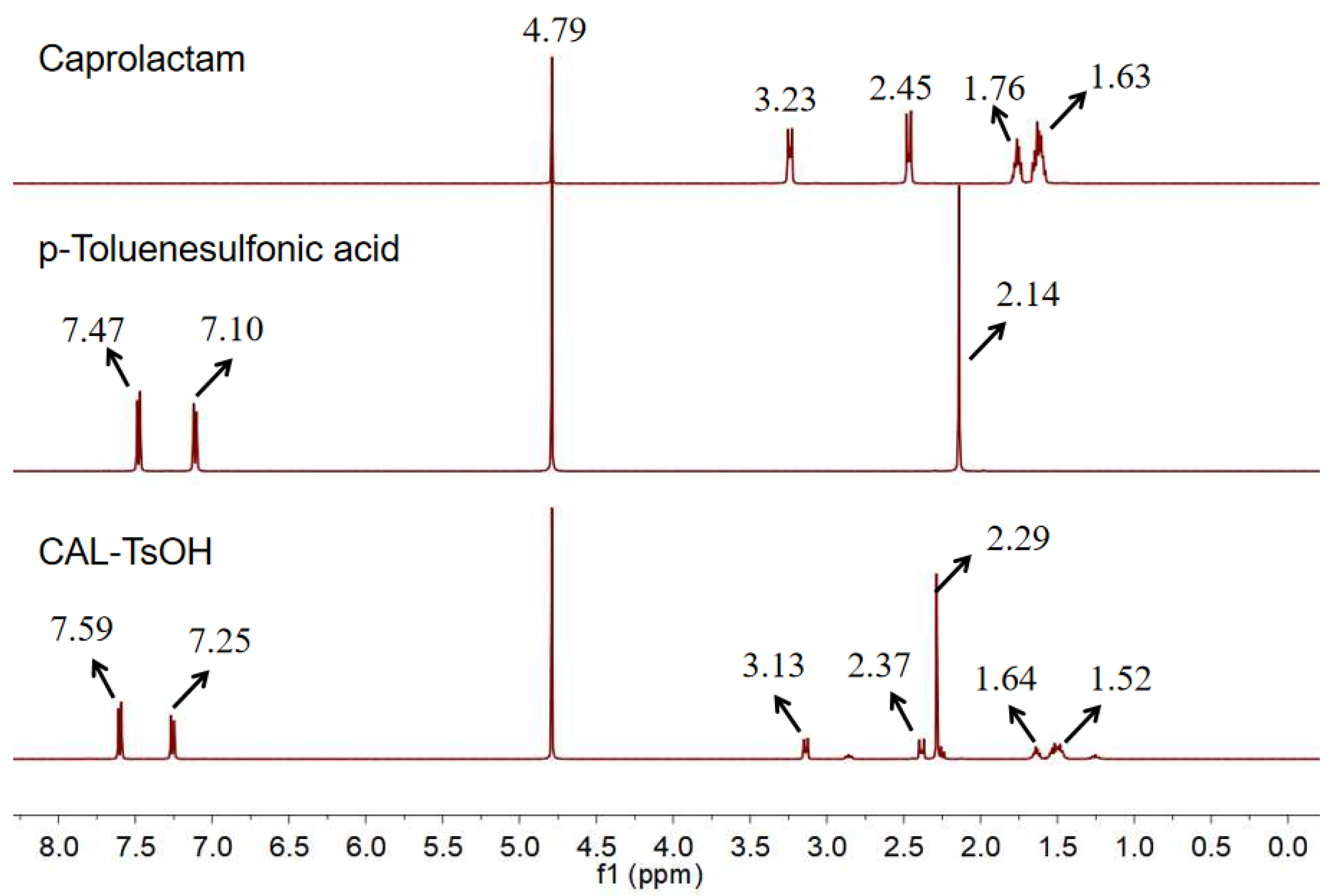
2.1.1. H NMR analysis for CAL-TsOH
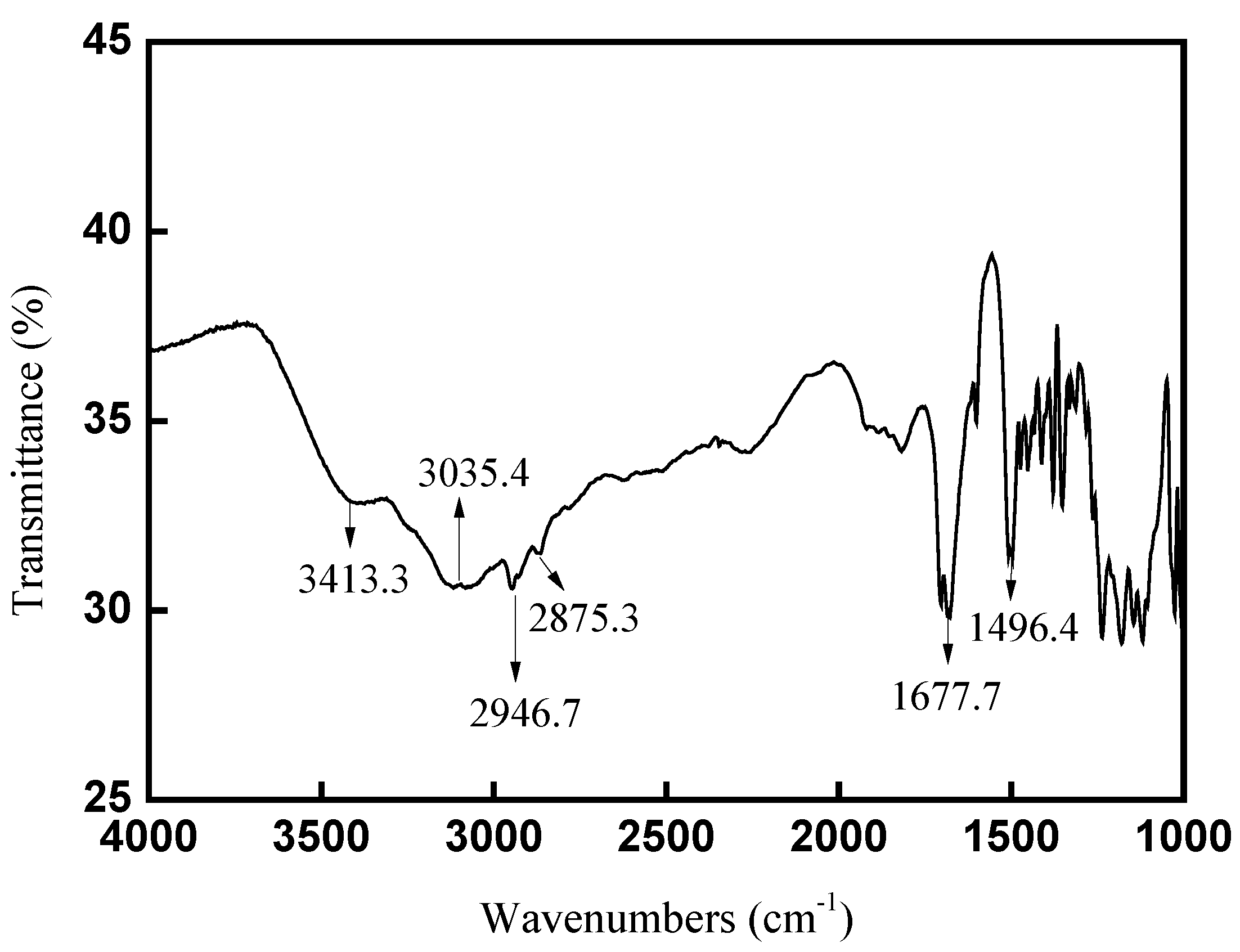
2.1.2. FT-IR spectra analysis for CAL-TsOH
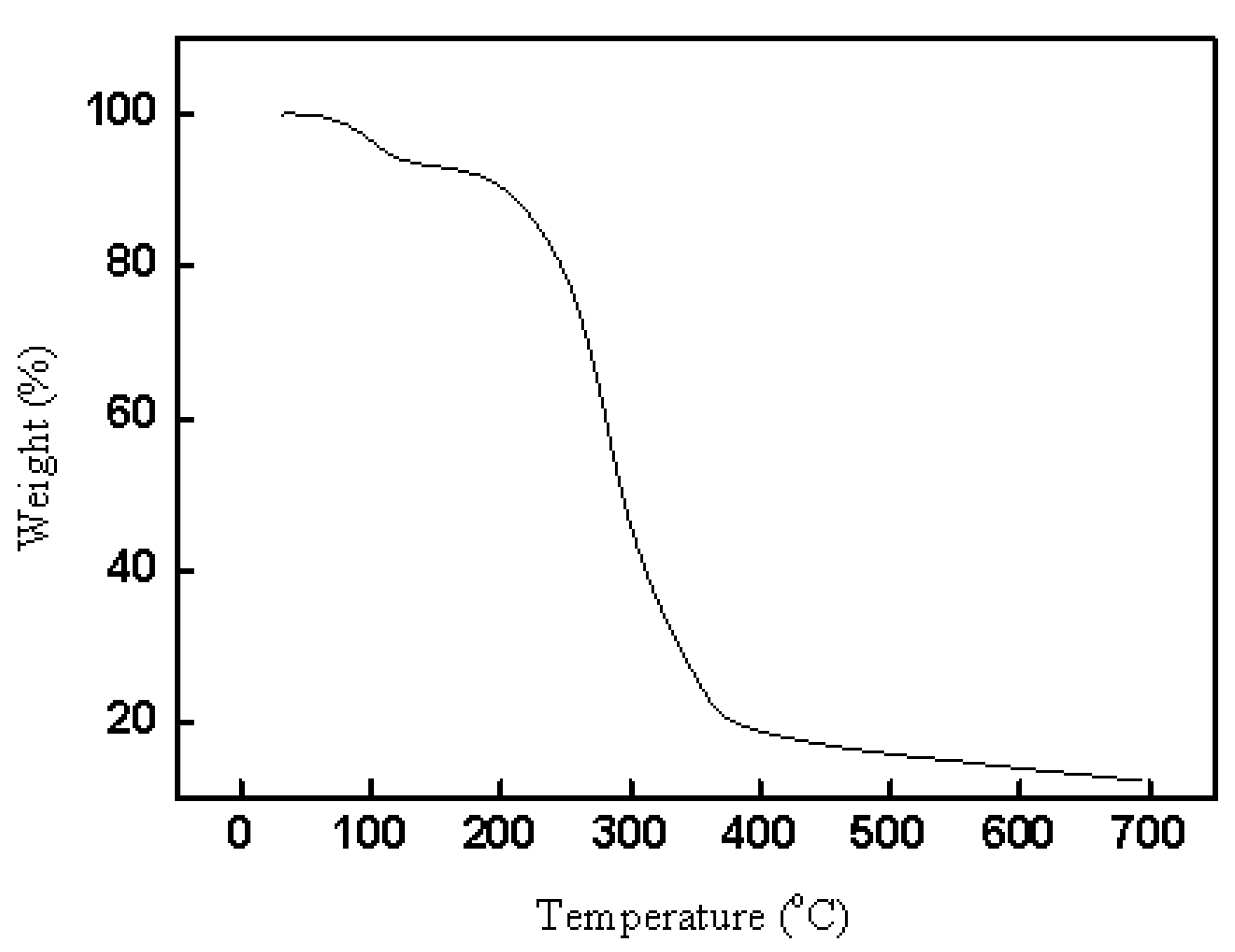
2.1.3. Thermogravimetric analysis for CAL-TsOH
2.2. Optimization of the conditions for the synthesis of 2-TBM
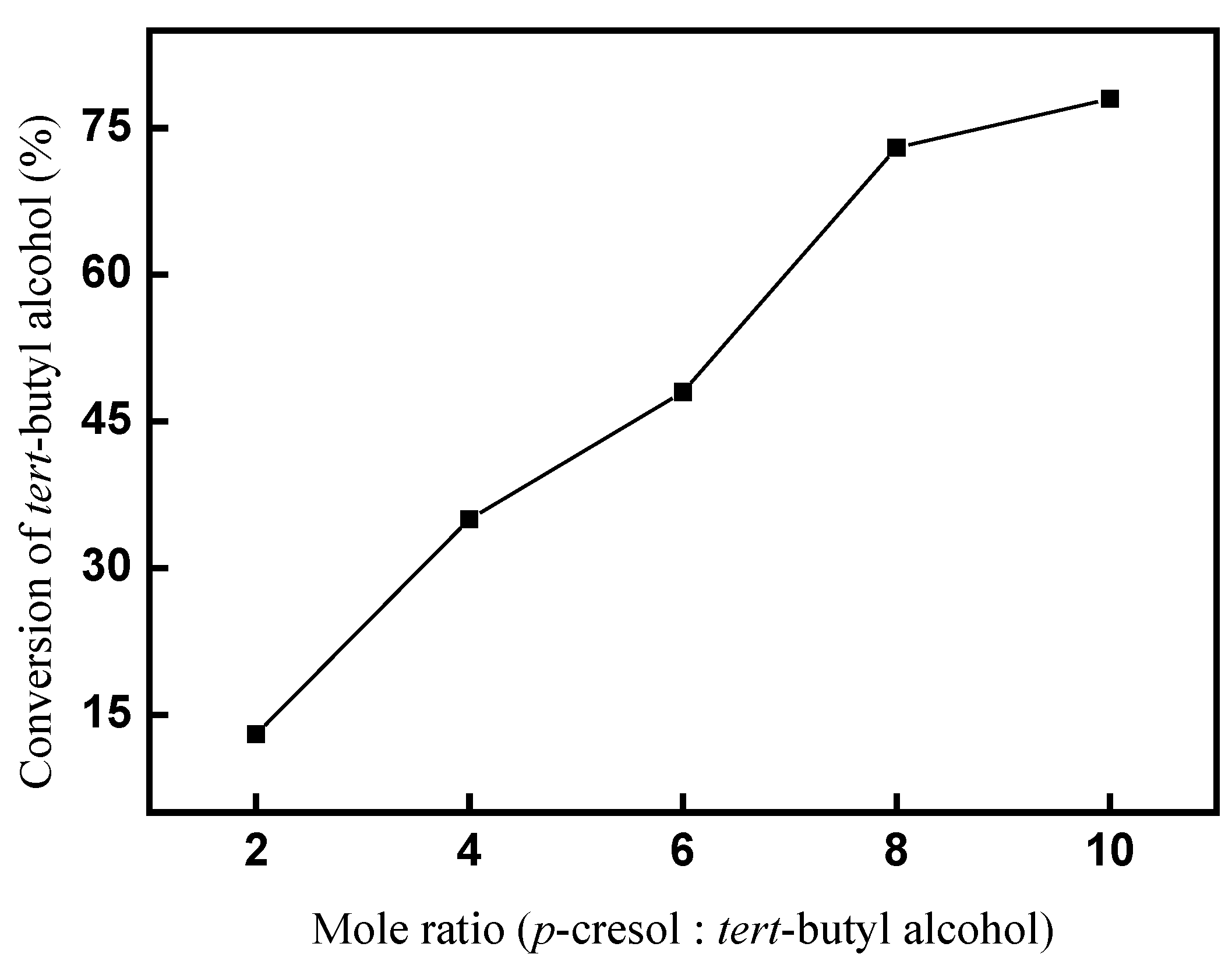
2.2.1. Effect of mole ratio (p-cresol: tert-butyl alcohol) on the conversion of tert-butyl alcohol
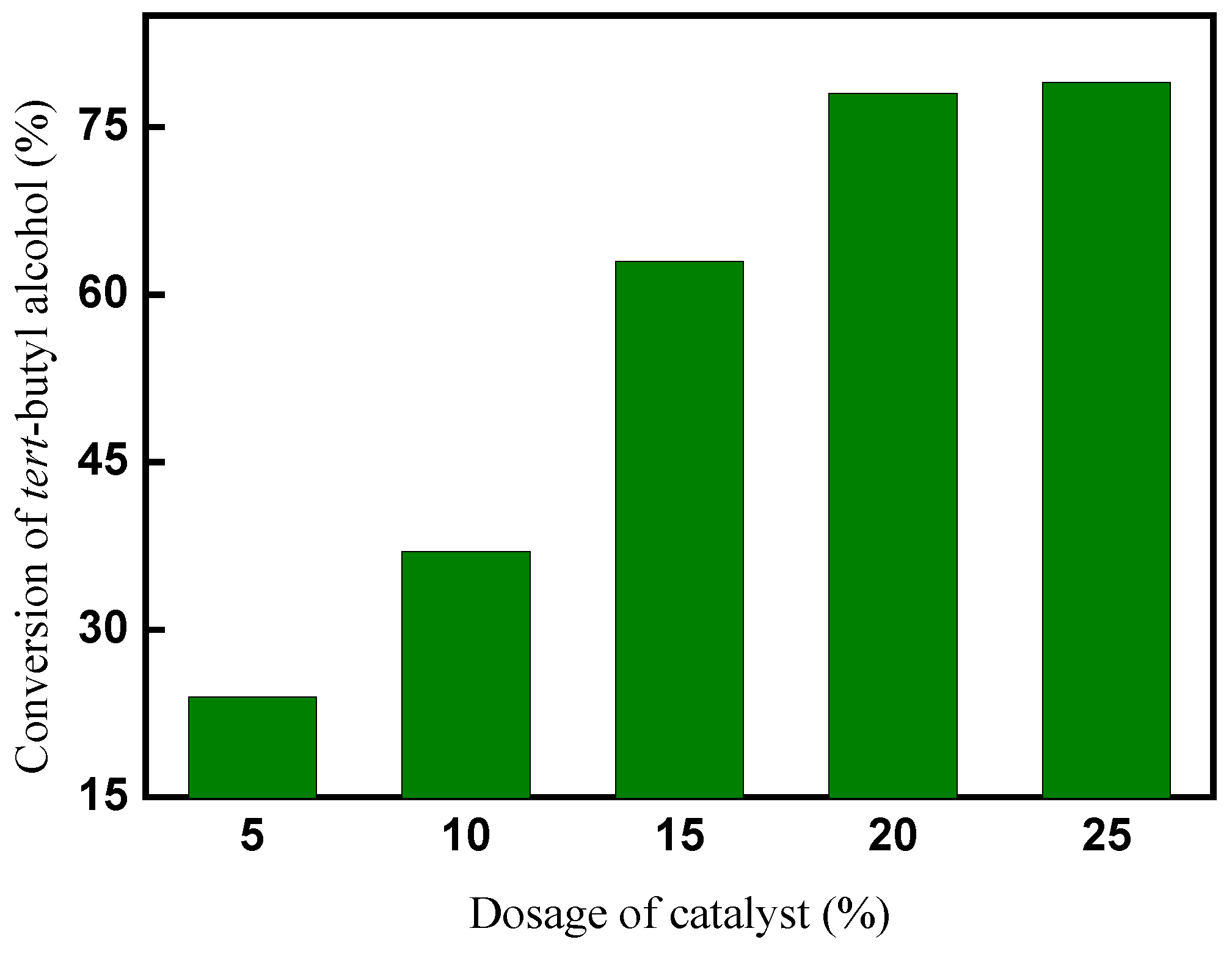
2.2.2. Effect of dosage of catalyst on conversion of tert-butyl alcohol
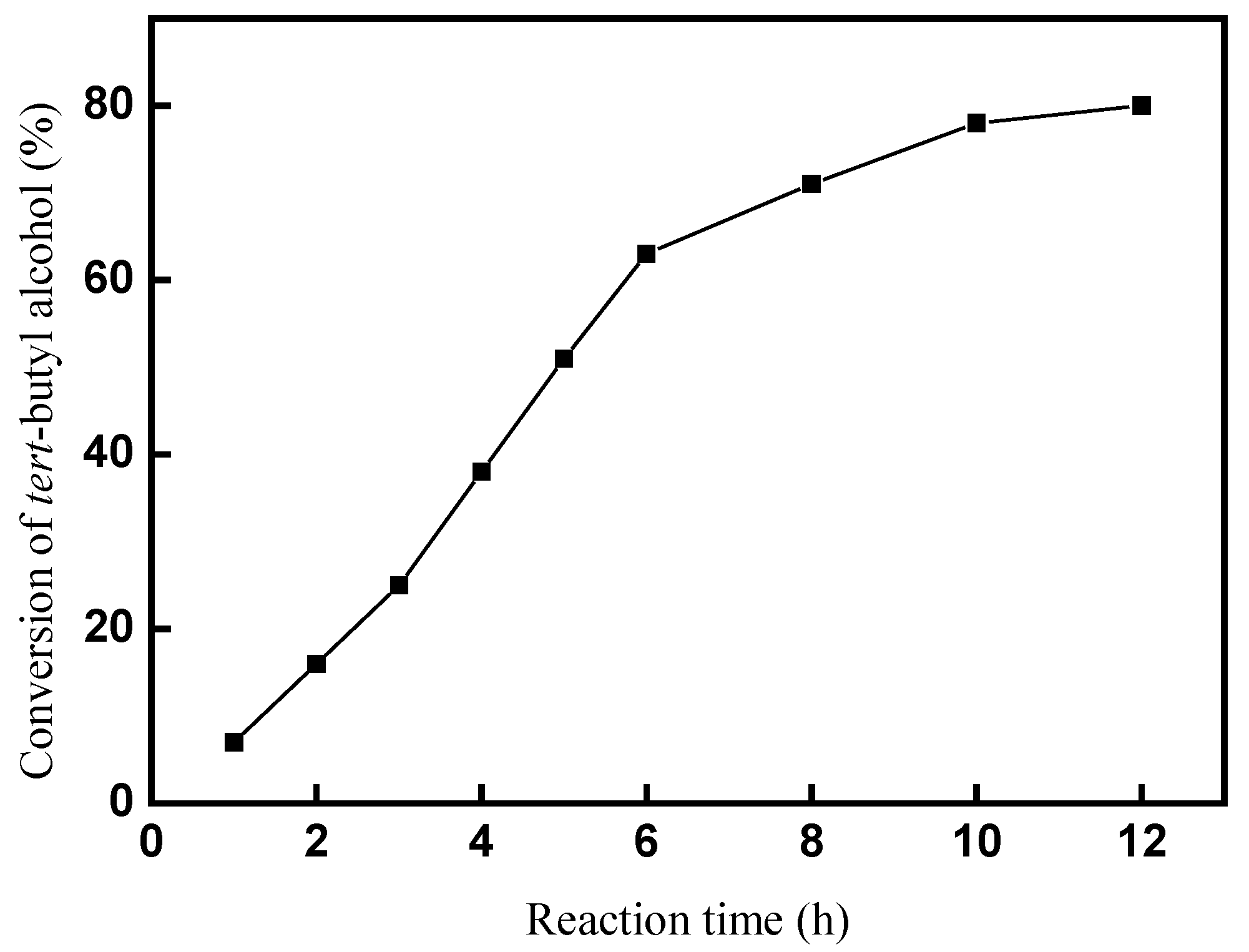
2.2.3. Effect of reaction time on the conversion of tert-butyl alcohol
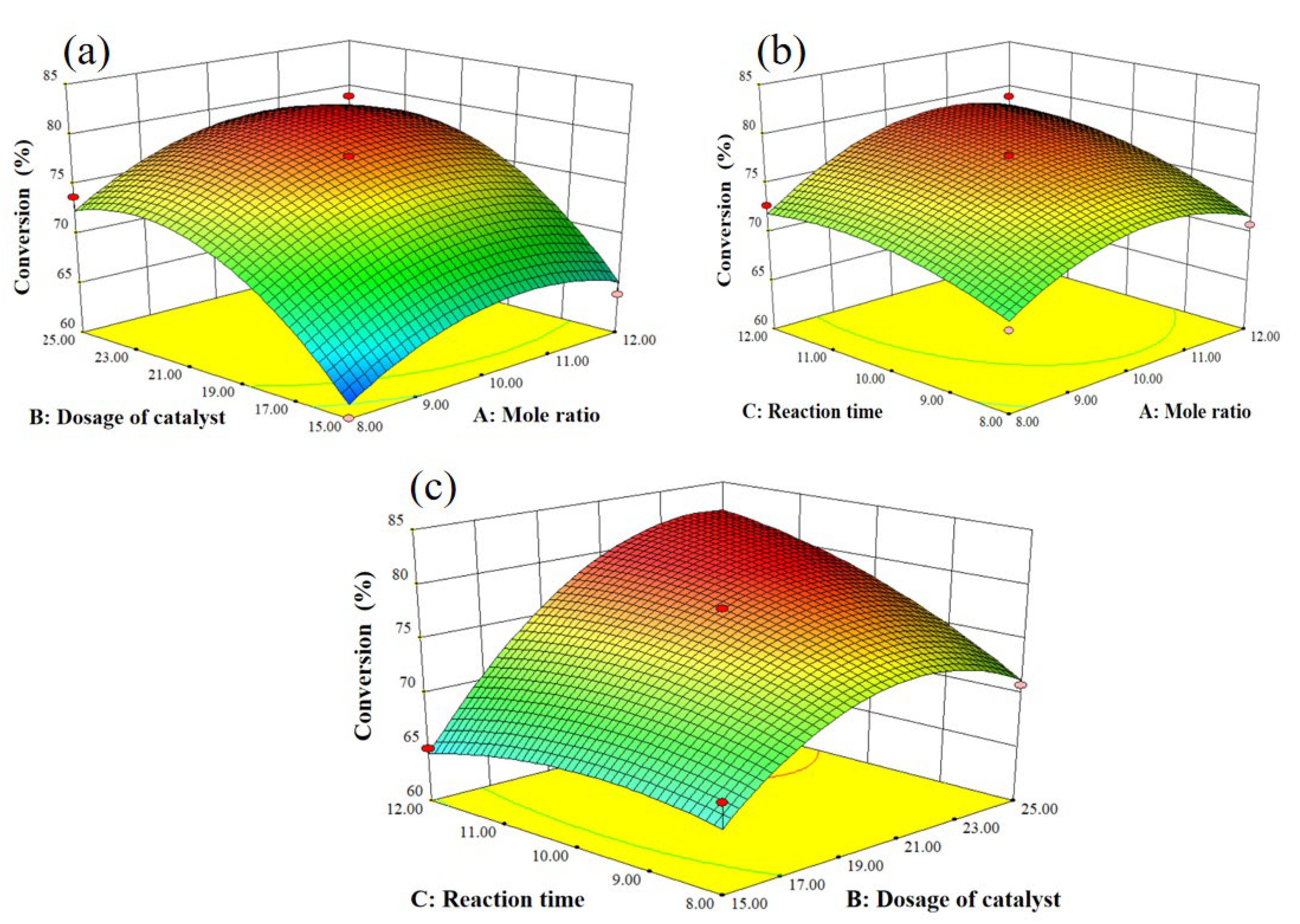
2.3. Response surface methodology for the alkylation of p-cresol and tert-butyl alcohol
2.4. Comparison of CAL-TsOH with other catalysts
2.5. Reaction kinetics for the alkylation of p-cresol and tert-butyl alcohol
2.6. Catalyst recovery for the CAL-TsOH
3. Materials and Methods
3.1. Materials and chemicals
3.2. Preparation of CAL-TsOH
3.3. Characterization of CAL-TsOH
3.4. Procedure for the synthesis of 2-TBM
3.5. Single factor experiments
3.5.1. Effect of mole ratio on the conversion of tert-butyl alcohol
3.5.2. Effect of dosage of catalyst on the conversion of tert-butyl alcohol
3.5.3. Effect of reaction time on the conversion of tert-butyl alcohol
3.6. Experimental design of response surface methodology
3.6. Determination of the conversion of tert-butyl alcohol
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pospíšil, J. Mechanistic action of phenolic antioxidants in polymers-A review. Polym. Degrad. Stabil. 1988, 20(3), 181–202. [Google Scholar] [CrossRef]
- Alamdari, R. F.; Hosseinabadi, Z.; Khouzani, M. F. Synthesis, characterization and investigation of catalytic activity of Cu1- xCoxFe2O4 nanocatalysts in t-butylation of p-cresol. J. Chem. Sci. 2012, 124(4), 827–834. [Google Scholar] [CrossRef]
- Hunter, S. E.; Savage, P. E. Acid-Catalyzed Reactions in Carbon Dioxide-Enriched High-Temperature Liquid Water. Ind. Eng. Chem. Res. 2003, 42(2), 290–294. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, S.; Wu, Q.; Xie, Y.; Liu, C.; Wang, C.; Zhang, K.; Shi, H.; Zhuo, X.; Wang, H. Efficient and Easily Recyclable Catalyst for the Alkylation Reaction of Phenol and tert-Butyl Alcohol. ACS Omega 2022, 7(35), 31495–31501. [Google Scholar] [CrossRef] [PubMed]
- Harmer, M. A.; Sun, Q. Solid acid catalysis using ion-exchange resins. Appl. Catal. A: Gen. 2001, 221(1), 45-62. [CrossRef]
- Zhang, K.; Zhang, H.; Xu, G.; Xiang, S.; Xu, D.; Liu, S.; Li, H. Alkylation of phenol with tert-butyl alcohol catalyzed by large pore zeolites. Appl. Catal. A: Gen. 2001, 207(1), 183-190. [CrossRef]
- Sato, T.; Sekiguchi, G.; Adschiri, T.; Arai, K. Non-catalytic and selective alkylation of phenol with propan-2-ol in supercritical water. Chem. Commun. 2001, 17, 1566–1567. [Google Scholar] [CrossRef]
- Nandhini, K.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. t-Butylation of phenol over mesoporous aluminophosphate and heteropolyacid supported aluminophosphate molecular sieves. J. Mol. Catal. A: Chem. 2004, 223(1), 201-210. [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol. 2019, 37(9), 943–959. [Google Scholar] [CrossRef]
- Perna, F.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sust. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Abbott, A. P. Deep eutectic solvents and their application in electrochemistry. Curr. Opin. Green Sust. 2022, 36, 100649. [Google Scholar] [CrossRef]
- Chiara, A.; Francesco, G. G.; Stefano, M.; Letizia, A. M. R.; Stefano, S.; Alberto, S.; Paola, D. Reactive Deep Eutectic Solvents (RDESs): A New Tool for Phospholipase D-Catalyzed Preparation of Phospholipids. Catalysts 2021, 11 (6): 655. 6: (6).
- Melanie, I.; Johannes, S.; Luciana, V.; Manuela, K.; Daniel, V. O.; Cordt, Z.; Tobias, G.; Michael, R.; Burkhard, K.; Volker, S. Enhanced C2 and C3 Product Selectivity in Electrochemical CO2 Reduction on Carbon-Doped Copper Oxide Catalysts Prepared by Deep Eutectic Solvent Calcination. Catalysts 2021, 11 (5): 542.
- Kassian, T. T. A.; Yuan C. L.; Mohan, S. V.; Subham, H.; Vinoth, K. P.; Jhang S. R. Deep eutectic solvents in the transformation of biomass into biofuels and fine chemicals: a review. Environ. Chem. Lett. 2023, 21: 183-230.
- Nicolás, F. G. P.; Michael, J. L.; Joshua, M. W.; Ángel, L.; Joan, F. B.; Roberto, I. C. Physicochemical properties of choline chloride-based deep eutectic solvents and excess properties of their pseudo-binary mixtures with 1-butanol. J. Chem. Thermodyn. 2019, 133: 272-284.
- Abbott, A. P.; Capper, G.; Davies, D. L.; Rasheed, R. K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1: 70-71. [CrossRef]
- Smith, E. L.; Abbott, A. P.; Ryder, K. S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114(21), 11060–11082. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.; Wang, X.; Tu, J. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: challenges, opportunities, and future vision. J. Mater. Chem. A 2017, 5(18), 8209–8229. [Google Scholar] [CrossRef]
- Tang, X.; Zuo, M.; Li, Z.; Liu, H.; Xiong, C.; Zeng, X.; Sun, Y.; Hu, L.; Liu, S.; Lei, T.; Lin, L. Green Processing of Lignocellulosic Biomass and Its Derivatives in Deep Eutectic Solvents. ChemSusChem 2017, 10(13), 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Teng, C.; Yan, L. Applications of deep eutectic solvents in the extraction, dissolution, and functional materials of chitin: research progress and prospects. Green Chem. 2022, 24(2), 552–564. [Google Scholar] [CrossRef]
- Craveiro, R.; Meneses, L.; Durazzo, L.; Rocha, Â.; Silva, J. M.; Reis, R. L.; Barreiros, S.; Duarte, A.; Paiva, A. Deep Eutectic Solvents for Enzymatic Esterification of Racemic Menthol. ACS Sustain. Chem. Eng. 2019, 7(24), 19943–19950. [Google Scholar] [CrossRef]
- Williamson, S. T.; Shahbaz, K.; Mjalli, F. S.; AlNashef, I. M.; Farid, M. M. Application of deep eutectic solvents as catalysts for the esterification of oleic acid with glycerol, Renew. Energ. 2017, 114, 480–488. [Google Scholar] [CrossRef]
- Pan, Y.; Alam, M. A.; Wang, Z.; Wu, J. Zhang, Y.; Yuan, Z. Enhanced esterification of oleic acid and methanol by deep eutectic solvent assisted Amberlyst heterogeneous catalyst. Bioresource Technol. 2016, 220, 543–548. [Google Scholar] [CrossRef]
- Hayyan, A.; Hizaddin, H.; Abed, K.; Mjalli, F.; Hashim, M.; Abo-Hamad, A.; Saleh, J.; Aljohani, A.; Alharbi, Y.; Alhumaydhi, F.; Ahmad, A.; Yeow, A.; Aldeehani, A.; Alajmi, F.; Nashef, I. Encapsulated deep eutectic solvent for esterification of free fatty acid. Biomass Convers. Bior. 2022, 12(9), 3725–3735. [Google Scholar] [CrossRef]
- Azizi, N.; Khajeh, M.; Alipour, M. Rapid and Selective Oxidation of Alcohols in Deep Eutectic Solvent, Ind. Eng. Chem. Res. 2014, 53(40), 15561–15565. [Google Scholar] [CrossRef]
- Bhise, R.; Patel, K.; Ghorpade, P.; Shankarling, G. Task-Specific Deep Eutectic Solvent for Selective Oxidation of Aromatic Methyl to Aldehyde. ChemistrySelect 2021, 6(24), 5893–5898. [Google Scholar] [CrossRef]
- Pateli, I.; Abbott, A.; Jenkin, G.; Hartley, J. Electrochemical oxidation as alternative for dissolution of metal oxideep eutectic solventin deep eutectic solvents. Green Chem. 2020, 22(23), 8360–8368. [Google Scholar] [CrossRef]
- Bušić, V.; Molnar, M.; Tomičić, V.; Božanović, D.; Jerković, I.; Gašo-Sokač, D. Choline Chloride-Based Deep Eutectic Solvents as Green Effective Medium for Quaternization Reactions. Molecules 2022, 27(21), 7429. [Google Scholar] [CrossRef]
- Yu, F.; Gu, Y.; Gao, X.; Liu, Q.; Xie, C.; Yu, S. Alkylation of isobutane and isobutene catalyzed by trifluoromethanesulfonic acid-taurine deep eutectic solvents in polyethylene glycol. Chem. Commun. 2019, 55(33), 4833–4836. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Xie, Y.; Li, L.; Ren, J.; Lin, X.; Qiu, T. Design and synthesis of novel Brønsted-Lewis acidic ionic liquid and its application in biodiesel production from soapberry oil. Energ. Convers. Manage. 2018, 166, 318–327. [Google Scholar] [CrossRef]
- Elavarasan, P.; Kondamudi, K.; Upadhyayula, S. Statistical optimization of process variables in batch alkylation of p-cresol with tert-butyl alcohol using ionic liquid catalyst by response surface methodology. Chem. Eng. J. 2009, 155(1), 355–360. [Google Scholar] [CrossRef]
- Patra, T.; Parveen, F.; Upadhyayula, S. Mechanistic insights into solvent induced alkylation of p-cresol with tert-butyl alcohol using Brönsted acidic ionic liquids. Mol. Catal. 2017, 433, 175–184. [Google Scholar] [CrossRef]
- Liu, X. , Zhou, J., Guo, X., Liu, M.; Ma, X.; Song,C.; Wang, C. SO3H-Functionalized Ionic Liquids for Selective Alkylation of p-Cresol with tert-Butanol. Ind. Eng. Chem. Res. 2008, 47(15), 5298–5303. [Google Scholar] [CrossRef]
- Kondamudi, K.; Elavarasan, P.; Dyson, P.; Upadhyayula, S. Alkylation of p-cresol with tert-butyl alcohol using benign Bronsted acidic ionic liquid catalyst, J. Mol. Catal. A: Chem. 2010, 321(1): 34-41. [CrossRef]
- Bao, S.; Quan, N.; Zhang, J.; Yang, J. Alkylation of p-Cresol with tert-Butanol Catalyzed by Novel Multiple-SO3H Functioned Ionic Liquid. Chinese J. Chem. Eng. 2011, 19(1), 64–69. [Google Scholar] [CrossRef]
- Sarish, S.; Devassy, B.; Halligudi, S. tert-Butylation of p-cresol over WOx/ZrO2 solid acid catalysts. J. Mol. Catal. A: Chem. 2005, 235(1), 44-51. [CrossRef]
- Devassy, B.; Shanbhag, G.; Lefebvre, F.; Halligudi, S. Alkylation of p-cresol with tert-butanol catalyzed by heteropoly acid supported on zirconia catalyst, J. Mol. Catal. A: Chem. 2004, 210(1), 125-130. [CrossRef]
- Kumbar, S.; Shanbhag, G.; Lefebvre, F.; Halligudi, S. Heteropoly acid supported on titania as solid acid catalyst in alkylation of p-cresol with tert-butanol. J. Mol. Catal. A: Chem. 2006, 256(1), 324-334. [CrossRef]
- Li, X.; Cao, R.; Lin, Q. Long-chain double SO3H-functionalized Brønsted acidic ionic liquids catalyzed selective alkylation of phenol andp-cresol with tert-butanol, Green Chem. Lett. Rev. 2014, 7(2), 179–183. [Google Scholar] [CrossRef]
- Yadav, G.; Pujari, A.; Joshi, A. Alkylation of p-cresol with methyl tert-butyl ether (MTBE) over a novel solid acid catalyst UDCaT-1. Green Chem. 1999, 1(6), 269–274. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.; Man, Z.; Khan, A.; Muhammad, N.; Sarwono, A. Preparation and kinetics study of biodiesel production from waste cooking oil using new functionalized ionic liquids as catalysts. Renew. Energ. 2017, 114, 755–765. [Google Scholar] [CrossRef]
- Booramurthy, V.; Kasimani, R.; Subramanian, D.; Pandian, S. Production of biodiesel from tannery waste using a stable and recyclable nano-catalyst: An optimization and kinetic study. Fuel 2020, 260, 116373. [Google Scholar] [CrossRef]

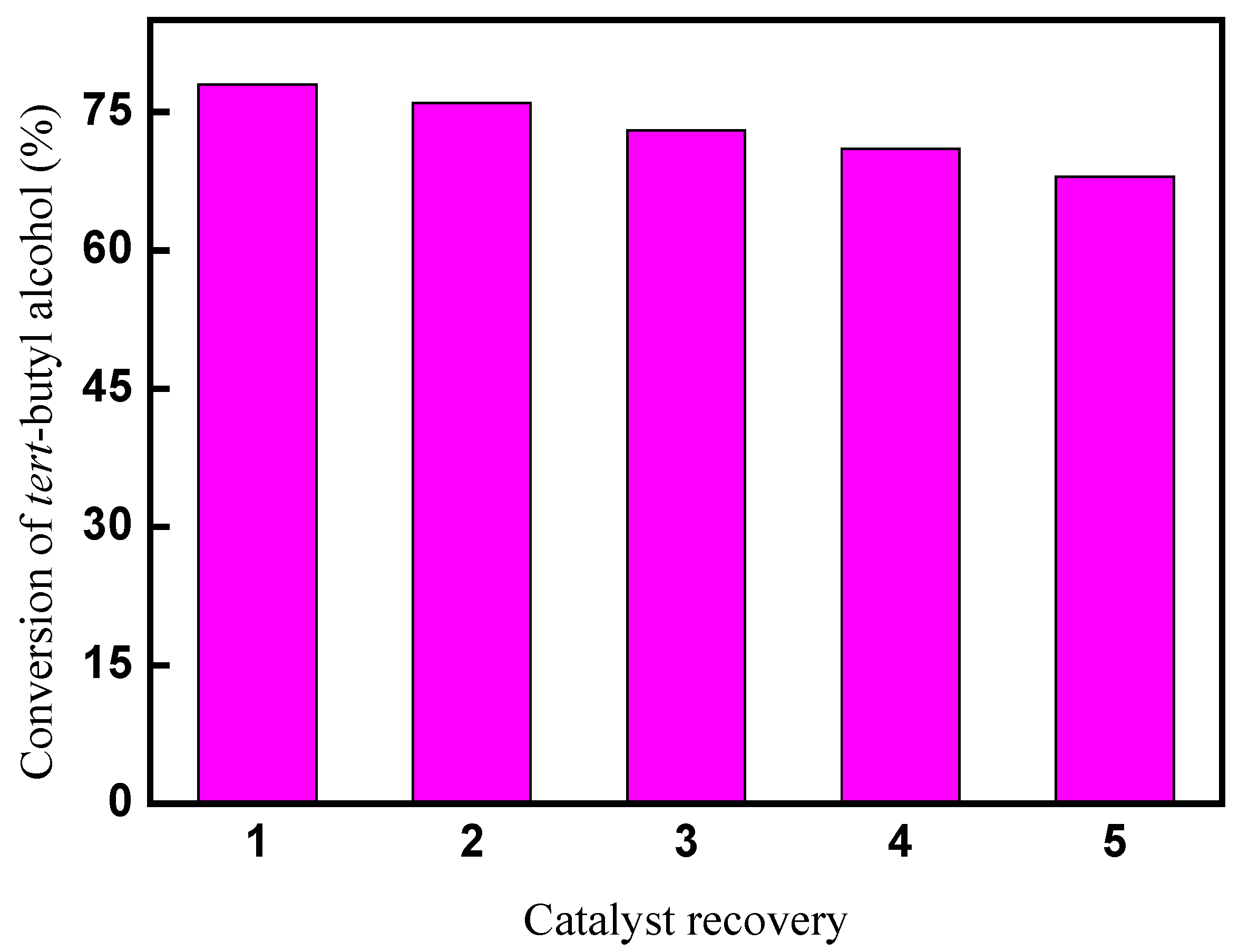
| Entry | Mole ratio | Dosage of catalyst/mol% | Reaction time/h | Conversion/% |
|---|---|---|---|---|
| 1 | 10 | 20 | 10 | 78 |
| 2 | 10 | 15 | 8 | 68 |
| 3 | 10 | 25 | 12 | 80 |
| 4 | 12 | 25 | 10 | 79 |
| 5 | 10 | 25 | 8 | 71 |
| 6 | 8 | 15 | 10 | 60 |
| 7 | 10 | 20 | 10 | 78 |
| 8 | 12 | 20 | 12 | 79 |
| 9 | 8 | 20 | 8 | 68 |
| 10 | 10 | 20 | 10 | 78 |
| 11 | 8 | 25 | 10 | 74 |
| 12 | 8 | 20 | 12 | 73 |
| 13 | 12 | 15 | 10 | 64 |
| 14 | 10 | 20 | 10 | 78 |
| 15 | 10 | 15 | 12 | 65 |
| 16 | 12 | 20 | 8 | 71 |
| 17 | 10 | 20 | 10 | 78 |
| Source | Sum of squares | df | Mean square | F-value | P-value | |
|---|---|---|---|---|---|---|
| Model | 597.69 | 9 | 66.41 | 21.88 | 0.0003 | significant |
| A-mole ratio | 40.5 | 1 | 40.5 | 13.34 | 0.0082 | |
| B-dosage of catalyst | 276.13 | 1 | 276.13 | 90.96 | <0.0001 | |
| C-reaction time | 45.13 | 1 | 45.13 | 14.86 | 0.0062 | |
| AB | 0.25 | 1 | 0.25 | 0.082 | 0.7824 | |
| AC | 2.25 | 1 | 2.25 | 0.74 | 0.4178 | |
| BC | 36.00 | 1 | 36.00 | 11.86 | 0.0108 | |
| A² | 51.58 | 1 | 51.58 | 16.99 | 0.0044 | |
| B² | 116.05 | 1 | 116.05 | 38.23 | 0.0005 | |
| C² | 12.89 | 1 | 12.89 | 4.25 | 0.0783 | |
| Residual | 21.25 | 7 | 3.04 | |||
| Lack of Fit | 21.25 | 3 | 7.08 | 7.43 | 0.1346 | not significant |
| Pure Error | 0.00 | 4 | 0.00 |
| Entry | Catalyst | Temperature (K) | Conversion$$$(%) | Refs |
|---|---|---|---|---|
| 1 | CAL-TsOH | 298 | 78 | This work |
| 2 | N-(1,4-sulfonic acid) butyl triethylammonium hydrogen sulfate | 348 | 89.4 | [31] |
| 3 | IL-CF3SO3 | 343 | 86.2 | [32] |
| 4 | SO3H ionic liquids | 343 | 79 | [33] |
| 5 | SO3H-functionalized Bronsted acidic ionic liquid | 343 | 80 | [34] |
| 6 | mutipule-SO3H ionic liquid | 343 | 85.3 | [35] |
| 7 | WOx/ZrO2 | 403 | 69.8 | [36] |
| 8 | TPA/ZrO2 | 403 | 61 | [37] |
| 9 | TPA/TiO2 | 403 | 82 | [38] |
| 10 | BAIL-1 | 343 | 93.2 | [39] |
| 11 | UDCaT-1 | 373 | 45 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





