Submitted:
06 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Gunkel, S.; Meusemann, K.; Kozlov, A.; Podsiadlowski, L.; Petersen, M.; Lanfear, R.; et al. Evolutionary history of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World, 2nd ed.; The Johns Hopkins University Press: Baltimore, USA, 2007. [Google Scholar]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; Vanbergen, A.J. Safeguarding pollinators and their values to human well-being. Nature 2016, 540(7632), 220–229. [Google Scholar] [CrossRef]
- Cane, J.H.; Sipes, S. Characterizing floral specialization by bees: analytical methods and revised lexicon for oligolecty. In Plant-pollinator Interactions: from Specialization to Generalization; Waser, N.M., Ollerton, J., Eds. The University of Chicago Press: Chicago, USA, 2006; pp. 99–121. [Google Scholar]
- Goulson, D. Bumblebees: Behaviour, Ecology, and Conservation; Oxford University Press Inc.: New York, USA, 2010. [Google Scholar]
- Minckley, R.L.; Roulston, T.H. Incidental mutualisms and pollen specialization among bees. In Plant-pollinator Interactions: from Specialization to Generalization; Waser, N.M., Ollerton, J., Eds. The University of Chicago Press: Chicago, USA, 2006; pp. 69–98. [Google Scholar]
- Bossert, S.; Wood, T.J.; Patiny, S.; Michez, D.; Almeida, E.A.B.; Minckley, R.L.; Packer, L.; Neff, J.L.; Copeland, R.S.; Straka, J.; et al. Phylogeny, biogeography and diversification of the mining bee family Andrenidae. Syst. Entomol. 2022, 47(2), 283–302. [Google Scholar] [CrossRef]
- Larkin, L.L.; Neff, J.L.; Simpson, B.B. The evolution of pollen diet: host choice and diet breadth of Andrena bees (Hymenoptera: Andrenidae). Apidologie 2008, 39, 133–145. [Google Scholar] [CrossRef]
- Wood, T.J.; Roberts, S.P.M. An assessment of historical and contemporary diet breadth in polylectic Andrena bee species. Biol. Conserv. 2017, 215, 72–80. [Google Scholar] [CrossRef]
- Luan, F.; Zeng, J.; Yang, Y.; He, X.; Wang, B.; Gao, Y.; Zeng, N. Recent advances in Camellia oleifera Abel: A review of nutritional constituents, biofunctional properties, and potential industrial applications. J. Funct. Food. 2020, 75, 104242. [Google Scholar] [CrossRef]
- Wang, X.N. Research on phenology and blossom biology of oil-tea Camellia. Master Thesis, Central South University of Forestry and Technology, Changsha, China, 2011. [Google Scholar]
- Huang, D.Y.; Ding, L.; Zhang, Y.Z.; Huang, H.R.; Yu, J.F.; Hao, J.S.; Zhu, C.D. Life history and relevant biological features of Andrena camellia Wu (Hymenoptera: Andrenidae). Acta Entomol. Sin. 2008, 51(7), 778–783. [Google Scholar]
- Xie, Z.; Chen, X.; Qiu, J. Reproductive failure of Camellia oleifera in the plateau region of China due to a shortage of legitimate pollinators. Int. J. Agric. Biol. 2013, 15, 458–464. [Google Scholar]
- Zhao, S.W. Management measure for honey colony in flowering period of Camellia oleifera. Apicult. China 1993, 5, 19–20. [Google Scholar]
- Ding, L.; Huang, D.Y.; Zhang, Y.Z.; Huang, H.R.; Li, J.; Zhu, C.D. Observation on the nesting biology of Andrena camellia Wu (Hymenoptera: Andrenidae). Acta Entomol. Sin. 2007, 50(10), 1077–1082. [Google Scholar]
- He, B.; Su, T.J.; Niu, Z.Q.; Zhou, Z.Y.; Gu, Z.Y.; Huang, D.Y. Characterization of mitochondrial genomes of three Andrena bees (Apoidea: Andrenidae) and insights into the phylogenetics. Int. J. Biol. Macromol. 2019, 127, 118–125. [Google Scholar] [CrossRef]
- Su, T.J.; He, B.; Zhao, F.; Jiang, K.; Lin, G.; Huang, Z. Population genomics and phylogeography of Colletes gigas, a wild bee specialized on winter flowering plants. Ecol. Evol. 2022, 12(4), e8863. [Google Scholar] [CrossRef]
- Wu, Y.R. The pollinating bees on Camellia olifera with descriptions of 4 new species of the genus Andrena. Acta Entomol. Sin. 1977, 20, 199–204. [Google Scholar]
- Li, H.Y.; Luo, A.C.; Hao, Y.J.; Dou, F.Y.; Kou, R.M.; Orr, M.C.; Zhu, C.D.; Huang, D.Y. Comparison of the pollination efficiency of Apis cerana with wild bees in oil-seed camellia fields. Basic Appl. Ecol. 2021, 56, 250–258. [Google Scholar] [CrossRef]
- Kang, X.D.; Fan, Z.Y. Toxic contents of nectar of oil-tea flowers to honey bees. J. Bee 1991, 1, 8–10. [Google Scholar]
- Li, Z.; Huang, Q.; Zheng, Y.; Zhang, Y.; Li, X.; Zhong, S.; Zeng, Z. Identification of the toxic compounds in Camellia oleifera honey and pollen to honey bees (Apis mellifera). J. Agric. Food Chem. 2022, 70, 13176–13185. [Google Scholar] [CrossRef]
- Holden, H.M.; Rayment, I.; Thoden, J.B. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J. Biol. Chem. 2003, 278(45), 43885–43888. [Google Scholar] [CrossRef]
- Vinson, C.C.; Mota, A.P.Z.; Porto, B.N.; Oliveira, T.N.; Sampaio, I.; Lacerda, A.L.; Danchin, E.G.J.; Guimaraes, P.M.; Williams, T.C.R.; Brasileiro, A.C.M. Characterization of raffinose metabolism genes uncovers a wild Arachis galactinol synthase conferring tolerance to abiotic stresses. Sci. Rep. 2020, 10(1), 15258. [Google Scholar] [CrossRef]
- Elango, D.; Rajendran, K.; Van der Laan, L.; Sebastiar, S.; Raigne, J.; Thaiparambil, N.A.; El Haddad, N.; Raja, B.; Wang, W.; Ferela, A.; Chiteri, K.O.; Thudi, M.; Varshney, R. K.; Chopra, S.; Singh, A.; Singh, A.K. Raffinose family oligosaccharides: friend or foe for human and plant health? Front. Plant Sci. 2022, 13, 829118. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34(17), i884–i890. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31(10), 1674–1676. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: architecture and applications. BMC Bioinformatics 2009, 10, 421. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32(1), 268–274. [Google Scholar] [CrossRef]
- Slater, G. S. C.; Birney, E. Automated generation of heuristics for biological sequence comparison. BMC Bioinform. 2005, 6, 31. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34(12), 3299–3302. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal omega. Curr. Protoc. Bioinf. 2014, 48, 3.13.1–3.13.16. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Yang, Z.; Wong, W.S.W.; Nielsen, R. Bayes empirical Bayes inference of amino acid sites under positive selection. Mol. Biol. Evol. 2005, 22, 1107–1118. [Google Scholar] [CrossRef]
- Zhang, J.; Nielsen, R.; Yang, Z. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol. Biol. Evol. 2005, 22, 2472–2479. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29(7), 644–U130. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22(13), 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lomsadze, A.; Borodovsky, M. Identification of protein coding regions in RNA transcripts. Nucl. Acid. Res. 2015, 43(12), e78. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20(1), 238. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14(4), 417–419. [Google Scholar] [CrossRef] [PubMed]
- Kucukural, A.; Yukselen, O.; Ozata, D.M.; Moore, M.J.; Garber, M. DEBrowser: interactive differential expression analysis and visualization tool for count data. BMC Genomics 2019, 20(1), 6. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25(16), 2078–2079. [Google Scholar] [CrossRef]
- Shen, W.; Le, S.; Li, Y.; Hu, F. SeqKit: A cross-platform and ultrafast toolkit for FASTA/Q File manipulation. PLoS ONE 2016, 11(10), e0163962. [Google Scholar] [CrossRef]
- Wen, Y.; Su, S.C.; Ma, L.Y.; Yang, S.Y.; Wang, Y.W.; Wang, X.N. Effects of canopy microclimate on fruit yield and quality of Camellia oleifera. Sci. Horticult. 2018, 235, 132–141. [Google Scholar]
- Feng, J.L.; Jiang, Y.; Yang, Z.J.; Chen, S.P.; El-Kassaby, Y.A.; Chen, H. Marker-assisted selection in C. oleifera hybrid population. Silvae Genet. 2020, 69(1), 63–72. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, X.; Liu, Y. The role of native bees on the reproductive success of Camellia oleifera in Hunan Province, Central South China. Acta Ecol. Sin. 2010, 30, 4427–4436. [Google Scholar]
- Huang, D.; Su, T.; Qu, L.; Wu, Y.; Gu, P.; He, B.; Xu, X.; Zhu, C. The complete mitochondrial genome of the Colletes gigas (Hymenoptera: Colletidae: Colletinae). Mit. DNA Part A 2016, 27(6), 3878–3879. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; He, B.; Gu, P.; Su, T.J.; Zhu, C.D. Discussion on current situation and research direction of pollination insects of Camellia oleifera. J. Environ. Entomol. 2017, 39, 213–220. [Google Scholar]
- Zhou, Q.S.; Luo, A.; Zhang, F.; Niu, Z.Q.; Wu, Q.T.; Xiong, M.; Orr, M.C.; Zhu, C.D. The first draft genome of the plasterer bee Colletes gigas (Hymenoptera: Colletidae: Colletes). Genome Biol. Evol. 2020, 12(6), 860–866. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.C.; Klein, A. Glycoprotein lysosomal storage disorders: K- and L-mannosidosis, fucosidosis and K-N-acetylgalactosaminidase deficiency. Biochim. Biophys. Acta 1999, 1455, 69–84. [Google Scholar] [CrossRef]
- Vallender, E.J.; Lahn, B.T. Positive selection on the human genome, Human Mol. Genet. 2004, 13, R245–R254. [Google Scholar]
- Pasquali, M.; Yu, C.; Coffee, B. Laboratory diagnosis of galactosemia: a technical standard and guideline of the American college of medical genetics and genomics (ACMG). Genet. Med. 2018, 20, 3–11. [Google Scholar] [CrossRef]
- Verdino, A.; D’Urso, G.; Tammone, C.; Scafuri, B.; Marabotti, A. Analysis of the structure-function-dynamics relationships of GALT enzyme and of its pathogenic mutant p.Q188R: a molecular dynamics simulation study in different experimental conditions. Molecules 2021, 26, 5941. [Google Scholar] [CrossRef]
- Timson, D.J.; Reece, R.J. Functional analysis of disease-causing mutations in human galactokinase. Eur. J. Biochem. 2003, 270(8), 1767–1774. [Google Scholar] [CrossRef]
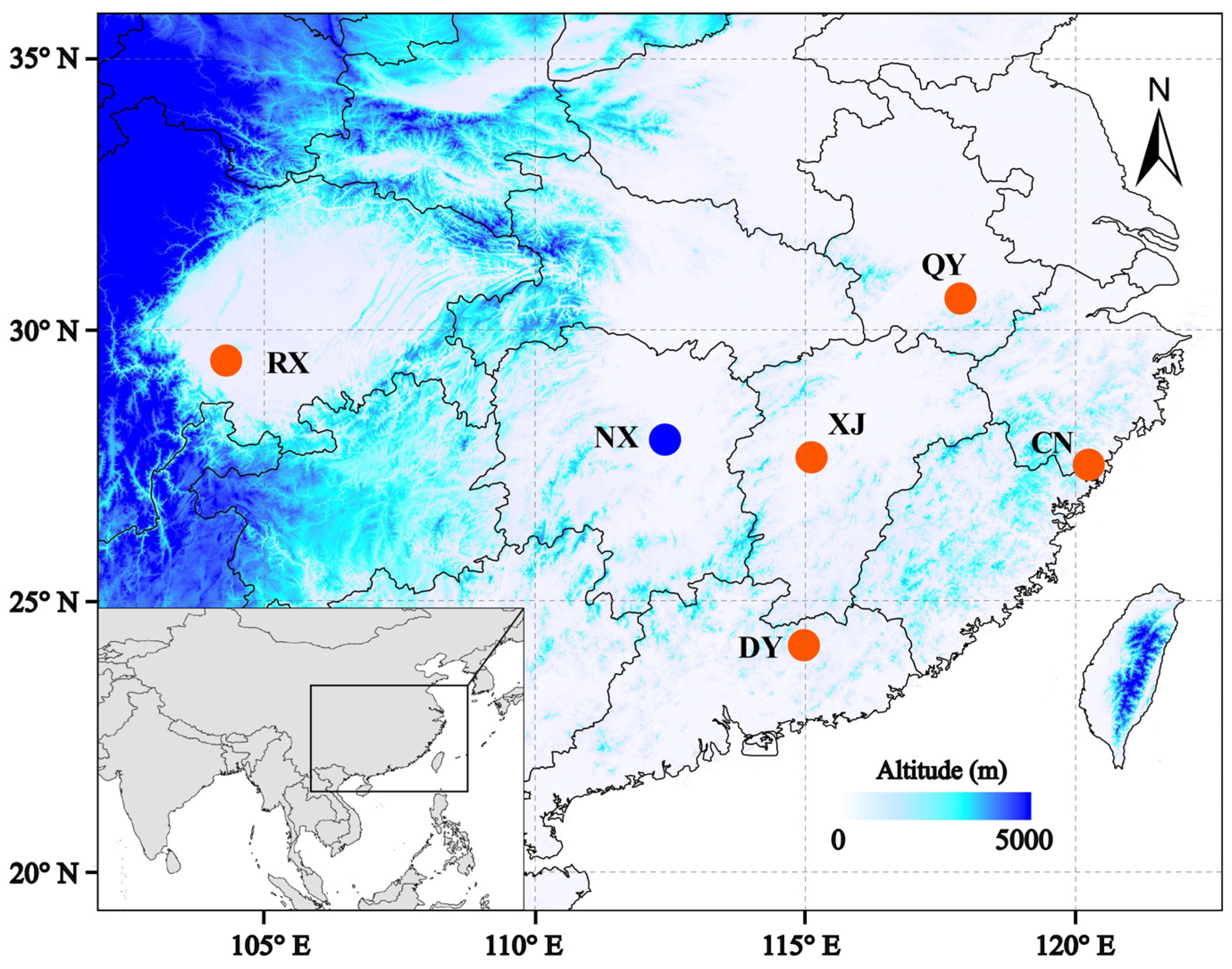
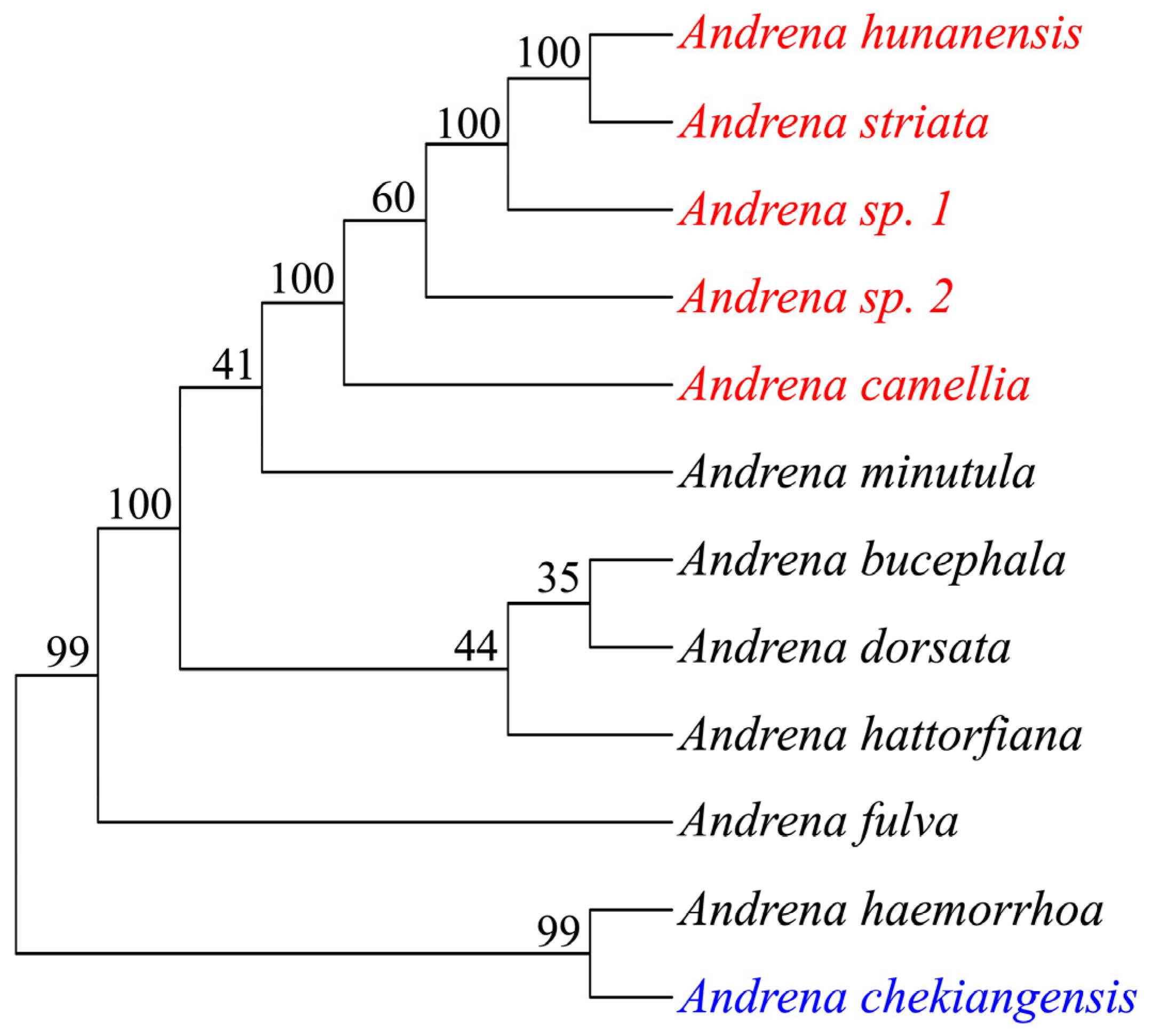
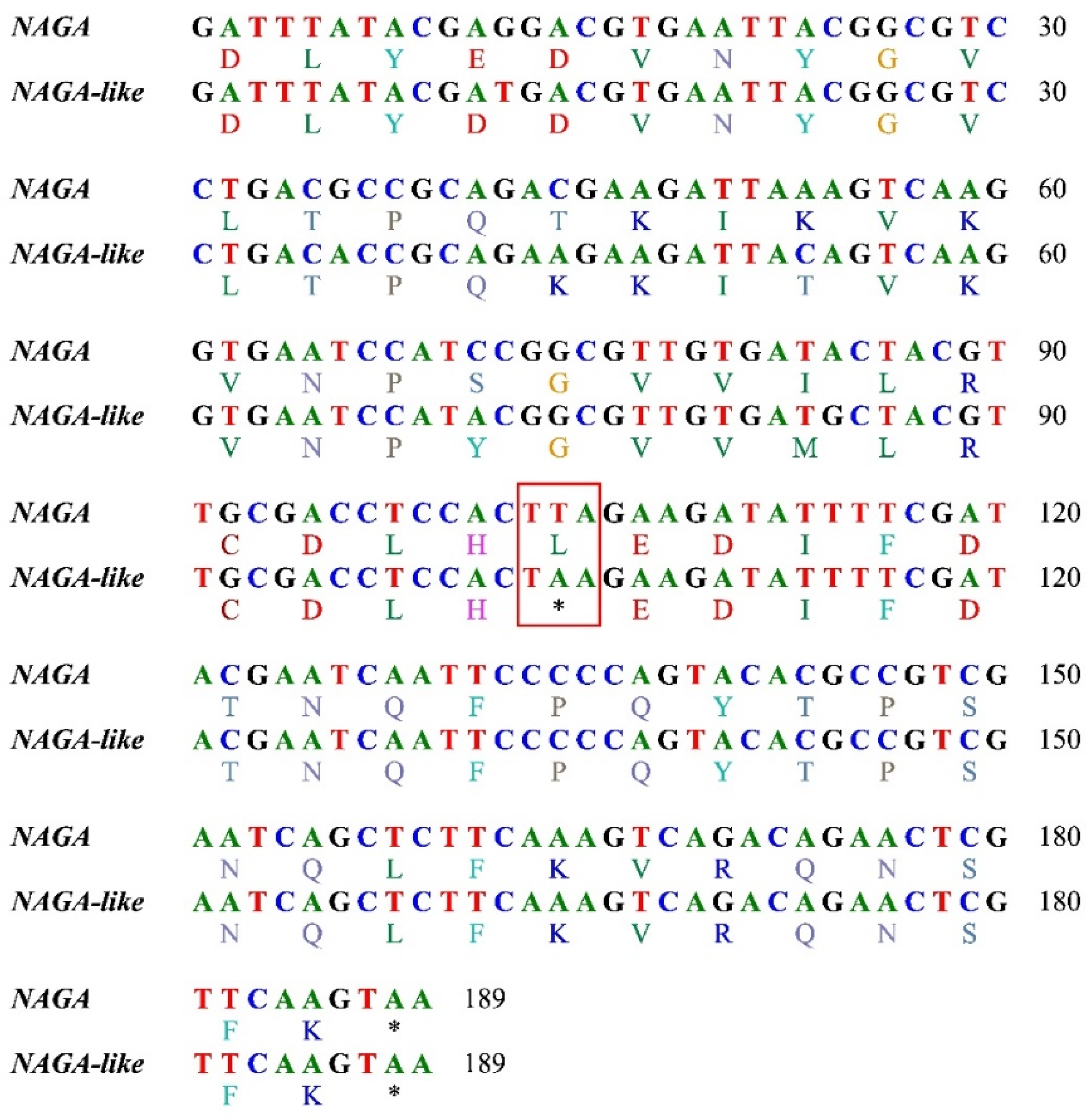
| Sample | Species | Location | Longitude | Latitude |
|---|---|---|---|---|
| XJ01 | Andrena camellia | Xiajiang, Jiangxi | 115.1285 | 27.6546 |
| QY01 | Andrena hunanensis | Qingyang, Anhui | 117.8796 | 30.5977 |
| RX01 | Andrena striata | Rongxian, Sichuan | 104.2913 | 29.4377 |
| CN02 | Andrena sp. 1 | Cangnan, Zhejiang | 120.2556 | 27.4591 |
| DY03 | Andrena sp. 2 | Dongyuan, Guangdong | 114.9792 | 24.1905 |
| NX04 | Andrena chekiangensis | Ningxiang, Hunan | 112.4206 | 27.9832 |
| Species | Reads | Assembly | ||
|---|---|---|---|---|
| Length (Gb) |
Accession | Length (Mb) |
N50 (Kb) |
|
| Andrena camellia | 10.38 | SRR23869504 | 369.7 | 11.1 |
| Andrena hunanensis | 11.01 | SRR23869503 | 384.3 | 8.2 |
| Andrena striata | 9.79 | SRR23869502 | 393.1 | 8.5 |
| Andrena sp. 1 | 10.75 | SRR23869501 | 363.2 | 9.6 |
| Andrena sp. 2 | 9.74 | SRR23869500 | 353.5 | 14.7 |
| Andrena chekiangensis | 10.02 | SRR23869499 | 365.7 | 11.6 |
| Gene | Length | Variable sites | Percent of variable sites |
|---|---|---|---|
| NAGA | 1,320 | 16 | 1.21 |
| NAGA-like | 1,239 | 33 | 2.66 |
| galM | 1,077 | 29 | 2.69 |
| galK | 1,182 | 24 | 2.03 |
| galT | 1,152 | 15 | 1.30 |
| galE | 1,098 | 8 | 0.73 |
| Gene | Foreground | Background | 2ΔlnL | p (df = 1) |
|---|---|---|---|---|
| NAGA | 0.049 | 0.023 | 1.412 | 0.234 |
| NAGA-like | 0.680 | 0.021 | 145.673 | <1.000e-10 |
| galM | 0.251 | 0.360 | 1.283 | 0.257 |
| galK | 0.864 | 0.161 | 24.279 | 8.336e-7 |
| galT | 0.387 | 0.088 | 12.761 | 3.540e-4 |
| galE | 0.129 | 0.063 | 0.913 | 0.339 |
| Sample | Accession | Length (Gb) |
Q30 (%) |
GC (%) |
|---|---|---|---|---|
| Acam1 | SRR8335252 | 7.54 | 91.55 | 45.84 |
| Acam2 | SRR8335251 | 9.50 | 92.41 | 46.35 |
| Acam3 | SRR8335254 | 7.64 | 91.76 | 46.23 |
| Acam4 | SRR8335253 | 9.04 | 92.35 | 45.97 |
| Ache1 | SRR23869498 | 9.03 | 95.82 | 41.95 |
| Ache2 | SRR23869497 | 8.88 | 96.10 | 42.46 |
| Ache3 | SRR23869496 | 7.82 | 96.16 | 41.61 |
| Ache4 | SRR23869495 | 9.36 | 95.81 | 43.69 |
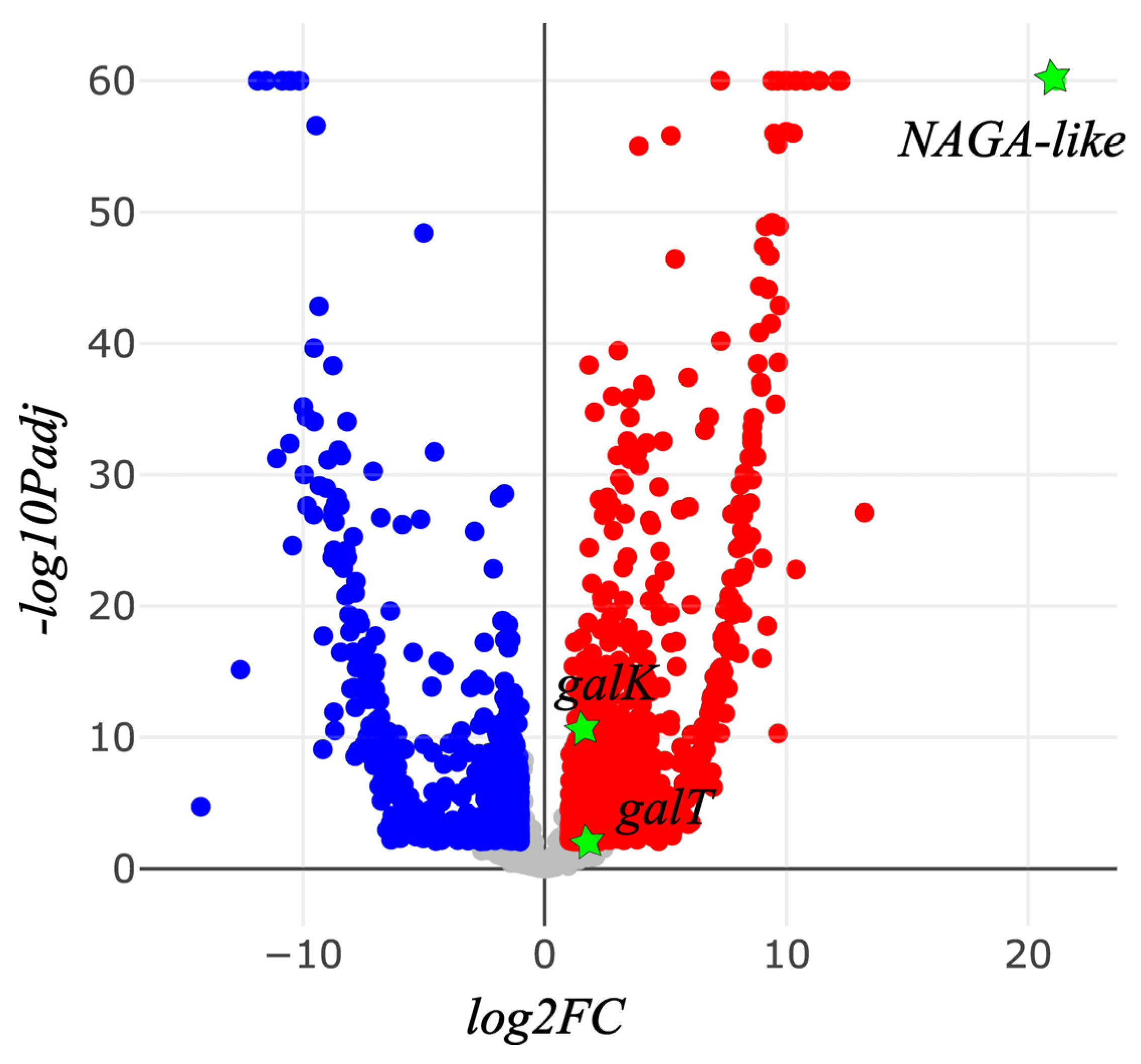
| Gene | TPM (Mean ± SD) | FC | Padj | |
|---|---|---|---|---|
| Andrena camellia | Andrena chekiangensis | |||
| NAGA | 574.88 ± 162.33 | 740.45 ± 470.32 | 0.639 | 0.387 |
| NAGA-like | 69,465.93 ± 7690.83 | 0 | +∞ | -∞ |
| galM | 537.64 ± 78.69 | 210.82 ± 211.28 | 2.14 | 0.118 |
| galK | 172.87 ± 47.92 | 41.77 ± 34.05 | 3.35 | 0.016 |
| galT | 987.77 ± 139.04 | 318.55 ± 57.03 | 2.99 | 1.781E-12 |
| galE | 32.45 ± 10.07 | 21.82 ± 14.73 | 1.23 | 0.624 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





