1. Introduction
Most heavy metals exist naturally in the environment, but some come from anthropogenic sources, such as industries, agriculture, burning of fossil fuels, insecticides, car exhaust, and sewage. These heavy metals in large quantities can become hazardous to the biological system. Particularly, cadmium (Cd), lead (Pb), zinc (Zn), and copper (Cu) affect the environment due to their non-biodegradability and accumulated toxicity [
1].
On the ground, all the inorganic elements that are necessary and essential for the normal growth and development of plants exist. Despite the fact that some heavy metals, such as copper (Cu), zinc (Zn), etc., are necessary for various enzymatic functions, excessive concentration of heavy metals could cause serious problems [
2,
3,
4], as they can become toxic and dangerous with serious environmental implications. Toxic heavy metals vary in nature and mode of accumulation, either in the soil or in the plants. Some of the most common sources of heavy metals in the soil are fertilizers, pesticides, and sewage sludge [
5].
Toxic metals such as cadmium (Cd) and lead (Pb), as well as many others, can easily end up in the higher members of the biological food chain and therefore in humans, causing serious diseases such as gastrointestinal tract (GIT) infection, cardiovascular problems, bone problems, and even becoming carcinogenic [
6,
7]. On the other hand, equally serious are the effects that heavy metals cause on the environment, such as soil pollution from heavy metals which is one of the most important problems of the planet. The term “soil pollution” refers to the concentration of polluting substances in it in quantities that cause a change in the composition of the soil, resulting in disturbances in the ecosystem. Some of these effects are summarized in
Table 1.
In order to limit the negative effects that heavy metals have on both humans and the environment, it is necessary to accurately determine the concentrations of heavy metals in their sources of accumulation.
Over the years, various techniques have been established for the detection of heavy metal ions (HMIs), including inductively coupled plasma mass spectrometry (ICP-MS) [
17], inductively coupled plasma optical emission spectrometry (ICP-OES) [
18], inductively coupled plasma atomic emission spectrometry (ICP-AES) [
19], flame atomic absorption spectrophotometry (FAAS) [
20], and atomic absorption spectroscopy (AAS) [
21], where sometimes the emphasis is on the parameters and sometimes on the choice of the analysis method. Spectrometric methods, such as atomic absorption (AAS) or inductively coupled plasma mass spectrometry (ICP-MS), although they provide high accuracy and sensitivity, are accompanied by certain limitations such as high cost and the fact that they are time-consuming and do not allow on-site measurements.
However, as already mentioned, researchers' interest in recent years has focused on the identification of heavy metals with the help of various electrochemical methods, particularly voltammetry, as it is an easy, fast, and relatively inexpensive way to determine them compared to other analytical methods. Although previous techniques are very sensitive and selective due to the limitations they cause, electrochemical methods such as voltammetry are preferred for the detection of heavy metals, which, in contrast to previous techniques, have the advantages of low cost, simplicity, ease of operation, fast analysis, portability, the ability to be applied for monitoring environmental samples in the field, as well as high sensitivity and selectivity. Electrochemical techniques, especially voltammetry, involve electroanalytical methods for the determination of one or more analytes by measuring the current as a function of potential. However, voltammetry is the only electrochemical method that has high sensitivity and can be applied for on-site recognition and detection of heavy metals [
22].
There are various types of voltammetry, such as cyclic voltammetry (CV), square wave voltammetry (SWV), linear sweep voltammetry (LSV), and differential pulse voltammetry (DPV) [
23]. The difference between these techniques lies in the time waveforms generated by the corresponding functional application [
24]. Additionally, DPV and SWV exhibit better detection sensitivity. Furthermore, electrochemical-stripping analysis (ESA) has historically been widely recognized as a powerful technique for detecting Pb, due to its remarkable sensitivity that allows the detection of Pb at trace and ultra-trace levels. It can also be easily combined with inexpensive and user-friendly instruments.
In order to detect heavy metals and examine their toxicity on both the environment and human health, we combine electrochemical techniques with certain modifiers that facilitate the detection process due to their characteristic properties.
Since Heyrovsky invented polarography in 1922, mercury has been established as the preferred electrode modifier for electrochemical-stripping analysis, due to its high hydrogen overpotential, which allows for its use in useful negative potentials. However, the need for alternative electrode modifiers arose, as everyone's attention began to turn more and more towards green chemistry, which aims to reduce the use and production of hazardous substances, such as mercury [
25], and since certain European regulations prohibit the export and storage of metallic mercury. Since then, several metals have been tested for their ability to replace mercury (e.g. bismuth). In addition, different organic or inorganic membranes have been evaluated for their potential application in detecting Pb with ESA.
In recent years, nanoparticles (NPs) have emerged as a promising area of research, replacing various electrode shaping media, thanks to their unique physicochemical properties that differ from their bulk counterparts. NPs are defined as particles with a dimension of at least 100 nm and are generally classified into four categories, including metal and metal oxide, semiconductors, polymers, and lipids. They exhibit a high surface area to volume ratio, which enhances their reactivity and allows them to interact with biological systems and the environment in unique ways. The ability of NPs to cross biological membranes and barriers has attracted considerable attention in various fields, including medicine, environmental science, and engineering, and they have been widely used for heavy metal detection, such as Cd, Pb, Zn, and Cu, due to their unique optical, electrical, and magnetic properties.
This review discusses mainly the use of voltammetry in the simultaneous detection of the presence of two or more heavy metals in different media using modified electrodes and presents a comprehensive overview of modifiers for various electrodes. There is a historical review from the abolition of mercury, to its replacement and the discovery of innovative nanoparticles, and presents their applications in chemistry and the environment. The present review also aims to summarize the different types of nanoparticles, such as metallic, semiconducting, and carbon-based nanoparticles, and their application in various electroanalytical techniques, including voltammetry.
2. Electrodes
Initially, the measurement technique is selected, with the most commonly used measurement techniques for detecting heavy metals being SW, SWASV, and DPASW. In the next step, the appropriate working electrode is selected, where carbon-based electrodes (CBE) are dominant. They appear to further improve the performance of voltammetric methods, as they are flexible, offer a wide potential window, and have desirable conductive and surface properties that allow for the sensitive determination of analytes. The four most common CBEs are glassy carbon electrode (GCE), graphite electrode (GE), carbon paste electrode (CPE), and screen-printed carbon electrode (SPCE). These electrodes have been widely used for the determination of heavy metal ions concentrations (Cd2+, Pb2+, and Zn2+), while some of the most common types of modified electrodes include, among others: nanoparticles-modified electrodes, chemically modified electrodes using chemical modifiers such as bismuth (Bi) and formerly mercury (Hg), carbon-based modified electrodes, and enzyme-modified electrodes.
Therefore, the appropriate electrode modifier is further investigated, as in our case where we work with GCE and our working electrode is modified with bismuth. In a recent study for the determination of lead, which belongs to heavy metals, and with GCE as the working electrode, modification was done with BFS (blast furnace slag), which is an economically efficient process and a new material in the field of detection with many promising results [
26]. A typical electrochemical analytical system consists mainly of three parts: an electrochemical detection device, an electrochemical detection instrument, and an electrolyte. The electrochemical detection instrument usually consists of three electrodes: a working electrode (WE), a reference electrode (RE), and a counter electrode (CE). After modifying the surfaces of the WEs using different materials, they can be used for the specific detection of various types of metallic ions. In some WEs, surface modification is necessary to obtain accurate and correct results, as in the case of the glassy carbon electrode (GCE), where polishing the surface with 0.1 mm and 0.05 mm alumina powder using a polishing cloth is required to produce a mirror-like surface [
22]. The electrodes are an important part when we want to use them for electrochemical detection, and they can greatly affect the sensitivity and selectivity of the analysis.
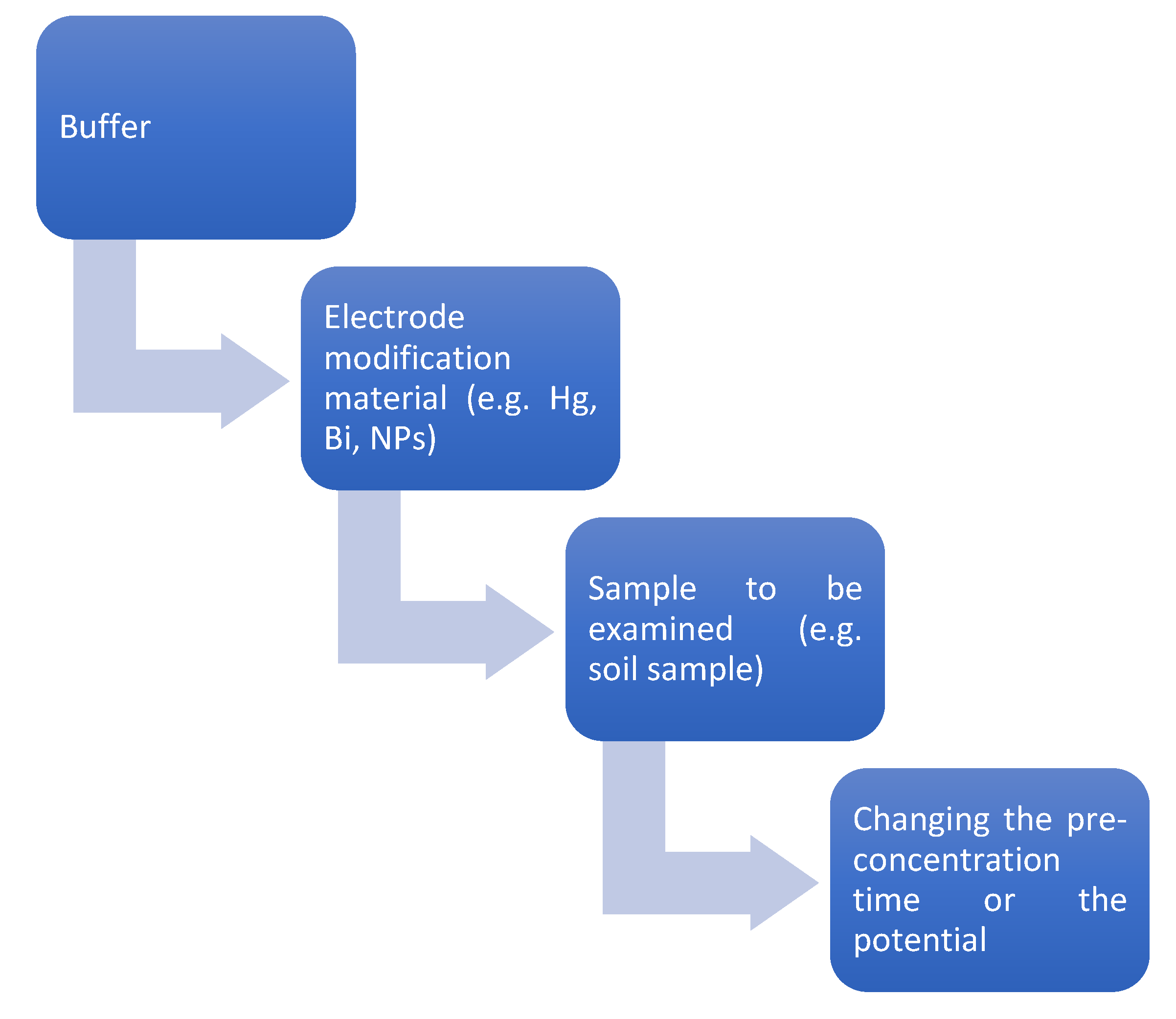
Figure 1.
Sample preparation procedures for measurement.
Figure 1.
Sample preparation procedures for measurement.
3. Modifiers
3.1. Mercury (Hg) and Bismuth as Electrode Modifiers for HMs Detection
In order to investigate the toxicity caused by heavy metals in soil, atmosphere, and consequently human health, and with the ultimate goal of limiting it, both techniques of Analytical Chemistry, specifically in our case voltammetry, and "supporting" means such as mercury (Hg), bismuth (Bi), while in recent years nanoparticles (NPs) have been used.
For many years, mercury was used as the material for modification working electrodes used in trace element detection due to its high sensitivity, reproducibility, and renewability. Mercury-shaped electrodes have been widely used for several decades in the detection of heavy metals using electrochemical techniques, thanks to their large cathodic window, reproducibility, sensitivity, and low background [
30,
31,
32].
However, mercury is a heavy metal that has become increasingly unpopular for use due to its intense toxicity and bioaccumulation in many species [
32,
33,
34,
35,
36]. The danger associated with mercury-shaped electrodes is their use, handling, and disposal due to their toxicity. In addition, it has been repeatedly shown that the absorption of Hg harms human health as it can lead to many serious problems, such as neurological consequences, as it penetrates the blood-brain barrier, memory loss, insomnia, neuromuscular changes, and various effects on the renal system.
Over the years, various materials have been proposed and tested to replace mercury in the electrode modification process, such as noble metals (Pt, Pd, Au, Ag) as well as other metals (Ru, Cu, Co, Ni, Pb, Sb, Bi, Al) [
37,
38]. Although it belongs to the heavy metals, the metal that prevailed due to its low toxicity [
32,
33,
35,
39,
40], as well as its similar electroanalytical properties to mercury, such as the wide potential window, simple preparation, partial insensitivity to dissolved oxygen, and the ability to form alloys with different metals [
32,
33,
34,
41,
42], is bismuth. Bismuth is also environmentally friendly [
43] and has mostly succeeded in replacing mercury successfully, as the latter is quite toxic. So, around 2000, electrodes modified with bismuth were introduced, which are constructed from a layer of bismuth deposited on a suitable substrate [
33,
44] and represent a very attractive alternative solution to the commonly used mercury electrodes [
45]. Many different materials have been used as electrode substrates, such as carbon, glassy carbon, carbon fibers, carbon paste, graphite, wax-impregnated graphite, gold, platinum [
34,
44]. The current peaks obtained in the voltammograms when using bismuth electrodes tend to be sharp and well-defined [
39], allowing for the reliable, fast, and economical recognition and quantification of metals present in the sample. Due to its characteristics, bismuth can be used as a film in electrodes, such as in glassy carbon electrodes (GCEs), which, in turn, find applications in various sample analyses (environmental, biological, etc.).
One application worth mentioning is the simultaneous detection of multiple heavy metals, which is carried out after the electrode of vitreous carbon is shaped with bismuth, and then various experimental parameters are optimized, such as the potential and deposition time, and finally the appropriate voltametric method is used, i.e. in this case the square wave voltammetry (SWV) [
46].
Electrochemical detection focuses on developing new electrode materials with better properties compared to commercial electrodes. The performance of voltametric determination of heavy metals depends heavily on the properties of the working electrode. Working electrodes can be modified with different materials to allow for specific recognition and concentration of metal ions. Additionally, it has been reported that the deposition of metal membranes on nanocarbon materials can further improve the electrochemically active surface [
25,
47]. Among these, the bismuth (Bi) film not only has low toxicity, high sensitivity, and strong response signal but can also form binary or multiple component alloys with heavy metal ions.
One of the earliest applications of a bismuth-modified electrode was for the determination of lead in water samples using Electrochemical Stripping Analysis (ESA), and because it is considered one of the least toxic metals, it has subsequently been used for analyses in the medical and pharmaceutical sectors [
48]. For approximately 20 years, bismuth-modified electrodes that emerged as a replacement for toxic mercury have found a wide range of environmental and clinical applications. Therefore, bismuth-Bi films are often combined with carbon materials for cooperative heavy metal detection. Hutton et al. [
49] used a bismuth film for stripping measurements of cobalt and cadmium internal soil extracts. Recently, Bi-modified electrodes have also been successfully used in electrochemical detection of nitrophenols, while bismuth oxides have been used in the detection of paracetamol.
3.2. Nanoparticles as Electrode Modifier for HMs Detection
As we have already mentioned, pollution from heavy metals is a significant issue, and currently, the addition of NPs with electrochemical sensors has developed a significant and innovative analytical technique for detecting heavy metals (HMs), as nanomaterials have been shown to offer remarkable properties as detection platforms. Nanomaterials could be considered a promising tool for the scientific community to detect toxic heavy metal ions, due to their sensitivity and selectivity. Over time, many different modification techniques have been explored. Recent studies have shown that NPs modified electrodes can be very useful in electrochemical sensor technology if designed and constructed correctly [
50]. Their surface area-to-volume ratio is high, and in combination with the particular characteristics exhibited by NPs, such as those based on metal and metal oxide, polymers, and carbon, make them beneficial for cleaning the environment from HMs [
51].
Nanotechnology and nanoparticles (NPs) have transformed science and technology. Today, this field has advanced to such a degree that it allows the development of production of nanoparticles using various physical, chemical, and even biological techniques. Among these techniques, the one that has stood out and is preferred more in the industrial sector for the production of nanoparticles due to its ease, the need for mild operating conditions, and the production of more environmentally friendly products and waste, is the biological method [
52]. Most industries today exploit the chemical properties of nanoparticles, as they are unique compared to their counterparts in volume, which are determined by their size, shape, composition, and surface chemistry and can be adapted to various applications. Some of the most important chemical properties of nanoparticles are [
53]:
The high surface-to-volume ratio: NPs have a high surface-to-volume ratio, which makes them extremely reactive. This property can be used in various applications, such as catalysis and sensors.
Surface energy: The surface energy of NPs is high due to the presence of unsaturated surface atoms. This property affects the agglomeration, stability, and dispersion of NPs.
Electromagnetic properties: NPs can exhibit unique electromagnetic properties due to their size, shape, and composition. For example, gold NPs exhibit localized surface plasmon resonance, which can be used for sensing and imaging applications.
Surface chemistry: The surface chemistry of NPs can be tailored by modifying their surface functional groups, which can change their surface reactivity and chemical properties.
Oxidation-reduction properties: NPs can exhibit unique oxidation-reduction properties due to their small size and large surface area. This property can be utilized in various applications, including energy storage and conversion.
The synthesis of NPs using the bioreduction method has drawn scientific interest, as it managed to overcome the drawbacks of using conventional chemical methods, such as thermodynamic stability, monodispersity, and particle formation [
54]. The biogenic synthesis of NPs presents some advantages over chemical synthesis, such as the absence of the need for high temperatures, toxic chemicals, pressure, energy, radiation processes, laser ablation, ultraviolet and ultrasonic fields, as well as the fact that the biomolecules required for NP synthesis are abundant and easily accessible, such as the availability in marine sources [
55]. On the other hand, NPs produced from precious metal groups such as gold (Au) and silver (Ag) exhibit interesting chemical and electromagnetic properties, such as chemical stability, conductivity, and good optical properties, due to their ability to interact with electromagnetic radiation, which produces many characteristic surface plasmon resonances (SPR), leading to their application in various fields such as gene therapy, biomedicine, and environmental improvement [
56,
57].
NPs, due to their large surface area, are excellent electron mediators and are suitable materials for improving electrodes. The use of materials based on silicon (Si), metallic nanoparticles (NPs), and carbon-based materials as electrode modifiers has been successful. As a result of the improved behavior of these NPs, sensors are constructed using a nanoscale electrode surface method that has increased the active surface area, catalytic activity, enhanced conductivity, and rapid movement of electrodes. These redesigned sensors can also exhibit size-dependent characteristics and have better functional units [
58]. Currently, the addition of these NPs to electrochemical sensors has developed a significant analytical technique for detecting heavy metals (HMs).
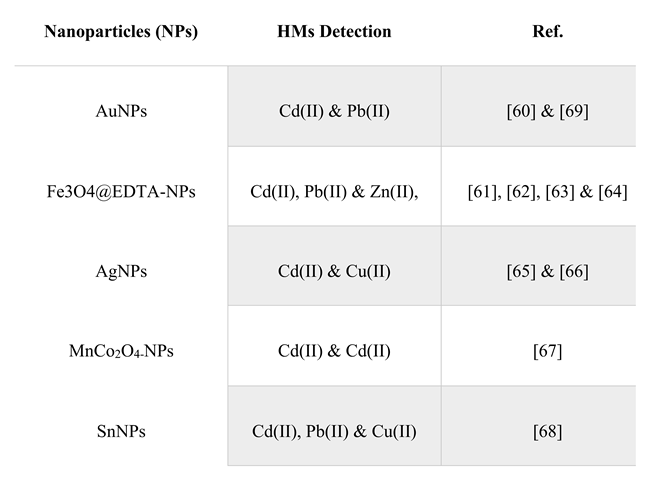
Gold is excellent for the fabrication of nanomaterials because gold nanoparticles (AuNPs) are characterized as excellent templates for the development of cutting-edge chemical and biological sensors, thanks to their unique physical and chemical properties. AuNPs can be easily produced and made very stable [
59]. They also have exceptional optical-electronic properties and, with the right linkers, offer a high surface-to-volume ratio, great biocompatibility, and finally, AuNPs provide a versatile substrate for attaching a wide variety of chemical or biological moieties, allowing for the selective capture and detection of small molecules and biological targets. However, different materials have been associated with AuNPs for the detection of HMs, particularly mercury and lead, while later, the same were used for the detection of Cd (II) and Pb(II) [
60].
Other NPs, such as superparamagnetic Fe3O4@EDTA, have been developed for the simultaneous adsorption and removal of Zn(II), Pb(II), and Cd(II) from different environmental water and soil samples. For this method, which has been proven to be simple, fast, effective, sensitive with high removal yields, good reproducibility and repeatability, electrodes modified with polymeric EDTA were used for the detection of various metallic ions at different pH values [
61,
62,
63]. Furthermore, after the adsorption process, easy separation is provided only by the application of an external magnetic field. In conclusion, this method is an effective and less time-consuming technique for the simultaneous removal of heavy metal ions targets in different environmental water and soil samples [
64].
While another category of NPs, AgNPs, which are used as electrode modifiers for the detection of heavy metal ions, such as Cd2, Cu2, have received significant attention due to some characteristics they exhibit, such as good electrical conductivity, high specific area, and easy synthesis method [
65,
66]. It is supported that when the electrochemical technique is combined with nanomaterials, very fast and efficient detection of heavy metals is obtained. For the simultaneous determination of lead and cadmium, MnCo2O4 nanoparticles were successfully used, which were morphed on a glassy carbon electrode. MnCo2O4 nanoparticles exhibit exceptional electrochemical properties such as fast current response, low detection limit, and good selectivity due to their unique structure [
67]. Lee et al. have used tin nanoparticles (SnNPs) with graphene oxide on a glassy carbon electrode to determine Cd2, Pb2, and Cu2 [
68].
Table 2.
Summary table of NPs-assisted detection of heavy metals.
Table 2.
Summary table of NPs-assisted detection of heavy metals.
As it seems, in addition to their electrocatalytic properties, these nanomaterial-based electrodes have the advantages of low cost, high sensitivity, and convenient functionality, making them highly promising for practical applications in heavy metal detection. However, further research is required to overcome potential issues and improve the stability and selectivity of these sensors.
4. Conclusions
Given that the global environmental burden from heavy metals and the associated impact on health and the environment are increasing, the interest in improving the quality of life and reducing their effects is ongoing. This review provides a general discussion on the field of electrochemical detection of HMs using bismuth-modified electrodes, which have replaced toxic mercury-modified electrodes, as well as nanomaterials as a more modern form of electrode modification, and their use in voltammetric experiments.
Certain materials, such as bismuth, have been distinguished for their ease of use, which is why researchers prefer them. The selection of suitable electrode modification materials is very important as they improve the electrochemical properties of the electrode, increase its effective surface area for the transfer of the electrochemical signal, and produce detectable signals suitable for the indirect detection of HMIs. For the detection of HMs, voltammetric methods have been distinguished as the most powerful, sensitive, and non-time-consuming.
In conclusion, the various nanoparticles that have been tested for the detection of heavy metals have shown significant results, due to the advantage of their large surface area compared to their size, as well as their electrocatalytic properties.
The purpose of the review is also to draw the attention of researchers working in electrochemistry, in order to develop new, improved morphology-controlled electrodes for the simultaneous detection of HMs at very low permissible limits (ppm, ppb) and thus reduce the quantity and toxicological burden of HMs in the environment [
70].
Abbreviations
HMs, Heavy metals; ICP-MS, Inductively coupled plasma mass spectrometry; FAAS, Flame atomic absorption spectrometry; AAS, Atomic absorption spectrometry; ICP-OES, Inductively Coupled Plasma Optical Emission Spectroscopy; ICP-AES, Inductively Coupled Plasma Atomic Emission Spectroscopy; LSV, Linear Sweep Voltammetry; WE, working electrode; ESA, Electrochemical-stripping analysis; RE, reference electrode; CE, counter electrode; EDTA, ethylenediamine tetraacetic acid; SPEs, screen printed electrodes; CTS, chitosan; AgNPs, Silver nanoparticle; AuNPs, Gold nanoparticles; NPs, Nanoparticles; SWASV, square wave anodic stripping voltammetry; CBE, carbon-based electrode; GE, graphite electrode; CPE, carbon paste electrode; SPCE, screen printed carbon electrode; BFs, blast furnace slag; Hg, Mercury; Bi, Bismuth; Cd, Cadmium; Pb, Lead; Zn, Zinc; Cu, Copper; Si, Silica; CV, Cyclic voltammetry; DPV, Different pulse voltammetry; GCE, Glassy carbon electrode; SWV, Square wave voltammetry
References
- G. Aragay, J. Pons, A. Merkoçi, (2011). “Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection”. Chem. Rev., 111, 3433-3458.
- Wang, C.; Li, W.; Guo, M.; Ji, J. Ecological risk assessment on heavy metals in soils: Use of soil diffuse reflectance mid-infrared Fourier-transform spectroscopy. Sci. Rep. 2017, 7, 40709. [Google Scholar] [CrossRef] [PubMed]
- Ming Li, Honglei Gou, Israa Al-Ogaidi & Nianqiang Wu, (2013). “ Nanostructured sensors for detection of heavy metals: a review”. ACS Sustain. ACS Sustainable Chemistry & Engineering, 1 (7), 713-723.
- Guascito, M.R.; Malitesta, C.; Mazzotta, E.; Turco, A. Inhibitive determination of metal ions by an amperometric glucose oxidase biosensor: Study of the effect of hydrogen peroxide decomposition. Sensors Actuators B: Chem. 2008, 131, 394–402. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hadi, P.; Chen, G.; McKay, G. Removal of cadmium ions from wastewater using innovative electronic waste-derived material. J. Hazard. Mater. 2014, 273, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent advances in metal-organic framework membranes for water treatment: A review. Sci. Total. Environ. 2021, 800, 149662. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A. Cadmium toxicity: effects on human reproduction and fertility. Rev. Environ. Heal. 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Bin Emran, T.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. - Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Wang, X. , Liu W., Li Z., Teng Y., Christie P. & Luo Y. (2020), “Effects of long-term fertilizer applications on peanut yield and quality and plant and soil heavy metal accumulation”. Pedosphere, 30, 555-562.
- Zhang, J. , Cao H., Zhang Y., Zhang Y., Ma J., Wang J., Gao Y., Zhang X., Zhang F. & Chu L., (2013). “Nephroprotective effect of calcium channel blockers against toxicity of lead exposure in mice”. Toxicol. Lett., 218, 273-280.
- Dotaniya, M.L. , Dotaniya C.K., Solanki P., Meena V.D. & Doutaniya R.K., (2020). “Lead Contamination and Its Dynamics in Soil-Plant System”. Part of the Radionuclides and Heavy Metals in the Environment book series. Lead in Plants and the Environment, 83-98.
- Martínez, C.E. & Motto H.L., (2000). “Solubility of lead, zinc and copper added to mineral soils”. Environ. Pollut., 107,153-158.
- Rattan, R.K. , Datta S.P., Chhonkar P.K., Suribabu K. & Singh A.K., (2005). “Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater-A case study”. Agric. Ecosyst. Environ., 109, 310322.
- Ciarkowska, K.; Gargiulo, L.; Mele, G. Natural restoration of soils on mine heaps with similar technogenic parent material: A case study of long-term soil evolution in Silesian-Krakow Upland Poland. Geoderma 2016, 261, 141–150. [Google Scholar] [CrossRef]
- Koelmel, J.; Amarasiriwardena, D. Imaging of metal bioaccumulation in Hay-scented fern (Dennstaedtia punctilobula) rhizomes growing on contaminated soils by laser ablation ICP-MS. Environ. Pollut. 2012, 168, 62–70. [Google Scholar] [CrossRef]
- A.M. Massadeh, A.A. Alomary, S. Mir, F.A. Momani, H.I. Haddad, Y.A. Hadad, (2016). “Analysis of Zn, Cd, As, Cu, Pb, and Fe in snails as bioindicators and soil samples near traffic road by ICP-OES”. Environ. Sci. Pollut. Res., 23 (13), 13424-13431.
- K. Sreenivasa Rao, T. Balaji, T. Prasada Rao, Y. Babu, G.R.K. Naidu, (2002). “Determination of iron, cobalt, nickel, manganese, zinc, copper, cadmium and lead in human hair by inductively coupled plasma-atomic emission spectrometry”. Spectrochim. Acta Part B: At. Spectrosc., 57 (8), 1333-1338.
- Daşbaşı, T.; Saçmacı. ; Çankaya, N.; Soykan, C. A new synthesis, characterization and application chelating resin for determination of some trace metals in honey samples by FAAS. Food Chem. 2016, 203, 283–291. [Google Scholar] [CrossRef]
- Siraj, K.; Kitte, S.A. Analysis of Copper, Zinc and Lead using Atomic Absorption Spectrophotometer in ground water of Jimma town of Southwestern Ethiopia. Int. J. Chem. Anal. Sci. 2013, 4, 201–204. [Google Scholar] [CrossRef]
- Yuanyuan Lua, Xinqiang Lianga, Christophe Niyungekoa, Junjie Zhoua, Jianming Xua & Guangming Tian, (2018), “A review of the identification and detection of heavy metal ions in the environment by voltammetry”. Talanta, 178, 324-338.
- Silva, J.J.; Paim, L.L.; Stradiotto, N.R. Simultaneous Determination of Iron and Copper in Ethanol Fuel Using Nafion/Carbon Nanotubes Electrode. Electroanalysis 2014, 26, 1794–1800. [Google Scholar] [CrossRef]
- J.G. Osteryoung, R.A. Osteryoung, (1985). “Square wave voltammetry”. Anal. Chem., 57 (1), 101A-110A.
- S. Armenta, S. Garrigues, M. de la Guardia, (2008) “Green Analytical Chemistry”. Trends Anal. Chem., 27, 497.
- Mourya, A.; Mazumdar, B.; Sinha, S.K. Determination and quantification of heavy metal ion by electrochemical method. J. Environ. Chem. Eng. 2019, 7, 103459. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T. Recent developments and applications of screen-printed electrodes in environmental assays—A review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Rico, M. .G.; Olivares-Marín, M.; Gil, E.P. Modification of carbon screen-printed electrodes by adsorption of chemically synthesized Bi nanoparticles for the voltammetric stripping detection of Zn(II), Cd(II) and Pb(II). Talanta 2009, 80, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Khaled, E.; Hassan, H.; Habib, I.; Metelka, R. Chitosan Modified Screen-Printed Carbon Electrode for Sensitive Analysis of Heavy Metals. Int. J. Electrochem. Sci. 2010, 5, 158–167. [Google Scholar] [CrossRef]
- Domingos, R.F.; Huidobro, C.; Companys, E.; Galceran, J.; Puy, J.; Pinheiro, J. Comparison of AGNES (absence of gradients and Nernstian equilibrium stripping) and SSCP (scanned stripping chronopotentiometry) for trace metal speciation analysis. J. Electroanal. Chem. 2008, 617, 141–148. [Google Scholar] [CrossRef]
- Aguilar, D.; Galceran, J.; Companys, E.; Puy, J.; Parat, C.; Authier, L.; Potin-Gautier, M. Non-purged voltammetry explored with AGNES. Phys. Chem. Chem. Phys. 2013, 15, 17510–17521. [Google Scholar] [CrossRef]
- L Cao and J Jia 2008 Electrochimica Acta 53 2177.
- C Kokkinos, I Raptis, A Economou and T Speliotis 2009 Procedia Chemistry 1 1039.
- A Economou 2005 Trends in Analytical Chemistry 24-4 334.
- C Kokkinos, A Economou 2007 Electrochemistry Communications 9 2795.
- E Hutton, B Ogoreve, S Hocevar, F Weldon, M R Smyth and J Wang 2001 Electrochemistry Communications 3 707.
- F. Arduini & J. Quintana, (2010) Trends in Analytical Chemistry, 2-11, 1295.
- R. Pauliukaite, S. Hocevar, B. Ogorevc & J. Wang (2004) Electroanalysis, 16, 719.
- J. Wang (2005) Electroanalysis 17, 1341.
- H. Xu & L. Zeng, (2008) Food Chemistry, 109, 834.
- S Hocevar S, I Svancara, K Vytras & I Svancara, (2005) Electrochemica Acta 51 706.
- L Baldrianova, I Svancara, M Vicek, A Economou & S Sotiropoulos, (2006).
- Kean Sam, (2011). “The Disappearing Spoon (and other true tales of madness, love, and the history of the world from the Periodic Table of Elements)”. New York/Boston: Back Bay Books, 158-160.
- C Kokinos and A Economous 2008 Electrochimica Acta 53 294.
- Joseph Wang, (2005). “Stripping Analysis at Bismuth Electrodes: A Review” Electroanalysis, 17(15-16), 1341-1346.
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Ceren Yıldız, Dilek Eskiköy Bayraktepe & Zehra Yazan (2021) “Highly sensitive direct simultaneous determination of zinc(II), cadmium(II), lead(II), and copper(II) based on in-situ-bismuth and mercury thin-film plated screen-printed carbon electrode”. Monatshefte für Chemie - Chemical Monthly volume 152, pages1527–1537.
- A.V. Naumov, (2007), “World market of bismuth: A review”. Russian Journal of Non-Ferrous Metals, 48 (1), 10.
- E. A. Hutton, J. T. van Elteren, B. Ogorevc, M. Smyth, (2004). “Validation of bismuth film electrode for determination of cobalt and cadmium in soil extracts using ICP-MS”. Talanta, 63, 849.
- Hassan, M.H.; Khan, R.; Andreescu, S. Advances in electrochemical detection methods for measuring contaminants of emerging concerns. Electrochem. Sci. Adv. 2021, 2, e2100184. [Google Scholar] [CrossRef]
- Ali, Z.; Ullah, R.; Tuzen, M.; Ullah, S.; Rahim, A.; Saleh, T.A. Colorimetric sensing of heavy metals on metal doped metal oxide nanocomposites: A review. Trends Environ. Anal. Chem. 2023, 37. [Google Scholar] [CrossRef]
- I.B.M. Ibraheem, B.E.E. Abd-Elaziz, W.F. Saad, W.A. Fathy, (2016) “Green biosynthesis of silver nanoparticles using marine Red Algae Acanthophora specifera and its antimicrobial activity”. J. Nanomed. Nanotechnol., 7 (409), 1-4.
- Nadeem Joudeh & Dirk Linke, (2022) “Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists”. Journal of Nanobiotechnology, 20 (262).
- S. Li, Y. Niu, H. Chen & P. He, (2021) «Complete genome sequence of an Arctic Ocean bacterium Shewanella sp. Arc9-LZ with capacity of synthesizing silver nanoparticles in darkness». Mar. Genom., 56 (100808).
- Abdel-Raoof, A.M.; El-Shal, M.A.; Said, R.A.M.; Abostate, M.H.; Morshedy, S.; Emara, M.S. Versatile Sensor Modified with Gold Nanoparticles Carbon Paste Electrode for Anodic Stripping Determination of Brexpiprazole: A Voltammetric Study. J. Electrochem. Soc. 2019, 166, B948–B955. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Panahi, A.; Levendis, Y.A.; Vorobiev, N.; Schiemann, M. Direct observations on the combustion characteristics of Miscanthus and Beechwood biomass including fusion and spherodization. Fuel Process. Technol. 2017, 166, 41–49. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Li, Y.; Zeng, T.; Wan, Q.; Yang, N. Morphology-controlled electrochemical sensing of environmental Cd2+ and Pb2+ ions on expanded graphite supported CeO2 nanomaterials. Anal. Chim. Acta 2020, 1126, 63–71. [Google Scholar] [CrossRef]
- Adeniji, T.M.; Stine, K.J. Nanostructure Modified Electrodes for Electrochemical Detection of Contaminants of Emerging Concern. Coatings 2023, 13, 381. [Google Scholar] [CrossRef]
- Sawan, S.; Maalouf, R.; Errachid, A.; Jaffrezic-Renault, N. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. TrAC Trends Anal. Chem. 2020, 131, 116014. [Google Scholar] [CrossRef]
- Üstündağ, Z.; Solak, A.O. EDTA modified glassy carbon electrode: Preparation and characterization. Electrochimica Acta 2009, 54, 6426–6432. [Google Scholar] [CrossRef]
- Md. Aminur Rahman, Misook Won, Y. Shim, (2003). “Characterization of an EDTA bonded conducting polymer modified electrode: its application for the simultaneous determination of heavy metal ions”. Anal. Chem., 75 (5), 1123-1129.
- Rahman, A.; Park, D.S.; Won, M.-S.; Park, S.-M.; Shim, Y.-B. Selective Electrochemical Analysis of Various Metal Ions at an EDTA Bonded Conducting Polymer Modified Electrode. Electroanalysis 2004, 16, 1366–1370. [Google Scholar] [CrossRef]
- Ensieh Ghasemi, Akbar Heydari & Mika Sillanpää, (2017). “Superparamagnetic Fe3O4@EDTA nanoparticles as an efficient adsorbent for simultaneous removal of Ag(I), Hg(II), Mn(II), Zn(II), Pb(II) and Cd(II) from water and soil environmental samples”. Microchemical Journal, 131, 51-56.
- Hassan, K.M.; Elhaddad, G.M.; AbdelAzzem, M. Voltammetric determination of cadmium(II), lead(II) and copper(II) with a glassy carbon electrode modified with silver nanoparticles deposited on poly(1,8-diaminonaphthalene). Microchim. Acta 2019, 186, 440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, N.; Zhang, L.; Xu, J.; Wang, H.; Wang, C.; Geng, T. Amperometric sensing of hydrogen peroxide using a glassy cabon electode modified with silver nanoparticles on poly(alizarin yellow R). Microchim. Acta 2011, 173, 135–141. [Google Scholar] [CrossRef]
- Antunović, V.; Ilić, M.; Baošić, R.; Jelić, D.; Lolić, A. Synthesis of MnCo2O4 nanoparticles as modifiers for simultaneous determination of Pb(II) and Cd(II). PLOS ONE 2019, 14, e0210904. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.M.; Chen, Z.; Li, L.; Liu, E. Reduced graphene oxide decorated with tin nanoparticles through electrodeposition for simultaneous determination of trace heavy metals. Electrochimica Acta 2015, 174, 207–214. [Google Scholar] [CrossRef]
- Sreerama Amrutha Lahari, Khairunnisa Amreen, Satish Kumar Dubey, R.N. Ponnalagu & Sanket Goel, (2023). “Optimized porous carbon-fibre microelectrode for multiplexed, highly reproducible and repeatable detection of heavy metals in real water samples”. Environmental Research, 220, 115192.
- Rassaei, L.; Marken, F.; Sillanpää, M.; Amiri, M.; Cirtiu, C.M.; Sillanpää, M. Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 2011, 30, 1704–1715. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).