1. Introduction
Primary cutaneous lymphomas are lymphoid cancers developing in the skin, in most cases running a relatively indolent course and rarely metastasizing to parenchymatous organs. Mycosis fungoides (MF) is the most common primary cutaneous T-cell lymphoma, manifesting itself clinically as scaly patches and plaques, occasionally progressing to tumors [
1]. The origin of MF has been a subject of considerable interest and remains controversial. The prevalent view is that MF represents a clonal expansion of a tissue-resident, mature memory T-cell [
2]. However, there is a considerable body of evidence that MF, like many other lymphomas, develops from immature T-cell progenitors, which differentiate into skin-homing, pre-malignant T-cells that colonize the skin, proliferate, and form MF lesions [
3,
4].
UV radiation is the most common environmental carcinogen implicated in the pathogenesis of most skin cancers. UV is mutagenic and plays a role during cancer initiation and promotion [
5]. The risk of malignant melanoma (MM) and non-melanoma (keratinocyte) skin cancers is strongly correlated with solar exposure and sunburns, and cancer cells show a high number of genomic UV-induced mutations [
6,
7,
8]. Similar dependency of UV would strongly support the origin of MF in the skin rather than from migratory, immature T-cell precursors. However, the evidence linking MF to UV exposure has been inconclusive. Some authors found epidemiological association between lymphoma risk and solar exposure [
9,
10,
11] but those correlations were weak and were not reproduced by other investigators [
12]. Other studies examined the patterns of mutations and reported putative UV signatures in specific oncogenes (TP53) and in the genomes in MF [
13,
14,
15]. However, those studies examined crude skin biopsies which might have contaminated the material with epidermal cells harboring UV mutations. Neither did they take into account the possible iatrogenic impact of phototherapy, which is a standard treatment for MF and is administered cyclically over extended periods of time.
Confirmation of a large number of UV mutations in MF genomes would indicate the causative role of solar radiation in its pathogenesis and favor the role of long-lived, skin-resident T-cells rather than migratory immature precursors as the cells of origin. We have therefore re-examined the question of whether UV mutational signatures are found in MF. To avoid the biases identified in other studies, we have carefully purified lymphoid tumor cells by microdissecting skin biopsies, analyzed the data using validated bioinformatic pipelines [
16,
17], and compared MF data to the mutation patterns of malignant melanoma, the well-established example of a UV-dependent tumor [
8].
2. Materials and Methods
Data sources
For analysis of MF mutations, we used whole-exome sequencing data which we generated previously from the microdissected biopsies from the lesional skin of the patients with confirmed diagnosis of mycosis fungoides (
Table 1) [
3,
18].
Briefly, tumor cell clusters were prepared using laser-capture microdissection of the skin biopsies, and DNA was isolated from the microdissected materials pooled for each biopsy as well as from matched peripheral blood mononuclear cells and buccal swabs. Whole-exome sequencing (WES) libraries were prepared with NEBNext Ultra II DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA), the exome and untranslated region DNA reads were captured using SSELXT Human All Exon V6 +UTR probes (Agilent Technologies, Santa Clara, CA) and sequenced using on Illumina HiSeq 1500 and Novaseq 6000 sequencer. Ethical approval HREBA.CC-16-0820-REN1 was obtained from the Health Research Ethics Board of Alberta Cancer Committee. Whole-exome sequencing data for samples of malignant melanoma data were downloaded from The Cancer Genome Atlas (TCGA) (
Table 2).
Data analysis
The raw fastq files generated from WES were processed through the GATK (version 4.0.10) best practices workflow and aligned to the hg38 reference genome. Somatic variants (SVs), including single somatic mutations and indels, were identified by MuTect2 variant caller (version 2.1). The synonyms and non-synonymous SVs were identified by the Variant Effect Predictor (VEP) (version 95.2). The VEP files were then used for identifying the single base substitution (SBS) and insertion and deletion (ID) signatures using SignatureAnalyzer (version 0.0.8) [
19]. Visual data representations were created using Prism (GraphPad software, version 9).
Data sharing
The MF exome-sequencing data is available on dbGaP under accession number phs001877.v1.p1.
3. Results
UV mutational signature is dominant in malignant melanoma
The UV signature of MM was investigated previously [
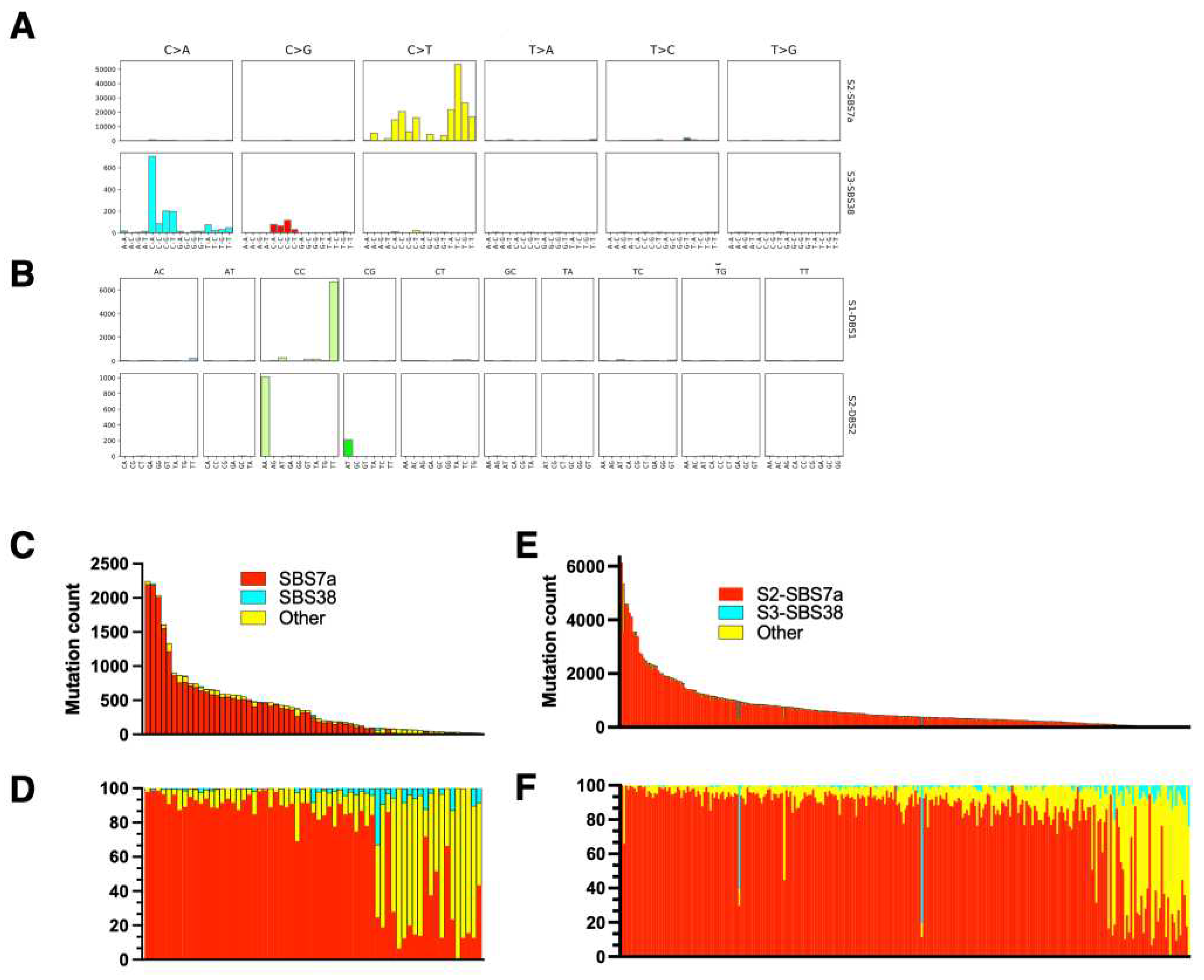
8] and we have performed an independent analysis of the TCGA dataset as a quality control of our analytic pipelines and as a reference for MF samples. For the single base substitutions (SBS) UV mutations we have detected SBS 7a and SBS 38 (
Figure 1). The UV signature 7a comprises C>T substitutions at TpC that are probably formed during repair of 6,4-photoproducts and was the dominant signature in melanoma across all the samples. SBS 38 results from indirect DNA damage by UV and is characterized by a high proportion of C>A substitutions [
8,
16]. Interestingly, we did not detect other subtypes of SBS 7, i.e. SBS7b (C>T substitutions at CpC, reflecting repair of cyclobutane pyrimidine dimers), or SBS 7c (high proportion of T>A and T>C substitutions reflecting, like SBS 38, indirect DNA damage), which were detected in smaller amounts relative to SBS7a in the previous benchmark study [
8]. However, SBS 7a and SBS 7b signatures are very similar and may be misattributed to each other, especially when analyzing WES datasets which are noisier and resolve fewer mutations than the whole-genome sequencing of Hayward et al. [
8]. Importantly, we have found an expected double base substitution DSB 1 (CC>TT) which is also a specific UV signature, albeit present in small quantities (<5% of SBS7a) [
6,
16,
17,
20]. In our material, DBS 1 and the less abundant DBS 2 (sometimes attributed to tobacco smoking) were the only DBS signatures and were present in 24 samples (
Figure 1). There was wide variation in the proportions and numbers of UV mutations across the samples, but on average, 92% of all mutations could be attributed to UV (SBS 7a + SBS 38 + DBS 1).
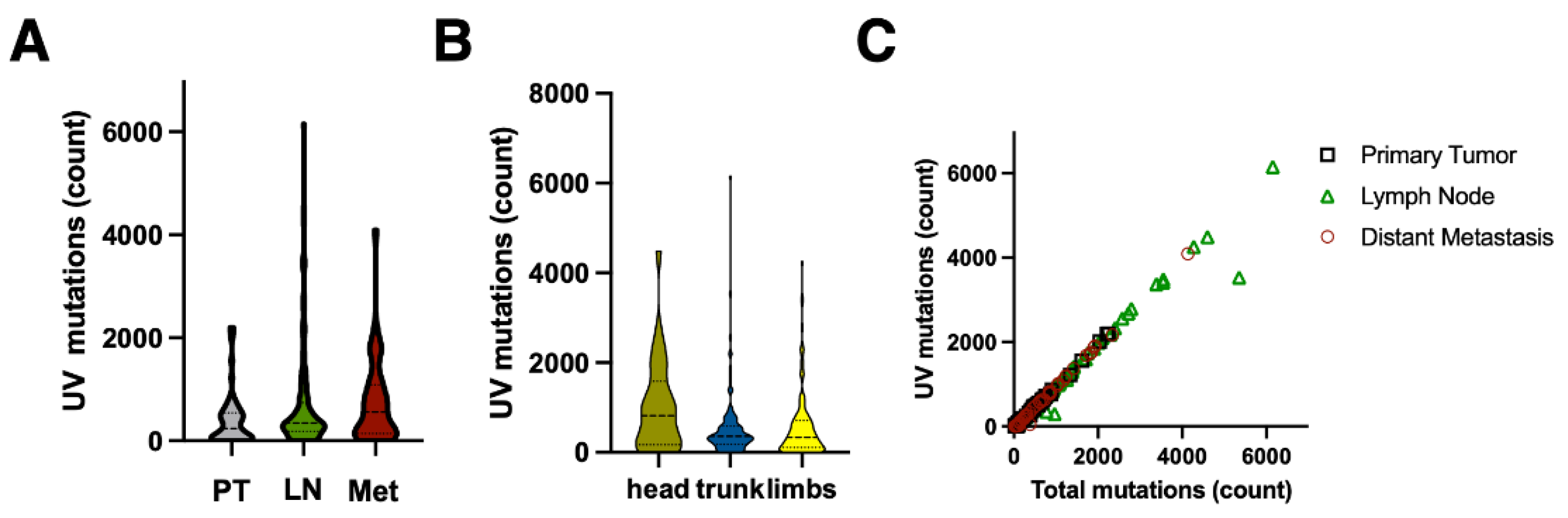
We have compared UV mutations between samples of cutaneous melanoma to the samples obtained from lymph nodes and distal metastases (
Figure 1C,E and
Figure 2A). As expected, the skin lesions had a lower UV mutation burden compared to lymph node and distal metastases (mean 395 [95% CI 268-522] vs 674 [CI 534-814] vs 738 [CI 490-987]). Melanomas on the sun-exposed skin on the head had a higher UV mutation burden than melanomas on trunk and limbs (1021 [CI 212-1063] vs 552 [CI 44-731]) but the difference was not statistically significant due to a large spread of the data (
Figure 2B). There was no difference between UV mutation burden between T3 and T4 stage cutaneous melanomas (not shown), but there was a good correlation between total mutation burden and number of UV mutations (SBS 7a + SBS 38 + DBS 1), as reported previously [
7,
8] (
Figure 2C).
UV mutations in mycosis fungoides
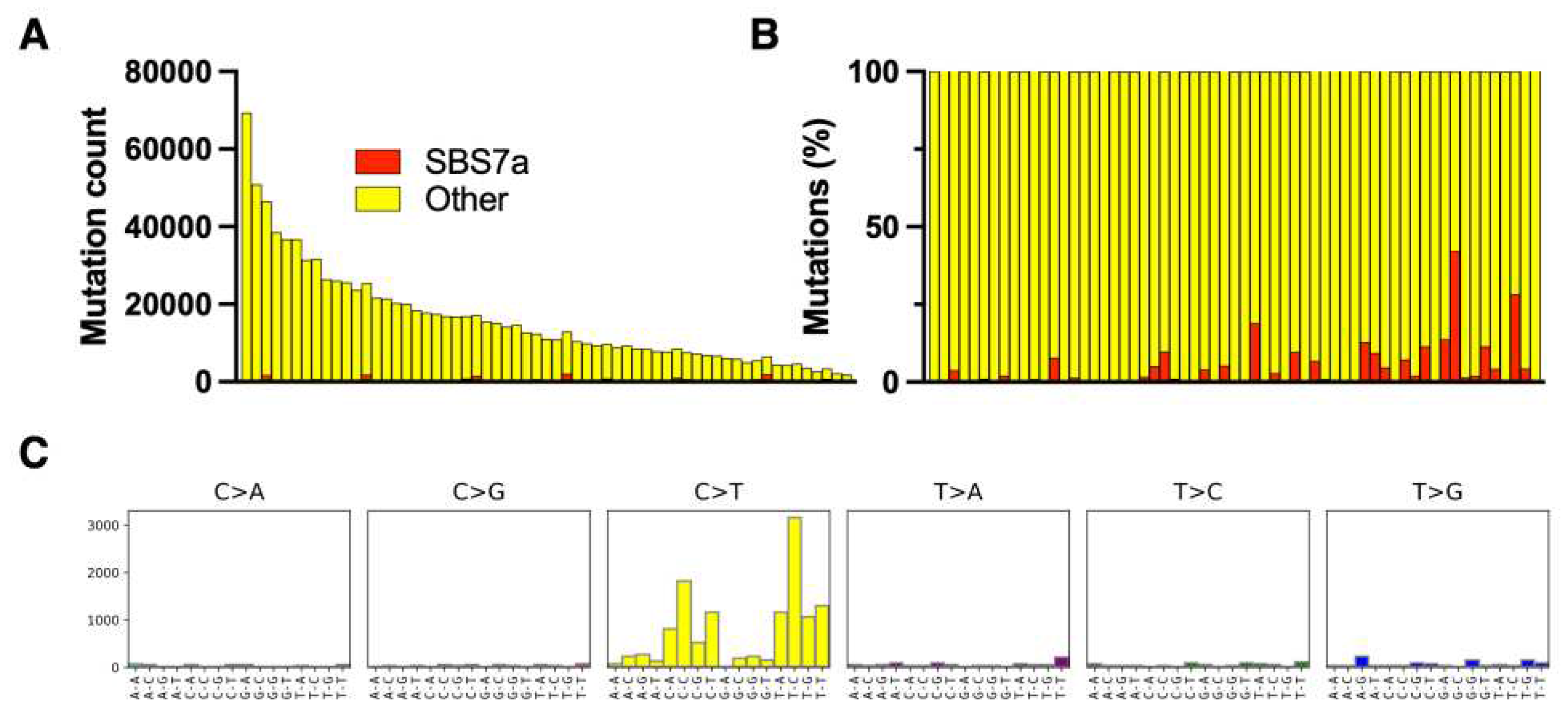
All samples included in the study were microdissected clusters of tumor cells and therefore they had a high tumor cell fraction and were not contaminated with epithelial tissue which is a rich source of mutated DNA [
3,
18]. MF had a high total mutation burden, comparable to MM, but the only UV-related mutation (SBS 7a) only comprised 4% [CI 2% - 6%] of the mutation burden (
Figure 3).
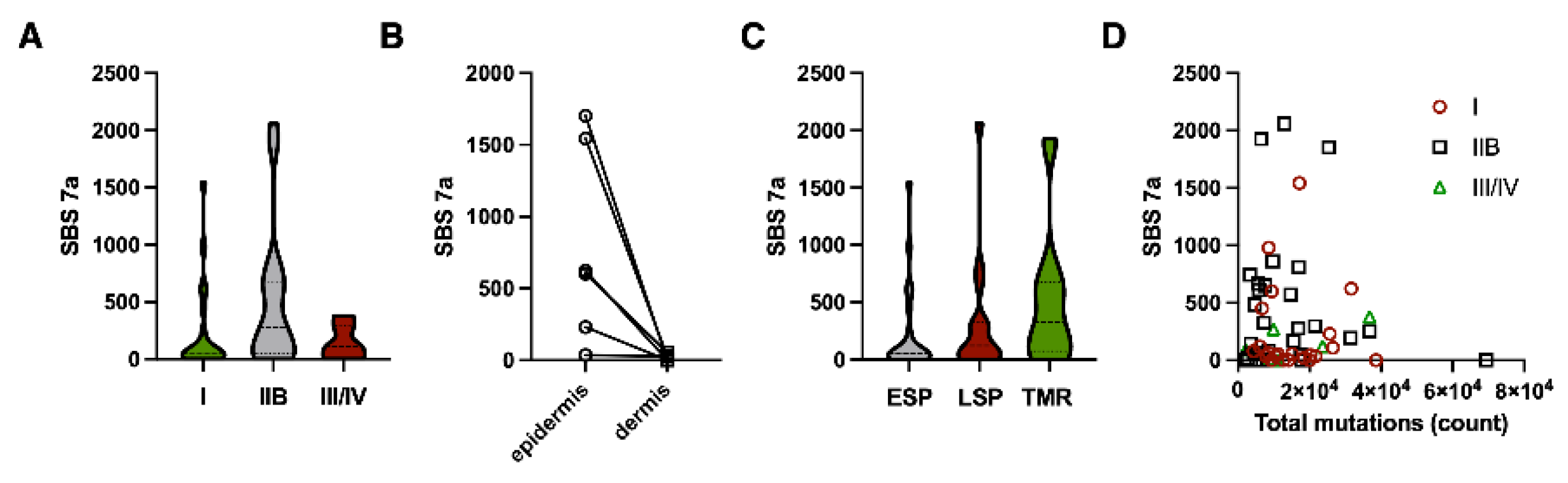
The low UV mutation burden indicated that they are unlikely to occur early in MF development but were rather passively accumulated due to exposure of the lesions to UV (from the sun and during phototherapy). We have therefore hypothesized that long-standing lesions will have a higher number of UV mutations than the lesions in the early-stage disease. As the duration of the lesions are roughly correlated with the stage of the disease, we have compared samples from early-stage patients (stage IA and IB) to advanced stage (IIB-IV). As predicted, stage I disease had a lower number of UV mutations than stage IIB (231 [CI 57-404] vs. 486 [CI 250-721], p=0.05 Mann Whitney test). The UV mutations were not correlated to the total mutation burden (
Figure 4A,B).
Interestingly, lesions from stage III (erythrodermic patients) and IV (distal metastases) had a lower UV mutation burden, which might be caused by the rapid evolution and growth of the skin lesions in those stages and dilution of the accumulated UV mutations. To further investigate this, we have divided our samples into three categories, according to their morphology and the stage of the disease [
18,
21,
22,
23]. The early-stage plaques (ESP) are found in patients with early-stage disease (stage IA/IB). Samples from patients with advanced MF (stage ≥ IIB) were classified based on their morphology as tumors (TMR) or late-stage plaques (LSP). LSP morphologically resembles the lesions in early disease and are sometimes carried over from early MF and sometimes develop de novo in patients already in stage ≥ IIB. Comparison of the UV mutation load between those lesions revealed a trend towards higher UV burden in the more advanced, long-standing lesions (ESP = 231 [CI 57-404]; LSP = 327 [CI 139-538]; TMR = 504 [135-572], p=0.04 for the difference between ESP and TMR) (
Figure 4C).
Finally, if the UV mutations were mainly caused by the cumulative exposure of the established lesions to UV, we hypothesized that the tumor cells in the more superficial layers of the skin (epidermis) would have a higher UV mutation load than the cells in the dermis. We were able to microdissect intraepidermal malignant lymphocytes (Pautier abscesses) from the dermal malignant cells in 6 samples and compared their SBS 7a signatures (
Figure 4D). As could be predicted, the epidermal malignant lymphocytes had a strikingly higher UV mutation burden than dermal cells in the same biopsies.
4. Discussion
Ultraviolet is a well-characterized environmental mutagen and a complete carcinogen playing a prominent role in the pathogenesis of skin cancers. Common skin cancers, such as malignant melanoma, basal cell carcinoma, and squamous cell carcinoma originate most likely from immature melanocytic and keratinocyte stem cells, residing in the bulge area of the hair follicle and also in the interfollicular epidermis [
24,
25,
26,
27,
28,
29] Those cells are non-migratory and slowly proliferating and remaining in the same anatomical niche for decades, they are exposed to environmental UV radiation and slowly accumulate mutations leading eventually to malignant transformation. Therefore, the UV mutation load is high in early skin cancers and resembles the mutation load in non-neoplastic, UV-damaged skin [
30,
31].
Thus, a high percentage of UV mutations is a hallmark of all skin cancers where UV plays a decisive pathogenic role. Melanomas which develop in sun protected areas, such as the mucosa or the uveal melanoma, are not dependent on UV as a carcinogen and their UV signature mutations are absent or present in small quantities [
32,
33]. In our own analysis, >90% of all mutations found in malignant melanoma had a UV signature SBS 7a with smaller component of SBS 38 and DBS 1. We also confirmed that the UV signature correlated with the expected level of exposure to UV, tending to be higher for MM localized in the face versus the trunk and limbs [
7]. Interestingly, those signatures persisted in metastatic tumors in the lymph nodes and in parenchymal organs without the ongoing UV exposure. Similar findings were reported before and it was even suggested that UV mutations can be used to determine whether organ metastases without clinically identifiable primaries melanoma arise from occult primary cutaneous lesions [
34]. Thus, analyses of the UV mutation signatures confirmed the pattern characteristic for all UV-dependent cancers: high percentage of UV mutations which persist during tumor evolution, and which are correlated with the degree of exposure of the skin region to solar radiation.
It has been a matter of long debate whether UV plays a role in the development of cutaneous lymphomas, such as mycosis fungoides (MF). Epidemiological evidence has not been convincing, and MF tends to develop primarily in the sun-protected areas (buttocks, inner thighs), unlike UV-dependent cancers that have predilection to the areas exposed to UV or suffering previous sun burns. Moreover, the migratory nature of the lymphocytes argues against the role of UV because those cells are unlikely to dwell in the skin long enough to accumulate UV mutations. However, the discovery and characterization of skin-resident T-cells rekindled the debate about the relevance of UV as a carcinogen in MF. The resident memory T-cells do not readily recirculate but persist in the skin and are therefore exposed to UV to the same degree as the native skin cells, like keratinocytes or melanocytes [
35,
36]. Those skin-resident T-cells were proposed to be the cell of origin for MF [
2] and C>T mutations were found in MF (including TP53 gene [
13,
15]), further supporting the importance of UV [
14].
The results of our analysis of UV mutation signatures in MF argue against the role of ultraviolet in the pathogenesis of this lymphoma. The UV signature mutations (SBS 7a) are found in small quantities and their contribution to the total mutational load is negligible (≤5%), in a striking contrast to the UV-dependent tumor such as melanoma. This small proportion of UV mutations could easily be explained as a result of the normal solar exposure of MF lesions, and they would persist as passenger mutations. Since patients with MF are usually treated with phototherapy, the therapeutic exposure to UV would further contribute to UV mutations.
The fact that UV signature mutations are more frequent in the epidermal lymphocytes as compared to the lymphocytes in the dermis further supports this interpretation. The carcinogenic UVB radiation penetrates to the levels of papillary dermis, but its energy rapidly dissipated by the stratum corneum (scattering and reflection of UV) and by the epidermal melanin that provides 20%-50% of the photoprotection [
37,
38]. Thus, both epidermal and superficial dermal lymphomatous infiltrates are exposed to UV, but the exposure levels are likely to be significantly higher in the epidermal lymphocytic abscesses, which are not efficiently photo-protected by epidermal melanin.
We have also found that the amount of UV mutations increases slightly during the progression of the disease, most likely due to cumulative effects of sun exposure and ongoing phototherapy. Unfortunately, we were unable to determine the duration of the biopsied lesions which would make the analysis more reliable.
The discrepancy between our findings and the results of other groups reporting significant UV signatures in MF [
14] may be explained by the methods of sample preparation and analysis. Most sequencing studies in MF use entire skin biopsy. We have shown that lymphoma cells contribute to the minority of cells in the bulk biopsies (usually less than 20% in stage I disease), the rest being reactive lymphocytes, macrophages and normal skin cells such as fibroblasts and epidermal cells. Considering the high UV mutation level in normal epidermis [
31], even small amounts of keratinocytes would significantly increase the UV signature in the sample. We avoided this pitfall by carefully microdissecting malignant lymphoid cells and enriching the tumor cell fraction from median 19.3% in crude biopsies to 69.3% (range 21% -98%) [
4].
One of the limitations of our paper is the use of whole exome sequencing data, rather than the whole genome. WES captures only the protein-coding comprising 1%-2% of the genome whereas whole genome sequencing covers 99% of the genome. Thus, the number of mutations per genome available to analysis is much lower which may impact the results. Moreover, UV mutagenesis is not a random event but depends on chromatin methylation. It was shown that UV-induced cyclobutane pyrimidine dimer DNA lesions are reduced within the demethylated CpG areas of gene promoters, which results in a unique trinucleotide UV signature with reduced TCG > TTG transitions [
39]. In this study we were unable to analyze such local variations in the UV signatures. Finally, to get better insight in the kinetics of UV damage in lymphoma cells it would be desirable to compare the levels of mutations to the epidermis in the same area as the tumor. Epidermal UV mutations can serve as a molecular UV dosimeter and would allow the capture of regional, cumulative UV mutagenesis.
5. Conclusions
Mutations caused by ultraviolet radiation are unlikely to cause cutaneous T-cell lymphoma (MF). The specific UV mutational signature (SBS 7a) has very low frequency and most probably represents passenger mutations induced by environmental and therapeutic ultraviolet exposure. Our data support the epidemiological evidence which did not show correlations between UV and the risk of lymphoma.
Author Contributions
R.G. collected clinical samples, performed data analysis, supervised research, and wrote the manuscript. A.I. was responsible for data analyses and data assembly. D.H. performed the bioinformatic data analysis and assembled bioinformatic pipelines. SO’K was responsible for sample processing, DNA/RNA extraction, and sequencing library prep. All authors contributed to study design, manuscript editing, data interpretation and approved the final version of the paper.
Funding
This research was funded by unrestricted grants from the University of Alberta (Canada) and Bispebjerg Hospital (Copenhagen, Denmark), Danish Cancer Society (Kræftens Bekæmpelse; R124-A7592 Rp12350), Canadian Dermatology Foundation (CDF), and University Hospital Foundation (University of Alberta). A.I. was funded under the Accelerate Postdoctoral Fellowship under a joint grant from the Mitacs and Sun Pharma Global FZE.
Data Availability Statement
The MF exome-sequencing data is available on dbGaP under accession number phs001877.v1.p1.
Conflicts of Interest
R.G. served as a paid consultant and received advisory board honoraria from Mallinckrodt, Recordati and Kyowa Kirin. The other authors present no conflict of interest.
References
- Cerroni, L. Mycosis Fungoides-Clinical and Histopathologic Features, Differential Diagnosis, and Treatment. Semin. Cutan. Med. Surg. 2018, 37, 2–10. [Google Scholar] [CrossRef]
- Campbell, J.J.; Clark, R.A.; Watanabe, R.; Kupper, T.S. Sezary Syndrome and Mycosis Fungoides Arise from Distinct T-Cell Subsets: A Biologic Rationale for Their Distinct Clinical Behaviors. Blood 2010, 116, 767–771. [Google Scholar] [CrossRef]
- Iyer, A.; Hennessey, D.; O’Keefe, S.; Patterson, J.; Wang, W.; Wong, G.K.-S.; Gniadecki, R. Skin Colonization by Circulating Neoplastic Clones in Cutaneous T-Cell Lymphoma. Blood 2019. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Hennessey, D.; Gniadecki, R. Clonotype Pattern in T-Cell Lymphomas Map the Cell of Origin to Immature Lymphoid Precursors. Blood Adv 2022, 6, 2334–2345. [Google Scholar] [CrossRef]
- Ziegler, A.; Jonason, A.S.; Leffell, D.J.; Simon, J.A.; Sharma, H.W.; Kimmelman, J.; Remington, L.; Jacks, T.; Brash, D.E. Sunburn and p53 in the Onset of Skin Cancer. Nature 1994, 372, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E. UV Signature Mutations. Photochem. Photobiol. 2015, 91, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Dousset, L.; Poizeau, F.; Robert, C.; Mansard, S.; Mortier, L.; Caumont, C.; Routier, É.; Dupuy, A.; Rouanet, J.; Battistella, M.; et al. Positive Association Between Location of Melanoma, Ultraviolet Signature, Tumor Mutational Burden, and Response to Anti-PD-1 Therapy. JCO Precis Oncol 2021, 5. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-Genome Landscapes of Major Melanoma Subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Adami, J.; Frisch, M.; Yuen, J.; Glimelius, B.; Melbye, M. Evidence of an Association between Non-Hodgkin’s Lymphoma and Skin Cancer. BMJ 1995, 310, 1491–1495. [Google Scholar] [CrossRef]
- Morales-Suárez-Varela, M.M.; Olsen, J.; Johansen, P.; Kaerlev, L.; Guénel, P.; Arveux, P.; Wingren, G.; Hardell, L.; Ahrens, W.; Stang, A.; et al. Occupational Sun Exposure and Mycosis Fungoides: A European Multicenter Case-Control Study. J. Occup. Environ. Med. 2006, 48, 390–393. [Google Scholar] [CrossRef]
- DeStefano, C.B.; Desale, S.; Fernandez, S.J.; Shenoy, A.G. The Impact of Environmental Ultraviolet Exposure on the Clinical Course of Mycosis Fungoides. J. Am. Acad. Dermatol. 2019, 81, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Newton, R. Solar Ultraviolet Radiation Is Not a Major Cause of Primary Cutaneous Non-Hodgkin’s Lymphoma. BMJ 1997, 314, 1483–1484. [Google Scholar] [CrossRef]
- McGregor, J.M.; Crook, T.; Fraser-Andrews, E.A.; Rozycka, M.; Crossland, S.; Brooks, L.; Whittaker, S.J. Spectrum of p53 Gene Mutations Suggests a Possible Role for Ultraviolet Radiation in the Pathogenesis of Advanced Cutaneous Lymphomas. J. Invest. Dermatol. 1999, 112, 317–321. [Google Scholar] [CrossRef]
- Jones, C.L.; Degasperi, A.; Grandi, V.; Amarante, T.D.; Genomics England Research Consortium; Mitchell, T.J.; Nik-Zainal, S.; Whittaker, S.J. Spectrum of Mutational Signatures in T-Cell Lymphoma Reveals a Key Role for UV Radiation in Cutaneous T-Cell Lymphoma. Sci. Rep. 2021, 11, 3962.
- Wooler, G.; Melchior, L.; Ralfkiaer, E.; Rahbek Gjerdrum, L.M.; Gniadecki, R. Gene Status Affects Survival in Advanced Mycosis Fungoides. Front. Med. 2016, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium Pan-Cancer Analysis of Whole Genomes. Nature 2020, 578, 82–93. [CrossRef] [PubMed]
- Iyer, A.; Hennessey, D.; O’Keefe, S.; Patterson, J.; Wang, W.; Salopek, T.; Wong, G.K.-S.; Gniadecki, R. Clonotypic Heterogeneity in Cutaneous T-Cell Lymphoma (mycosis Fungoides) Revealed by Comprehensive Whole-Exome Sequencing. Blood Adv 2019, 3, 1175–1184. [Google Scholar] [CrossRef]
- SignatureAnalyzer. Available online: https://software.broadinstitute.org/cancer/cga/msp (accessed on 17 January 2022).
- Pfeifer, G.P.; You, Y.-H.; Besaratinia, A. Mutations Induced by Ultraviolet Light. Mutat. Res. 2005, 571, 19–31. [Google Scholar] [CrossRef]
- Iyer, A.; Hennessey, D.; O’Keefe, S.; Patterson, J.; Wang, W.; Wong, G.K.-S.; Gniadecki, R. Branched Evolution and Genomic Intratumor Heterogeneity in the Pathogenesis of Cutaneous T-Cell Lymphoma. Blood Adv 2020, 4, 2489–2500. [Google Scholar] [CrossRef]
- Sivanand, A.; Hennessey, D.; Iyer, A.; O’Keefe, S.; Surmanowicz, P.; Vaid, G.; Xiao, Z.; Gniadecki, R. The Neoantigen Landscape of Mycosis Fungoides. Front. Immunol. 2020, 11, 561234. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.Z.X.; Hennessey, D.; Iyer, A.; O’Keefe, S.; Zhang, F.; Sivanand, A.; Gniadecki, R. Transcriptomic Changes during Stage Progression of Mycosis Fungoides. Br. J. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Shi, X.; Lan, S.; Jin, H.; Wu, D. Effect of Melanoma Stem Cells on Melanoma Metastasis. Oncol. Lett. 2021, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Schatton, T.; Frank, M.H. Cancer Stem Cells and Human Malignant Melanoma. Pigment Cell Melanoma Res. 2008, 21, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Schatton, T.; Murphy, G.F.; Frank, N.Y.; Yamaura, K.; Waaga-Gasser, A.M.; Gasser, M.; Zhan, Q.; Jordan, S.; Duncan, L.M.; Weishaupt, C.; et al. Identification of Cells Initiating Human Melanomas. Nature 2008, 451, 345–349. [Google Scholar] [CrossRef]
- Peterson, S.C.; Eberl, M.; Vagnozzi, A.N.; Belkadi, A.; Veniaminova, N.A.; Verhaegen, M.E.; Bichakjian, C.K.; Ward, N.L.; Dlugosz, A.A.; Wong, S.Y. Basal Cell Carcinoma Preferentially Arises from Stem Cells within Hair Follicle and Mechanosensory Niches. Cell Stem Cell 2015, 16, 400–412. [Google Scholar] [CrossRef]
- Grachtchouk, M.; Pero, J.; Yang, S.H.; Ermilov, A.N.; Michael, L.E.; Wang, A.; Wilbert, D.; Patel, R.M.; Ferris, J.; Diener, J.; et al. Basal Cell Carcinomas in Mice Arise from Hair Follicle Stem Cells and Multiple Epithelial Progenitor Populations. J. Clin. Invest. 2011, 121, 1768–1781. [Google Scholar] [CrossRef]
- Jian, Z.; Strait, A.; Jimeno, A.; Wang, X.-J. Cancer Stem Cells in Squamous Cell Carcinoma. J. Invest. Dermatol. 2017, 137, 31–37. [Google Scholar] [CrossRef]
- Bonilla, X.; Parmentier, L.; King, B.; Bezrukov, F.; Kaya, G.; Zoete, V.; Seplyarskiy, V.B.; Sharpe, H.J.; McKee, T.; Letourneau, A.; et al. Genomic Analysis Identifies New Drivers and Progression Pathways in Skin Basal Cell Carcinoma. Nat. Genet. 2016, 48, 398–406. [Google Scholar] [CrossRef]
- Martincorena, I.; Roshan, A.; Gerstung, M.; Ellis, P.; Van Loo, P.; McLaren, S.; Wedge, D.C.; Fullam, A.; Alexandrov, L.B.; Tubio, J.M.; et al. Tumor Evolution. High Burden and Pervasive Positive Selection of Somatic Mutations in Normal Human Skin. Science 2015, 348, 880–886. [Google Scholar] [CrossRef]
- Royer-Bertrand, B.; Torsello, M.; Rimoldi, D.; El Zaoui, I.; Cisarova, K.; Pescini-Gobert, R.; Raynaud, F.; Zografos, L.; Schalenbourg, A.; Speiser, D.; et al. Comprehensive Genetic Landscape of Uveal Melanoma by Whole-Genome Sequencing. Am. J. Hum. Genet. 2016, 99, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; van der Weyden, L.; Schott, C.R.; Foote, A.; Constantino-Casas, F.; Smith, S.; Dobson, J.M.; Murchison, E.P.; Wu, H.; Yeh, I.; et al. Cross-Species Genomic Landscape Comparison of Human Mucosal Melanoma with Canine Oral and Equine Melanoma. Nat. Commun. 2019, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sanchez-Vega, F.; Chang, J.C.; Chatila, W.K.; Shoushtari, A.N.; Ladanyi, M.; Travis, W.D.; Busam, K.J.; Rekhtman, N. Lung-Only Melanoma: UV Mutational Signature Supports Origin from Occult Cutaneous Primaries and Argues against the Concept of Primary Pulmonary Melanoma. Mod. Pathol. 2020, 33, 2244–2255. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A. Resident Memory T Cells in Human Health and Disease. Sci. Transl. Med. 2015, 7, 269rv1. [Google Scholar] [CrossRef]
- Clark, R.A.; Watanabe, R.; Teague, J.E.; Schlapbach, C.; Tawa, M.C.; Adams, N.; Dorosario, A.A.; Chaney, K.S.; Cutler, C.S.; Leboeuf, N.R.; et al. Skin Effector Memory T Cells Do Not Recirculate and Provide Immune Protection in Alemtuzumab-Treated CTCL Patients. Sci. Transl. Med. 2012, 4, 117ra7. [Google Scholar] [CrossRef]
- Wulf, H.C.; Sandby-Møller, J.; Kobayasi, T.; Gniadecki, R. Skin Aging and Natural Photoprotection. Micron 2004, 35, 185–191. [Google Scholar] [CrossRef]
- Gniadecka, M.; Wulf, H.C.; Mortensen, N.N.; Poulsen, T. Photoprotection in Vitiligo and Normal Skin. A Quantitative Assessment of the Role of Stratum Corneum, Viable Epidermis and Pigmentation. Acta Derm. Venereol. 1996, 76, 429–432. [Google Scholar] [CrossRef]
- Lindberg, M.; Boström, M.; Elliott, K.; Larsson, E. Intragenomic Variability and Extended Sequence Patterns in the Mutational Signature of Ultraviolet Light. Proc. Natl. Acad. Sci. USA 2019, 116, 20411–20417. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).