Submitted:
16 May 2023
Posted:
18 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Quantitative RT-PCR
2.3. Agrobacterium-Mediated Infiltration
2.4. GUS Histochemical Staining
2.5. Subcellular Localization Analysis
2.6. Yeast Two-Hybrid
2.7. Bimolecular Fluorescence Complementarities
2.8. Firefly Luciferase Fragment Complementary Image Technique (LCI)
3. Results
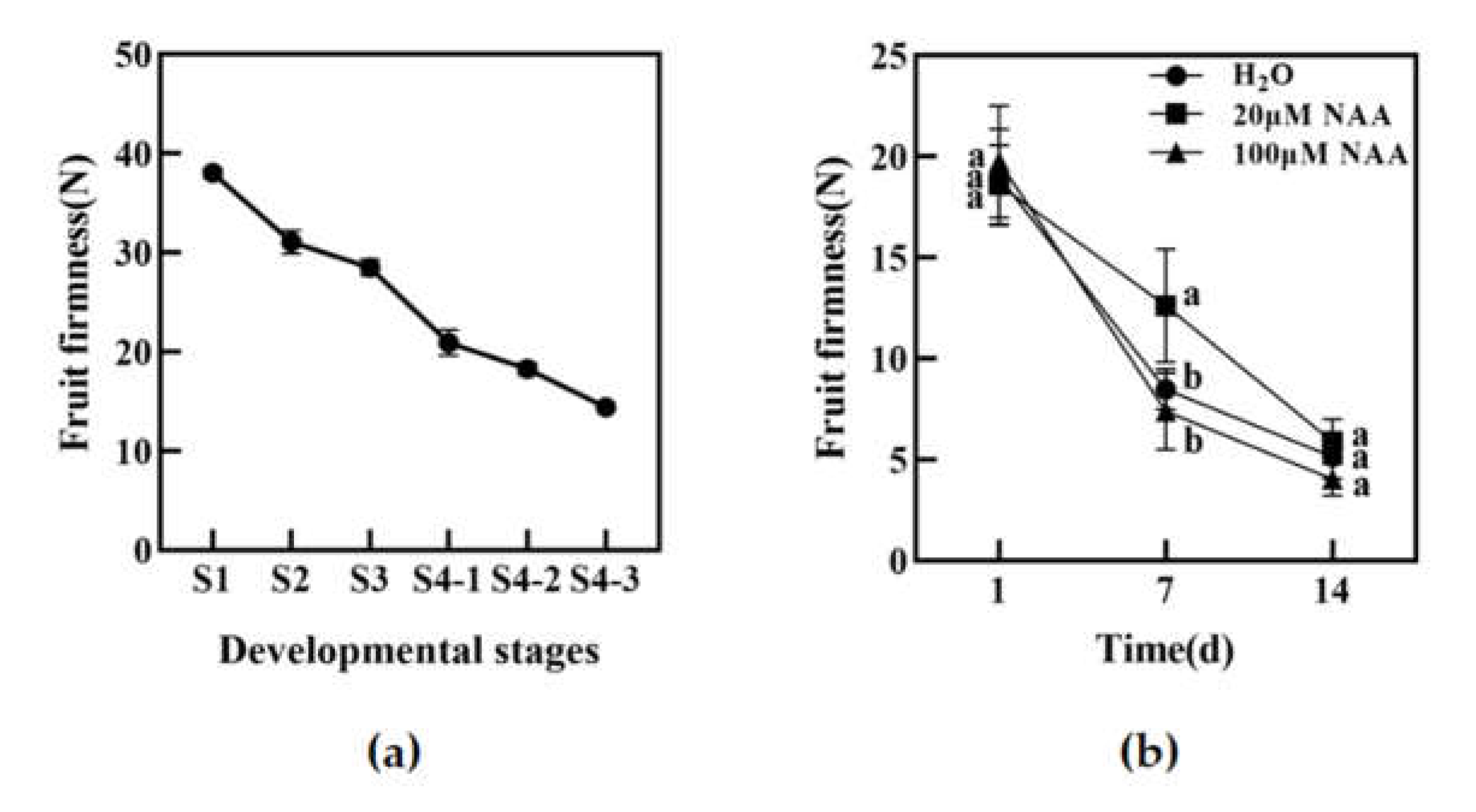
3.1. Fruit Firmness Decrease can be Delayed by Low Concentration NAA Treatment
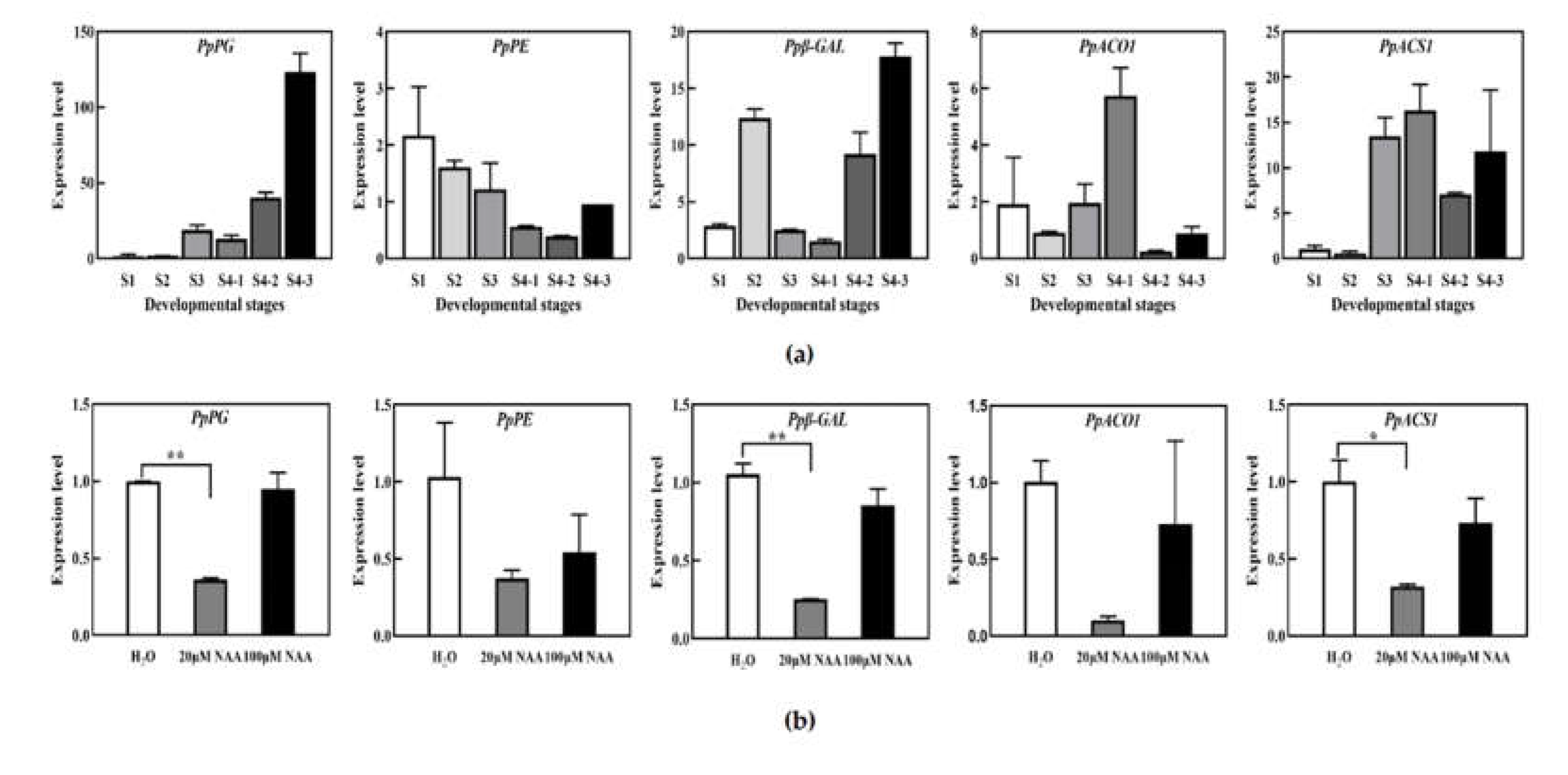
3.2. Low Concentration NAA Treatment Decreased the Activities of Softening-Related Enzymes in Peach Fruit
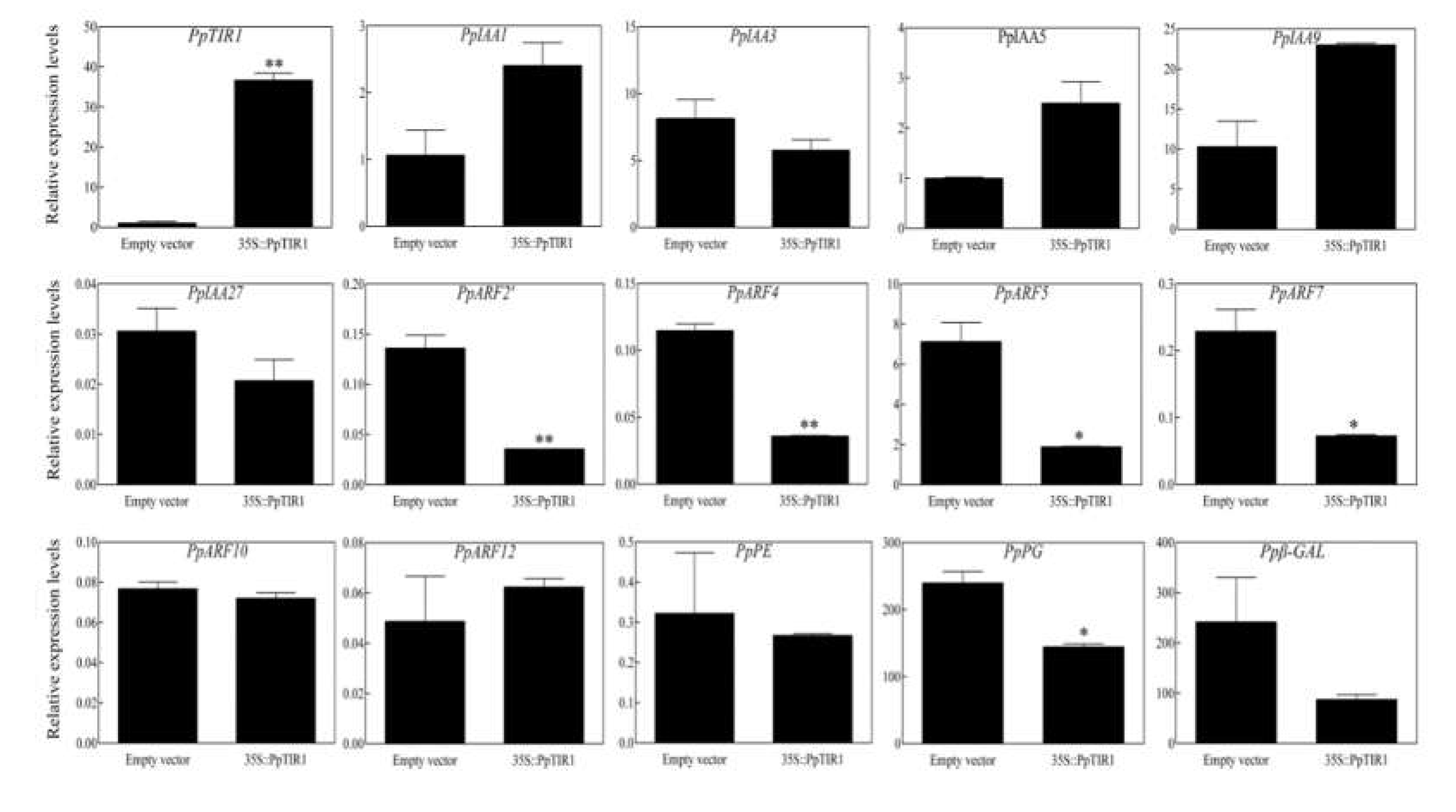
3.3. Transient Overexpression of PpTIR1 Gene Affects Expression of Auxin Signal Transduction Factors and Fruit Softening Related Genes
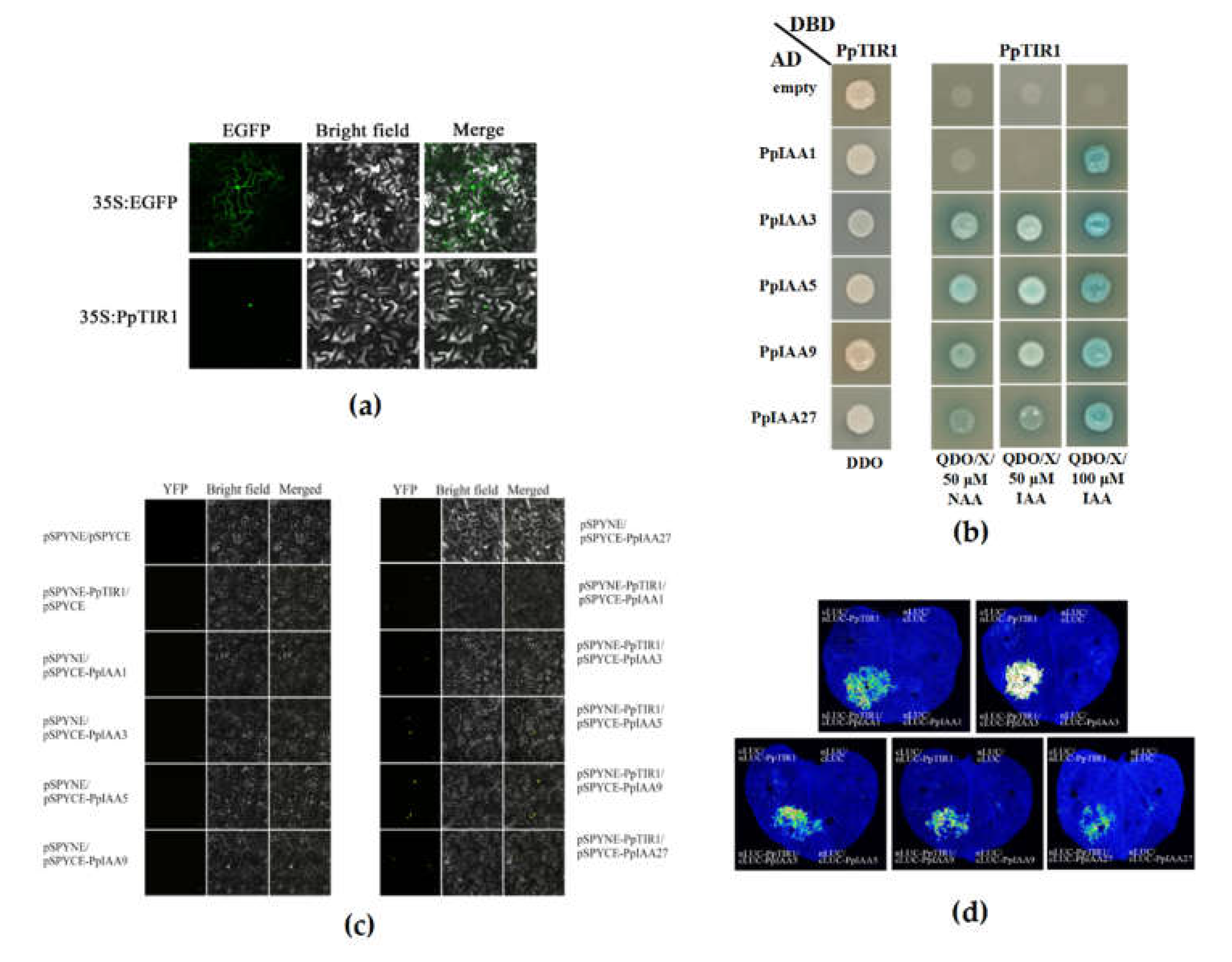
3.4. Yeast-2 Hybrid, BiFC, and Luciferase Reporter Assays Suggest that IAA and TIR1 Proteins may Directly Interact
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Castro, R.I.; González-Feliu, A.; Muñoz-Vera, M.; Valenzuela-Riffo, F.; Parra-Palma, C.; Morales-Quintana, L. Effect of exogenous auxin treatment on cell wall polymers of strawberry fruit. Int. J. Mol. Sci. 2021, 22, 6294. [Google Scholar] [CrossRef] [PubMed]
- Bttcher, C.; Boss, P.K.; Davies, C. , Delaying Riesling grape berry ripening with a synthetic auxin affects malic acid metabolism and sugar accumulation, and alters wine sensory characters. Funct. Plant Biol. 2012, 39, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Li. J.; Tao, X.; Li, L.; Mao, L.; Luo, Z.; Khan, Z.U.; Ying, T. Comprehensive RNA-Seq analysis on the regulation of tomato ripening by exogenous auxin. PLoS ONE. 2016, 11, e0156453. [Google Scholar]
- Yue, P.; Lu, Q.; Liu, Z.; Lv, T.; Li, X.; Bu, H.; 1, Liu, W. ; Xu, Y.; Yuan, H.; Wang, A. Auxin-activated MdARF5 induces the expression of ethylene biosynthetic genes to initiate apple fruit ripening. New Phytol. 2020, 226, 1781–1795. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, Y.X. Expression and regulation of pear 1-aminocyclopropane-1-carboxylic acid synthase gene (PpACS1a) during fruit ripening, under salicylic acid and indole-3-acetic acid treatment, and in diseased fruit. Mol. Biol. Rep. 2014, 41, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, I.; Sherif, S.M.; Jones, B.; Mila, I.; Kumar, P.P.; Bouzayen, M.; Jayasankar, S. TIR1-like auxin-receptors are involved in the regulation of plum fruit development. J. Exp. Bot. 2014, 65, 5205–5215. [Google Scholar] [CrossRef]
- Pan, L.; Zeng, W.; Liang, N.; Lu, Z.; Liu, H.; Cao, G.; Zhu, Y.; Chu, J.; Li, W.; Fang, W. PpYUC11, a strong candidate gene for the stony hard phenotype in peach (Prunus persica L. Batsch), participates in IAA biosynthesis during fruit ripening. J. Exp. Bot. 2015; 22, 7031–7044. [Google Scholar]
- Tadiello, A. , Ziosi, V., Negri, A.S., et al. On the role of ethylene, auxin and a GOLVEN-like peptide hormone in the regulation of peach ripening. BMC Plant Biol. 2016, 16, 44. [Google Scholar] [CrossRef]
- Miho, T.; Naoko, N.; Hiroshi, F.; Takehiko, S.; Michiharu, N.; Ken-Ichiro, H.; Hiroko, H.; Hirohito, Y.; Yuri, N. , Increased levels of IAA are required for system 2 ethylene synthesis causing fruit softening in peach (Prunus persica L. Batsch). J. Exp. Bot. 2013, 64, 1049–1059. [Google Scholar]
- Tatsuki, M.; Soeno, K.; Shimada,Y. ; Sawamura, Y.; Suesada, Y.; Yaegaki, H.; et al. Insertion of a transposonlike sequence in the 5′-flanking region of the YUCCA gene causes the stony hard phenotype. Plant J. 2018, 96, 815–827. [Google Scholar] [CrossRef]
- Trainotti, L.; Casadoro, L. The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J. Exp. Bot. 2007, 58, 3299–3308. [Google Scholar] [CrossRef]
- Leyser, O. Auxin signaling. Plant Physiol. 2018, 176:465–479.
- Ren, Z.; Li, Z.; Miao, Q.; Yang, Y.; Deng, W.; Hao, Y. , The auxin receptor homologue in Solanum lycopersicum stimulates tomato fruit set and leaf morphogenesis. J. Exp. Bot. 2011, 62, 2815–2826. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, J.; Cui, L.; Zhang, T.; Wu, Z.; Zhu, P.; Meng, Y.; Zhang, K.; Yu, X.; Lou, Q. New insights into the roles of cucumber TIR1 homologs and miR393 in regulating fruit/seed set development and leaf morphogenesis. BMC Plant Biol. 2017, 17, 1–14. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; Sherif, S.; El Kayal, W.; Jones, B.; Li, Z.; Sullivan, A.; Jayasankar, S. Overexpression of plum auxin receptor PslTIR1 in tomato alters plant growth, fruit development and fruit shelf-life characteristics. BMC Plant Biol. 2016, 16, 56. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; Sherif, S.M.; Jones, B.; Mila, I.; Kumar, P.; Bouzayen, M.; Jayasankar, S. TIR1-like auxin-receptors are involved in the regulation of plum fruit development. J. Exp. Bot. 2014, 65, 5205–5215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jones, B.; Li, Z.; Frasse, P.; Bouzayen, M. , The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell. 2005, 17, 2676–2692. [Google Scholar] [CrossRef] [PubMed]
- Bouzayen, M. Genome-wide identification, functional analysis and expression profiling of the Aux/IAA gene family in tomato. Plant Cell Physiol. 2012, 53, 659–672. [Google Scholar]
- Su, L.; Bassa, C.; Audran, C.; Mila, I.; Cheniclet, C.; Chevalier, C.; Bouzayen, M.; Roustan, J.; Chervin, C. The Auxin Sl-IAA17 transcriptional repressor controls fruit size via the regulation of endoreduplication-related cell expansion. Plant Cell Physiol. 2014, 55, 1969–1976. [Google Scholar] [CrossRef]
- Ding, Y.; Zeng, W.; Wang, X.; Wang, Y.; Niu, L.; Pan, L.; Lu, Z.; Cui, G.; Li, G.; Wang, Z. , Over-expression of peach PpIAA19 in tomato alters plant growth, parthenocarpy, and fruit Shape. J Plant Growth Regul. 2019, 38, 103–112. [Google Scholar] [CrossRef]
- Wu, F.; Guan, D.; Wang, W.; Wang, Q.; Yang, H.; Liu, Y. , Bioinformatics and expression pattern analysis of auxin receptor gene family in peach. Molecular plant breeding, 2021, 20, 6331–6340. [Google Scholar]
- Guan, D.; Hu, X.; Diao, D.; Wang, F.; Liu, Y. , Genome-wide analysis and identification of the Aux/IAA gene family in peach. Int. J. Mol. Sci. 2019, 20, 4703. [Google Scholar] [CrossRef]
- Diao, D.; Hu, X.; Guan, D.; Wang, F.; Yang, H.; Liu, Y. Genome-wide identification of the ARF (Auxin Response Factor) gene family in peach and their expression analysis. Mol. Biol. Rep. 2020, 47, 4331–4344. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Estelle, M. , Diversity and specificity: auxin perception and signaling through the TIR1/AFB pathway. Curr. Opin. Plant Biol. 2014, 21, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Valenzuela, T.; Muñoz-Espinoza, C.; Riveros, A.; Pedreschi, R.; Arús, P.; Campos-Vargas, R.; Meneses, C. Expression QTL (eQTLs) analyses reveal candidate genes associated with fruit flesh softening rate in peach [Prunus persica (L.) Batsch]. Front. Plant Sci. 2019, 10, 1581. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, Y. Expression and regulation of pear 1-aminocyclopropane-1-carboxylic acid synthase gene (PpACS1a) during fruit ripening, under salicylic acid and indole-3-acetic acid treatment, and in diseased fruit. Mol. Biol. Rep.. 2014, 41, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, Z.; Zhang, L.; Tan, D.; Wei, Y.; Yuan, H.; Li, T.; Wang, A. Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J. 2016, 88, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; González-Feliu, A.; Muñoz-Vera, M.; Valenzuela-Riffo, F.; Parra-Palma, C.; Morales-Quintana, L. Effect of exogenous auxin treatment on cell wall polymers of strawberry fruit. Int. J. Mol. Sci. 2021, 22, 6294. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Xie, Z.; Wang, C.; Shangguan, L.; Qian, N.; Cui, M.; Liu, Z.; Zheng, T.; Wang, M.; Fang, J. Abscisic acid, sucrose, and auxin coordinately regulate berry ripening process of the Fujiminori grape. Funct. Integr. Genomics. 2017, 17, 441–457. [Google Scholar] [CrossRef]
- Dal Santo, S.; Tucker, M.; Tan, H.; Burbidge, C.; Fasoli, M.; Böttcher, C.; Boss, P.; Pezzotti, M.; Davies, C. Auxin treatment of grapevine (Vitis vinifera L.) berries delays ripening onset by inhibiting cell expansion. Plant Mol. Biol. 2020, 103, 91–111. [Google Scholar] [CrossRef]
- Chen, H.; Ma, B.; Zhou, Y.; He, S.; Tang, S.; Lu, X.; Xie, Q.; Chen, S.; Zhang, J. , E3 ubiquitin ligase SOR1 regulates ethylene response in rice root by modulating stability of Aux/IAA protein. Proc. Natl. Acad. Sci. 2018, 115, 4513–4518. [Google Scholar] [CrossRef]
- Islam, E.S.; Sherif, S.M.; Brian, J.; Isabelle, M.; Kumar, P.P.; Mondher, B.; Subramanian, J. , TIR1-like auxin-receptors are involved in the regulation of plum fruit development. J. Exp. Bot. 2014, 65, 5205–5215. [Google Scholar]
- Chaabouni, S.; Jones, B.; Delalande, C.; Wang, H.; Li, Z.; Mila, I.; Frasse, P.; Latché, A.; Pech, J.; Bouzayen, M. , Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth. J. Exp. Bot. 2009, 60, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



