1. Introduction
Worldwide more than 1 million women are diagnosed annually with a gynecological cancer. Non-specific symptoms (such as in ovary cancer) and disparities in accessibility to health services (such as in cervical cancer) explain the differences in gynecological cancer outcome globally. Most gynecological cancer types are characterized by an accompanying stroma that not only supports malignant cells growth but is also largely responsible for the high resistance of the cancer to conventional and targeted therapies [
1,
2].
Epithelial ovarian cancer (EOC) is one of the most common gynecological cancers, with one of the highest mortality rates in women close to cervical and uterine cancer mortality rates [
3]. Globally, EOC is the seventh most common malignancy diagnosed among women accounting for more than two hundred thousand deaths globally [
4]. More than 300,000 women were estimated to have been diagnosed with EOC worldwide in 2020 [
4]. Due to the late onset of symptoms and the absence of early screening and detection modalities, EOC is usually diagnosed at an advanced stage and approximately 75% of women present with advanced stage disease (stage III or IV) [
5]. Whilst patients initially respond to chemotherapy, 80% of them rapidly develop chemoresistance and the recurrence is pretty high [
6,
7]. Exfoliated individual cells or spheroids of ovarian cancer cells disseminate through the peritoneal cavity colonizing the mesenterium, the omentum and the diaphragm and also the external layers of organs such as intestine and spleen [
8,
9].
Ovarian cancer shows a remarkable resistance to available therapies. Current management of ovarian cancer at presentation consists of cytoreductive surgery followed by chemotherapy that includes platinum and taxanes. However, almost 30% of patients have primary platinum resistance, 80% of patients will rapidly become refractory to the treatment and almost all patients will ultimately succumb of their disease [
7]. Interestingly, recent studies have shown that higher stroma proportion at the tumor at initial diagnosis of ovarian carcinoma, is associated with eventual emergence of platinum chemoresistance [
10]. With current 5-year survival rates under 50%, and 15% of women with ovarian cancer dying within few months of diagnosis, there is an urgent need for novel treatments for this deadly disease.
Many treatment options are being assessed in recurrent EOC settings including targeted therapy with the anti-VEGF antibody bevacizumab or poly (ADP ribose) polymerase inhibitor (PARPi) therapy that have shown some efficacy to extend progression-free survival rates but not overall survival [
11,
12]. Bevacizumab, combined with platinum/ taxane-based chemotherapy has been recommended by the National Comprehensive Cancer Network (NCCN) guidelines as a first-line treatment for EOC [
13]. Interestingly, the combination of the anti-PD1 and anti-CTLA4 check point inhibitors showed promising results in platinum resistant ovarian cancer at the six months-interim analyses with an overall response rate (ORR) of 34% (doubling the results of nivolumab monotherapy [
14,
15]. In November 2022, mirvetuximab soravtansine (a conjugated antibody targeting the folate receptor α to inhibit microtubules) was granted an accelerated approval by the FDA for the treatment of patients with folate receptor α positive, platinum-resistant EOC who have received 1-3 prior systemic treatment regimens; the median duration of response being less than 7 months [
16]. The efficacy of the different and novel targeted therapies reduces with each recurrence and even the more advanced targeted medicines are still far from providing reliable therapeutics for this deadly disease.
Oncolytic viruses are a state of the art therapeutic strategy for cancer treatment. Oncolytic adenoviruses (OAdV) can be engineered with tumor specific promoters (TSP) and transcriptionally targeted to selectively attack and kill target cells [
17,
18]. In previous studies we have shown that hybrid TSPs can be designed to target the cancer stromal cells compartment in addition to the malignant cell compartment [
18]; moreover, TSPs can be engineered with the addition of tumor microenvironment responsive motifs [
19]. In the present study we extend the studies of AR2011, a stroma targeted, tumor microenvironment responsive OAdV, by showing its lytic capacity on fresh explants obtained from different gynecological cancers (ovarian, uterus and cervical cancer). We also show its lytic capacity on human ovarian cancer cells obtained from peritoneal ascites combined with cisplatin. Finally, we describe the
in vivo efficacy in different murine models of a AR2011-derived version armed with cytokines’ genes.
2. Results
2.1. AR2011 in vitro lytic activity
2.1.1. On fresh explants obtained from human gynecological cancers
AR2011 is a stroma targeted, tumor microenvironment responsive, oncolytic adenovirus (OAdV) whose replication is driven by a triple hybrid promoter based on a 0.5 Kb selected fragment of the SPARC promoter combined with hypoxia, and NFkB-responsive elements; a parental version of this OAdV showed a remarkable efficacy in preclinical models of ovarian cancer [
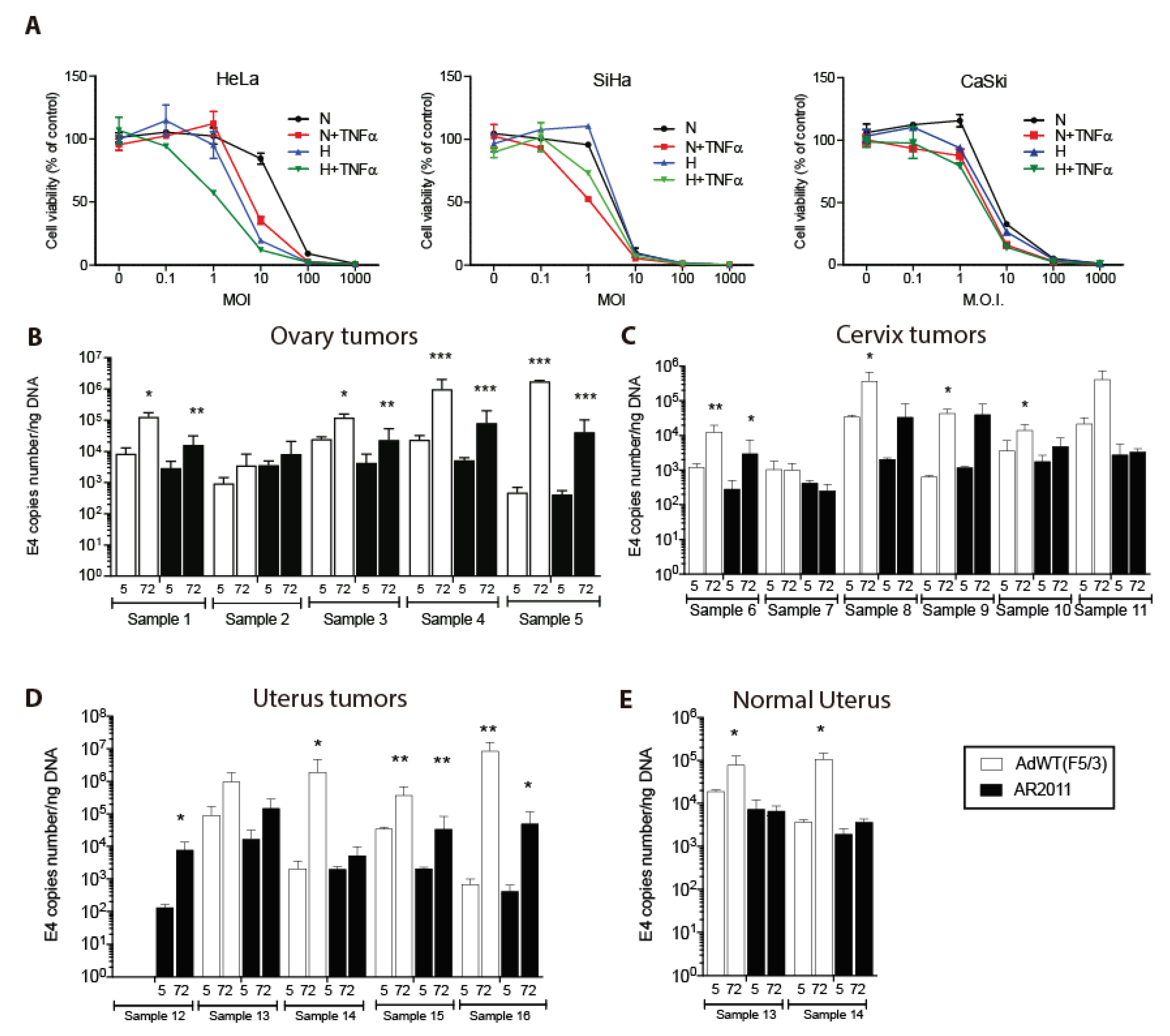
20]. In the present studies we initially aimed to establish if AR2011 lytic effect could be extended to other gynecological cancers beyond EOC. We first assessed AR2011 activity on cervical cancer cell lines. AR2011 exerted a remarkable lytic effect on the three cervical cancer cell lines assayed (
Figure 1A). Maximal lytic activity in the three cell lines was attained at 10-100 MOI (
Figure 1A). HeLa cells were the most responsive both to hypoxia conditions and to TNFα addition (
Figure 1A).
In previous studies we have shown that a former version of AR2011 was able to replicate and lyse fresh explants of human primary and metastatic ovarian cancer [
18]. Here, we extended those studies by assessing AR2011 replication in additional gynecological human cancers (
Table 1) by quantifying adenoviral E4 levels as a surrogate marker of virion particles. AR2011 was able to replicate in 4/5 fresh explants of ovarian cancer (range of E4 ratio at 72 hr. vs. 5 hr.: 2.3 - 100.4), in 3/6 fresh explants of cervical cancer (range of E4 ratio 72 hr. vs. 5 hr.: 2.7 - 33.4), and in 4/5 fresh explants of uterus cancer (range of E4 ratio 72 hr. vs. 5 hr.: 2.6 - 120.5) (
Figure 1B to D). AR2011 did not replicate at all in fresh explants of normal uterus (
Figure 1E). Although AdWT(F5/3) was able to replicate and lyse most gynecological cancer explants (
Figure 1B to D), it also replicated and lysed fresh explants of 2 normal uteri (E4 ratio 72 hr vs. 5 hr: 4.0 and 29.0) (
Figure 1E).
2.1.2. On malignant ovarian epithelial cells obtained from ascites fluid
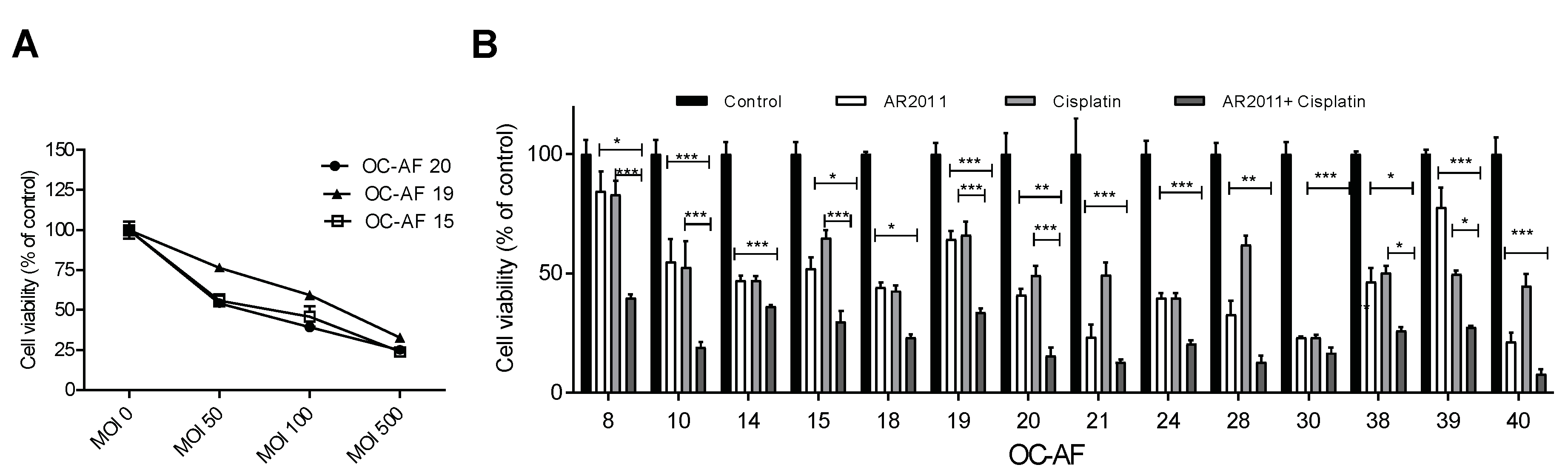
More than 70% of women with ovarian cancer are diagnosed with advanced disease, and surgery combined with chemotherapy regimens (including platinum analogues and taxanes) are still the mainstay option in the frontline setting. In order to establish the potential use of AR2011 in combination treatment options for advanced ovarian cancer, we assessed its lytic capacity on malignant cells obtained from ascites fluid (OC-AF). In initial studies performed on 3 different samples of OC-AF, we selected an MOI of 100 that was sufficient to induce 50% inhibition of malignant cells’ growth (
Figure 2A). From previous data from the literature [
21,
22] and our own data, we selected 2.5 ug/ml which is the IC50 of cisplatin on SKOV-3 that is accepted as a slightly resistant cell line to cisplatin. Fourteen OC-AF samples were exposed hence to the combination of AR2011 at 100 MOI and cisplatin at 2.5 μg/ml compared to exposure to each individual agent. We observed that the combination of AR2011 + cisplatin was able to kill malignant cells more efficiently than each single agent individually in all the samples (
Figure 2B). Moreover, in 9/14 samples the combination was synergistic according to the Bliss independence model [
21] used to analyze the drug combination data (
Table 2).
2.2. In vitro and in vivo studies of AR2011(404) a novel oncolytic vector engineered to express immunomodulatory genes
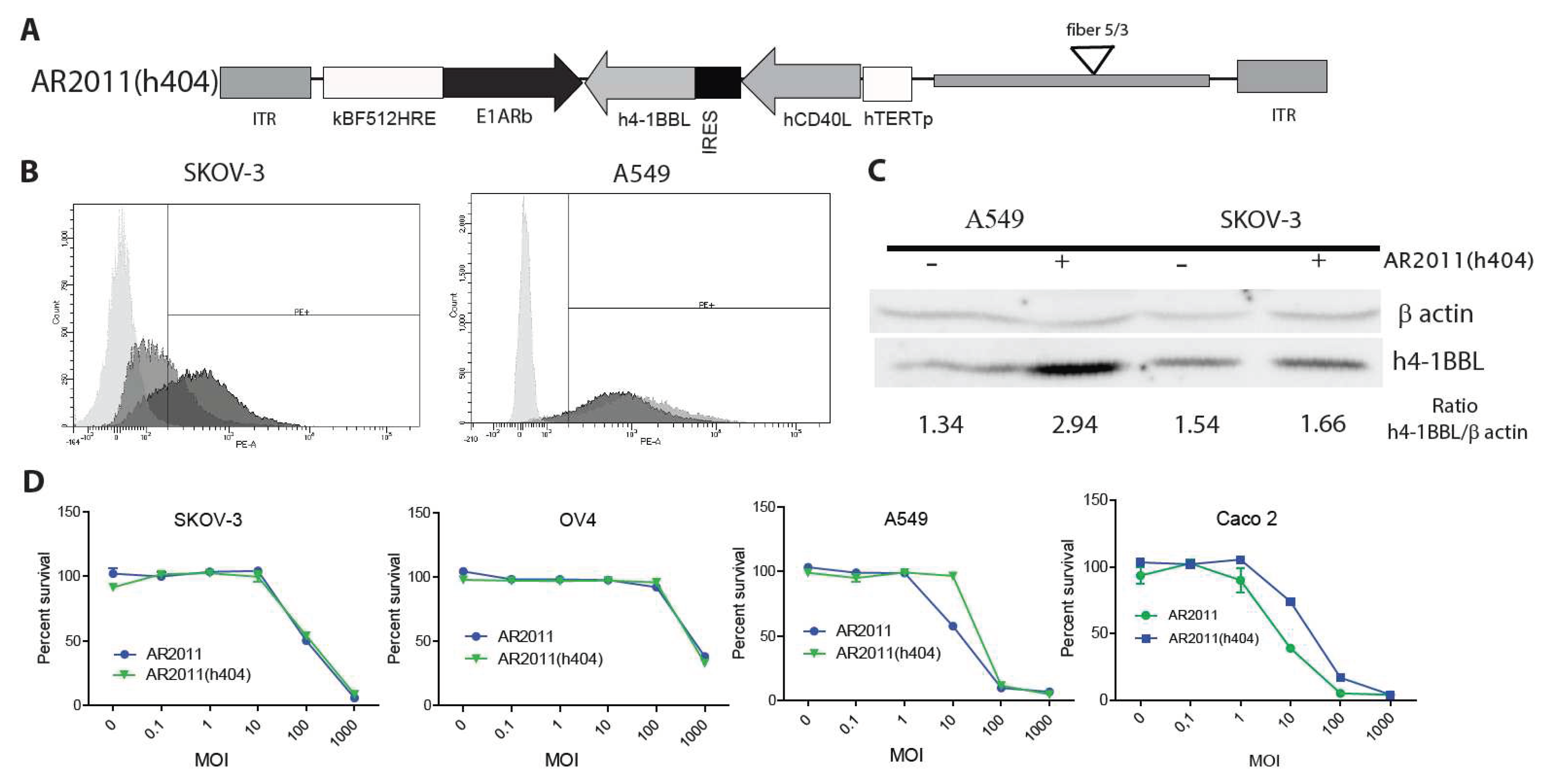
2.2.1. Vector construction and in vitro studies
Following the initial attack of the oncolytic virus it is expected that the immunomodulatory genes carried by the virus would help to expand the secondary antitumor immune response. Therefore, and as a further step to bring AR2011 closer to the clinic, we engineered AR2011 with the human versions of membrane bound, hCD40L and h4-1BBL under the control of the human telomerase (hTERT) promoter to obtain AR2011(h404) (
Figure 3A). By using a combination of flow cytometry and western blots, we were able to confirm that the hTERT promoter was able to drive the expression of hCD40L and h4-1BBL in SKOV-3 human ovarian cancer cells and in A549 human lung cancer cells, both expressing endogenous 4-1BBL (
Figure 3B and C). On the other hand, AR2011 induced a decrease in hCD40L and h4-1BBL expression in target cells likely due to its cells’ lytic effect (data not shown). In addition, we observed that AR2011 and AR2011(h404) were able to kill SKOV-3 and OV4 human ovarian cancer cells, A549 human lung cancer cells and CaCo 2 human colorectal cancer cells quite similarly indicating that expression of the cytokines did not hamper AR2011(h404) lytic capacity compared to the parental AR2011 OAdV (
Figure 3D).
2.2.2. In vivo studies in nude mice
In the absence of productive adenovirus replication in most murine organs and malignant cells, the
in vivo efficacy of oncolytic adenoviruses is usually tested in immunocompromised nude mice xenografted with human malignant cells. This clearly poses a limitation in terms of study the outcome of a secondary immune response following viral replication inside the tumor mass. With this limitation in mind, we performed different experiments in nude mice to assess the
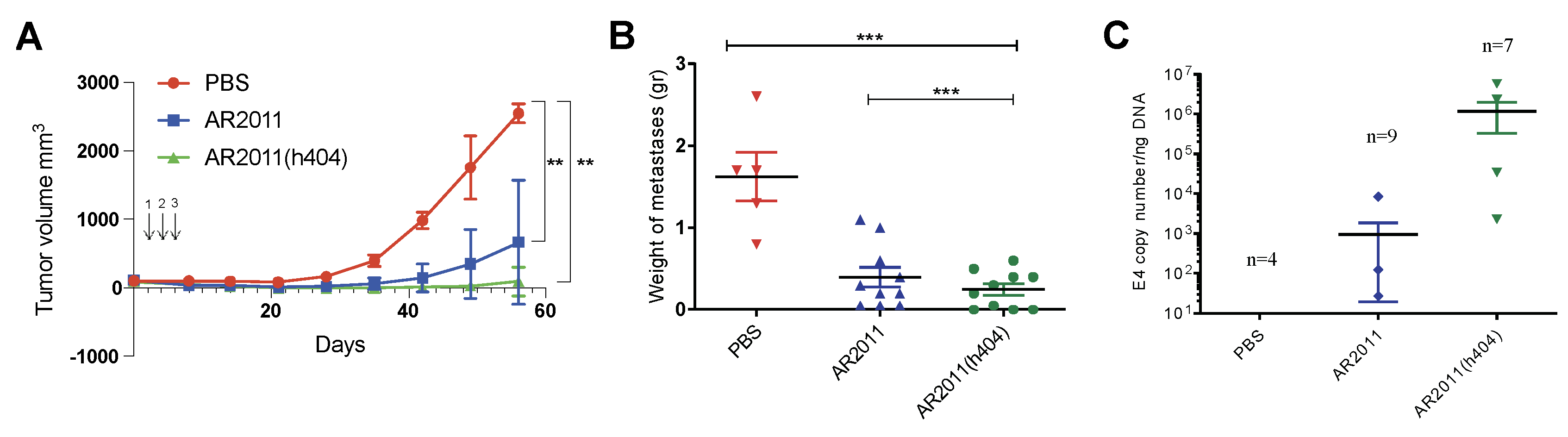
in vivo efficacy of AR2011(h404). Following preliminary studies to confirm 100% tumor take, nude mice were injected with tumorigenic inoculum of human ovarian cancer cells SKOV-3 in the flank. Three intratumor administrations of AR2011 led to a marked inhibition of tumor growth in most of the animals compared to control mice injected with PBS (
Figure 4A); notably, AR2011(h404) administration led to an even higher inhibition of tumor growth and 7/10 mice showed no detectable tumors at the end of the experiment (
Figure 4A). Treatment with each one of the OAdVs reached statistical significance compared to control PBS-treated mice (
Figure 4A). Macroscopic visualization showed complete disappearance of the tumor in most mice already 15 days after the first administration of AR2011(h404) (
Figure S1). Thus, expression of the cytokines not only did not hamper AR2011(h404) activity that exhibited a slightly increased efficacy that did not reach statistical significance compared to AR2011. We confirmed that the cytokines are expressed
in vivo; indeed, a new set of nude mice were injected s.c. with a tumorigenic inoculum of SKOV-3 cells followed by a single intratumor injection of either PBS, AR2011 or AR2011(h404); 72 hr. later, tumors were removed for assessment of cytokines’ expression. Western blot studies of the removed tumors showed that only samples obtained from mice treated with AR2011(h404) showed
in vivo expression of hCD40L (
Figure S2A). On the contrary, we were unable to see increased expression of h41BB-L
in vivo since SKOV-3 cells showed endogenous expression of the cytokine; in fact, PBS-treated mice showed the largest expression of h41BB-L while OAdVs-treated mice showed diminished expression of h4-1BBL most likely due to elimination of SKOV-3 cells due to OAdV
in vivo lytic effect (
Figure S2B).
We next moved on to assess the
in vivo efficacy of AR2011(h404) in avoiding peritoneal dissemination of malignant cells in nude mice xenografted with SKOV-3 ovarian cancer cells. Following preliminary studies to confirm 100 % tumor take, mice were injected i.p with a tumorigenic inoculum of 6 x 10
6 SKOV-3 cells. Five days later, mice were treated i.p. with 5x10
10 v.p. either of AR2011 or AR2011(h404) while control mice received PBS. OAdVs or PBS administration in the peritoneum was repeated at days 3 and 5. At the end of the study, when all control mice showed a swollen belly, mice were sacrificed following institutional guidelines. At necropsy mice were photographed and all the intraperitoneal tumors were excised, photographed and weighed. In addition to liquid ascites all the PBS-treated control mice showed an extended intraperitoneal dissemination including large metastatic nodules in liver, diaphragm, intestines and peritoneum walls (
Figure S3A). None of the mice treated with AR2011 was tumor free but 4/9 mice had tumors ≤ 0.1 gr; in addition, 4/9 mice developed ascites; interestingly, 3/10 mice treated with AR2011(h404) were tumor-free and an additional mouse had a tumor ≤ 0.1 g (
Figure S3B). Only 3/10 mice developed ascites. Overall, we observed a remarkable inhibition of tumor growth both in AR2011- as in AR2011(h404)-treated mice (
Figure 4B). Interestingly, only treatment with AR2011(h404) reached statistical significance compared to the PBS-treated control mice (
Figure 4B). Of note, AR2011(404) treatment also reached statistically significant difference compared to AR2011 treatment (
Figure 4B). In order to confirm that tumors were indeed targeted by the virus, we assessed E4 adenoviral gene levels as a surrogate marker of virion number. Assessment of E4 levels at the end of the experiment confirmed the presence of virions in the tumor mass of 3/9 mice treated with AR2011 and 4/7 mice treated with AR2011(h404) (
Figure 4C). Interestingly, E4 levels were higher (albeit not statistically significant
) in tumor samples obtained from mice treated with AR2011(h404) (
Figure 4C).
2.2.3. In vivo studies in syngeneic mice models
As mentioned above, most if not all murine tumor models do not support adenoviral replication and hence limit the use of adenoviruses to assess the induction of a secondary immune response further to adenovirus infection/replication in malignant target cells. Despite that limitation, we tried to establish whether we can make use of a syngeneic model that might be useful to demonstrate if the arming cytokines are active
in vivo. We constructed AR2011(m404) where the hTERT promoter drove the transcriptional activity of the murine versions of CD40L and 41BB-L to obtain AR2011(m404). The
in vitro and
in vivo effects of AR2011(m404) were compared with AR2011. To reduce liver uptake [
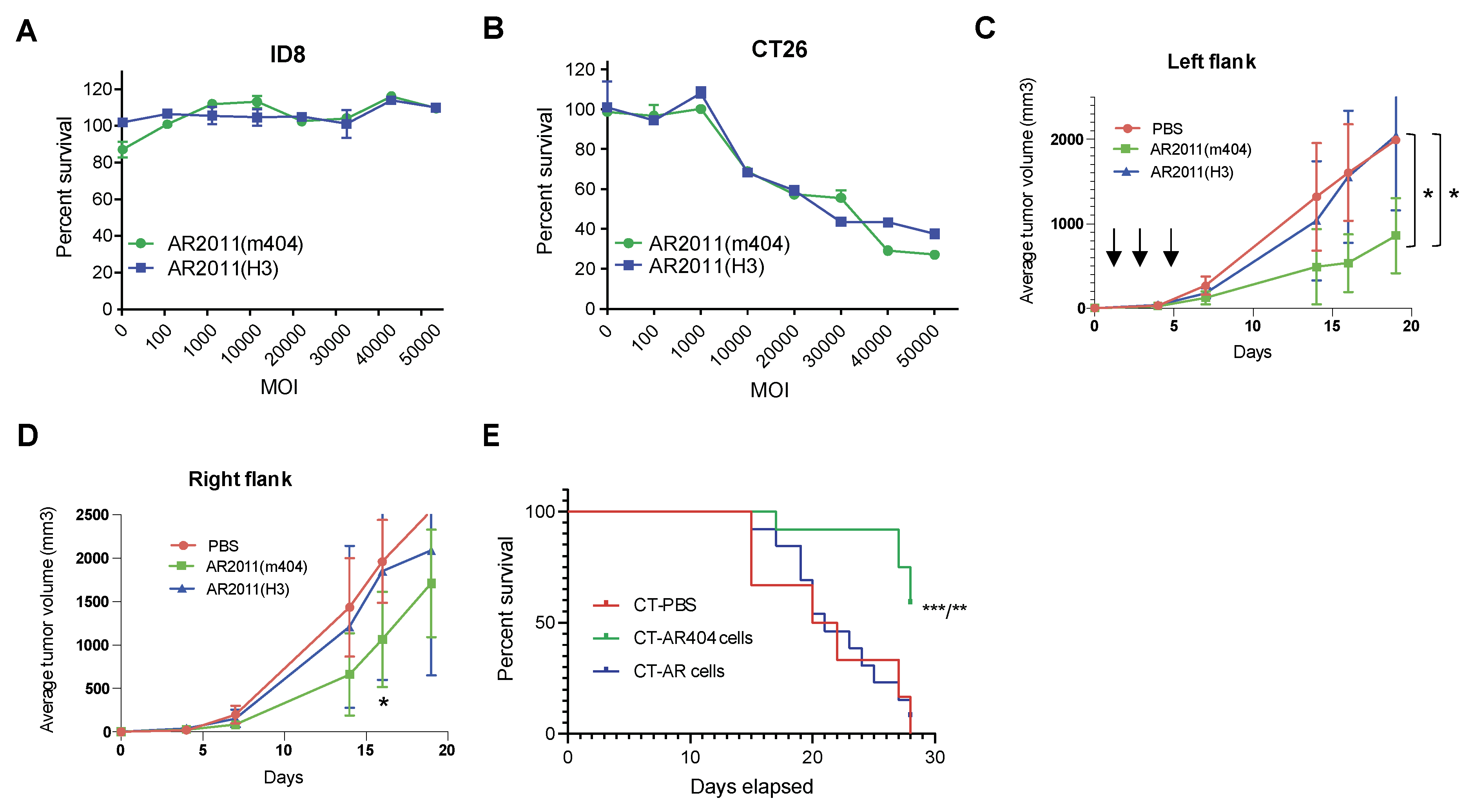
22], we exchanged the hexon protein in both OAdVs by engineering most of the hAdV3 hexon protein instead of the native one. We first observed that nor AR2011(H3) neither AR2011(m404) were able to kill ID8 murine ovarian malignant cells
in vitro up to an MOI of 50,000 (
Figure 5A) most likely related to the failure of adenovirus proteins synthesis in these murine cells [
23]. Following assessment of additional cell lines, we found that AR2011(H3) and AR2011(m404) were able to kill CT26 cells at a very similar level, starting from an MOI of 10,000 and reaching around 60% cell killing at MOI 50,000 (
Figure 5B). Based on these data, we decided to perform two different
in vivo studies with CT26 cells aiming to explore cytokines contribution to the antitumor viral effect
in vivo.
In the first approach, Balb/c mice were injected in both flanks with a tumorigenic inoculum of 5x10
5 syngeneic CT26 cells; nine days later, when tumors reached an average volume of 100 mm
3, mice were injected in the left flank i.t. with 7.5x10
10 v.p. of either AR2011(H3), AR2011(m404) or PBS. Mice received additional injections in the left tumor with the same amount of viral particles or PBS at days 3 and 5 after the first administration (
Figure 5C). Tumor size was followed in both flanks of each individual mouse; the experiment was ended when all the PBS-treated mice needed to be sacrificed due to an average tumor size of ~2000 mm
3 that occurred after 19 days. Interestingly, intratumor administration of AR2011(H3) had absolutely no effect on tumors’ growth compared to PBS-treated mice neither in the injected left flank nor in the non-injected right flank (
Figure 5C and D). Interestingly, AR2011(m404) treatment induced a statistically significant inhibition on tumor growth in the injected left flank compared either with AR2011(H3) or PBS-treated mice (
Figure 5C). Most interesting, AR2011(m404) treatment also induced a statistically significant inhibition of tumor growth in the non-injected right flank at day 18, although ultimately this difference waned at the end of the experiment (
Figure 5D). Since AR2011(H3) had no effect at all, it is compelling to suggest that the inhibition of tumor growth observed in both flanks with AR2011(m404) is due to cytokines expression.
In the next
in vivo study we injected Balb/c mice in the right flank with a tumorigenic inoculum of 5x10
5 CT26 cells; when tumors reached an average volume of 100 mm
3, mice were injected in the contralateral left flank with CT26 cells pre-infected overnight either with 30.000 MOI of AR2011(H3), (named CT-AR cells) or AR2011(m404), (named CT-AR404 cells). Control mice were injected in the contralateral flank with CT26 cells pre-treated with PBS (named CT-PBS). Contrary to the previous study, we observed no tumor growth in the left flank of mice injected either with CT-AR cells or with CT-AR404 cells (data not shown) while PBS pre-treated CT26 cells growth was unaffected. Mice were followed up to day 28 when all control mice (injected with CT-PBS cells) reached 2 cm
3 tumor size in the right flank and were sacrificed following institutional guidelines. In close coincidence with the previous
in vivo study, we observed no effect on mice survival following administration of CT-AR cells compared to CT-PBS-treated mice (
Figure 5E). On the contrary, most of the mice treated contralaterally with CT-AR404 cells were still alive at the end of the experiment suggesting that cytokines’ expression were responsible for the observed effect (
Figure 5E).
3. Discussion
The potential of ovarian cancer cells to disseminate and metastasize into the peritoneal cavity is governed by, among others, the ECM composition. The outcome of oncogenic events in epithelial cells can be significantly modified by the nature of surrounding cancer associated fibroblasts and endothelial cells. Stromal cells could be implicated in the acquisition of a chemoresistant phenotype. An extensive infiltrative pattern with desmoplasia is one of the major features favouring metastases [
24]. In a recent study aimed to identify novel molecular subtypes of ovarian cancer by gene expression profiling a poor prognosis subtype was defined by a reactive stroma gene expression signature, correlating with extensive desmoplasia [
25]. In previous studies we have shown the therapeutic efficacy of a novel stroma-targeted oncolytic adenovirus, AR2011, on preclinical models of ovarian cancer including fresh human explants. We extend the previous data by showing here that AR2011 was able to replicate and lyse fresh explants obtained from additional gynaecologic cancers; moreover, it was able to replicate and eliminate
in vitro in combination with mainstay chemotherapeutic agents, ovarian cancer cells obtained from ascites of advanced stage disease; AR2011 armed to express hCD40L and h4-1BBL showed an extended
in vivo efficacy on human tumors established in the flanks of nude mice; moreover, the armed OAdV was able to arrest and even completely eliminate intraperitoneally disseminated human tumors. In preliminary studies, the armed OAdV expressing murine cytokines was able to exert a limited but significant abscopal effect in a syngeneic murine model.
Chemotherapy resistance is the major limitation of current treatments for ovarian cancer. Since most patients are treated with neoadjuvant and adjuvant chemotherapy, the development of OAdV aimed at reversing resistance and sensitizing cells to chemotherapy agents can be a major advance in the field. Different
in vitro and
in vivo approaches have been used combining non-replicative viruses expressing sensitizing agents to chemotherapy compounds. As an example, it was shown that ovarian cancer cells can be sensitized
in vitro and
in vivo to cisplatin by adenoviral expression of the manganese superoxide dismutase gene [
26]. Previous studies have also shown that myxoma virus can be combined
in vitro and in preclinical models with chemotherapy agents to treat mice with syngeneic ovarian tumors [
27]. It has been also shown that paclitaxel resistance can increase oncolytic virus lytic effect through a mechanism that involves upregulation of viral receptors [
26]. Previous studies from our group showed that AR2011 can replicate and lyse fresh explants of solid metastases arising from human ovarian cancer heavily pretreated with chemotherapeutic agents [
18]. Here, we extend the data by showing that AR2011 can synergize
in vitro with cisplatin to eliminate ovarian cancer cells obtained from patients’ ascites, even in those cases where patients have been previously treated with neoadjuvant chemotherapy.
A major limitation for adenovirus use in preclinical studies is the absence of syngeneic models that can recapitulate a human
scenario, in particular the secondary immune response that follows initial administration of an OAdV. In the absence of a full syngeneic ovarian cancer model in rodents [
28] most of the studies with OAdV are being performed in nude mice with the limitation of the absence of the full immune response. The lack of cross reactivity of hCD40L with murine models posed an additional limitation to assess the immune response associated with arming AR2011 with human CD40L. Interestingly, CD40-L has been shown to sensitize epithelial ovarian cancer cells to cisplatin treatment clearly indicating that activation of the CD40 intracellular pathway in cancer cells can be of relevance beyond CD40 effect in establishing the adaptive immune response [
29]. Despite the limitation with human CD40-L, it was interesting to note that AR2011(404) demonstrated a slightly higher
in vivo efficacy than AR2011 in nude mice studies. We cannot rule out that this enhanced
in vivo effect of AR2011(404) could be related to 4-1BBL expression. Although with markedly reduced affinity it was shown that human 4-1BBL can bind murine 4-1BBL receptor [
30]. This cytokine has been shown to expand T cells and NK cells and it is likely that NK activation could explain the slightly improved activity of AR2011(404)
in vivo in the nude mice model. Activation of the 4-1BB pathway in T cells restoration of effector functions has been hampered by the liver toxicity induced by soluble agonists [
31]. Therefore, it was compelling to express 4-1BBL locally under the regulation of the extremely specific hTERT promoter. Interestingly, activation of 4-1BB with agonists has been used in combination with other immune check points agents such as PD-1 and TIM-3 in murine models of ovarian cancer with remarkable efficacy [
32]. In this context it was interesting to see in preliminary studies, that expression of murine CD40-L and 4-1BBL in the CT26 model was able to induce not only a clear anti-tumor immune mediated response, locally, but also a modest but significant abscopal effect. Both the local and the abscopal effects were observed upon administration of AR2011(m404), while AR2011(H3) lacking cytokines expression was unable to induce any antitumor effect in this syngeneic model, not local neither systemic.
Diagnoses at a late stage of most ovarian cancers makes it imperative to develop innovative therapeutic approaches to tackle the disease. Both RNA and DNA oncolytic viruses have been used in the few clinical studies in ovarian cancer; all the clinical studies did not proceed after phase 1, or are recruiting patients for phase 1. Measless viruses expressing the carcinoembryonic antigen (CEA) were used in a phase I trial administered intraperitoneally in advanced stage cases [
33]. The treatment was well tolerated and resulted in dose-dependent biological activity in a cohort of heavily pre-treated recurrent ovarian cancer patients [
33]. Vaccinia virus and Reovirus reolysin reached also phase 1 trials but definitive reports are still lacking [
34]. Oncolytic adenoviruses have been used also in few clinical trials in ovarian cancer with unclear effects. After initial studies with the 55K-E1B-deleted dl1520 oncolytic adenovirus halted after phase 1 with no clear benefit [
35], few other groups reached phase 1 trials stage with modified oncolytic adenoviruses. Ad-delta24-RGD is an oncolytic adenovirus modified in the knob fiber domain to express an RGD moiety able to retarget the virus to integrins in a viral receptor-independent manner; this OAdV was well tolerated in a phase 1 trial after intraperitoneal administration and showed promising clinical activity [
36]. A variant of this OAdV expressing GM-CSF used in a compassionate mode in very few ovarian cancer patients was well tolerated and appeared to induce an anti-tumor immune response [
37]. Combination of this OAdV with a daily low dose of cyclophosphamide was also attempted in a phase 1 trial that involved few ovarian cancer patients with promising results [
38]. Few other trials are still under patients’ recruitment [
39]. We provide here novel data on the stroma targeted AR2011 OAdV that has been shown to kill ovarian cancer cells obtained from liquid ascites corresponding to patients pre-treated or not with chemotherapy. AR2011 could be combined with mainstay chemotherapy and was able to eliminate cells that seem to be refractory to chemotherapy. Moreover, AR2011 could be armed with transcriptionally targeted cytokines to be expressed only in tissues overexpressing the hTERT gene. The hTERT proximal promoter has been already used to drive specific adenoviral replication in malignant tissues and oncolytic cell death [
40]. OAdV driven by hTERT have entered clinical trials stages in different cancer cell types.
4. Materials and Methods
4.1. Cell lines and cell culture
The human ovarian cancer cell lines SKOV-3 and OV-4, A549 lung carcinoma cells, human embryonic retinoblasts 911 and the murine colon carcinoma cells CT26, were already described [
18] . The human ovarian cancer cells PA-1 (CRL-1572), and cervical cancer cells (HeLa, CCL-2; SiHa, HTB-35 and Ca Ski, CRL-1550) were obtained from the ATCC (Manassas, VA, USA). HEK293 cells were purchased from Microbix (Toronto, Canada). All the cell lines were grown in the recommended medium supplemented with 15% of fetal bovine serum (Natocor, Cordoba, Argentina), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin and maintained in a 37 °C atmosphere containing 5% CO
2.
4.2. Construction and Production of the oncolytic adenoviruses
The main features of AR2011 have been already described [
9]. AR2011(404) was derived from AR2011 and expressed either human (h) or murine (m) CD40-L and 4-1BBL. For AR2011(h404) construction, a 2.8 Kb
BglII fragment containing the hTERT promoter followed by the sequence encoding for hCD40L, an internal ribosome entry site (IRES) from encephalomyocarditis virus and h4-1BBL, was cloned into the
BglII site of pshuttle 2kbHREF512ΔRb in the 3’-5’ orientation. A similar design was used for cloning a 2.9 Kb fragment in AR2011(m404) that included the murine version of each cytokine under hTERT. Both CD40L and 4-1BBL are the full length membrane bound forms and have a deletion in the cleavage site FEMQK (in hCD40L) and FEMQR (in mCD40L). All the DNAs were synthetized at Genscript (NJ, USA) following our own design. All the constructs were confirmed by automatic sequencing at Macrogen (Seoul, Korea).
For the recombination steps, the pshuttle containing the sequences encoding for the human cytokines was linearized with
PmeI and co-transformed with pvK500C F5/3 in BJ5183 cells to obtain the viral plasmid corresponding to AR2011(h404). To obtain AR2011(m404), the pshuttle encoding the sequences for the murine cytokines was recombined with pVK500C F5/3 where the entire hexon protein was replaced by the hexon protein of hAdV3. For hexon exchange, an adenovirus 5 backbone without hexon named pARΔHexon was prepared as described [
41]. A 6.9 Kb fragment containing the entire hexon of hAdV3 and flanking regions from hexon 5 was released with
SfiI from pAd5H3 / GL [
41] and recombined with pVKΔHexon linearized with
AsisSI. As a control of AR2011(m404), we constructed AR2011(H3) modified in the hexon protein following a similar procedure. The different recombined adenoviral genomes were linearized with
PacI and transfected in 911 cells. The rescued adenoviruses were used to infect HEK293 cells to produce the viral stocks [
42]. All the constructs were confirmed by restriction pattern and automatic sequencing.
4.3. In vitro cytotoxicity assay
For determination of virus-mediated cytotoxicity, 1 x 10
4 cells were seeded in 24 well - tissue culture plates and infected with the oncolytic adenoviruses at the indicated MOI [
42]. Hypoxic and normoxic conditions as well as addition of TNFα to recreate an inflammatory environment was already described [
19]. After 6 days, cell viability was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS assay; Promega, Madison, WI).
Fresh human explants were obtained at the Hospital Municipal de Oncología Marie Curie, Buenos Aires, Argentina, following institutional review board approval. Written informed consent was obtained from each patient. The declaration of Helsinki was followed in all the protocols. Samples were kept in RPMI medium on ice (Invitrogen, Carlsbad, CA). Time from harvest to slicing was kept at an absolute minimum (<2 hours). Between 6 to 10 slices, 1-2 mm depth, were placed in 24-well plates followed by the addition of the virus at 500 MOI in 500 μl of DMEM/F12 including 2% v/v FBS, 1% antibiotics and 1% L-glutamine [
43]. Infections were allowed to proceed for 5 hours where 3-5 slices were harvested for E4 quantification. In the remaining slices the medium was replaced with fresh DMEM/F12 containing 10 % FBS until the end of the experiment at 72 hours. For assessment of E4 levels as a surrogate of viral particles, DNA was obtained from tissue slices and qPCR for E4 was performed as described [
18].
4.4. Isolation of malignant cells from ovarian cancer liquid ascites
Samples obtained from the Hospital Marie Curie were centrifuged at 1500 RPM for 10 minutes to clear the ascites from cells. Cells were incubated in DMEM/F12 supplemented with 15% of FBS, brought to confluence and stored in liquid nitrogen until use. Each cells’ isolate was named as OC-LA followed by the respective number.
4.5. In vitro lytic assays in combination with cisplatin
Malignant cells obtained from liquid ascites were seeded in 96 well tissue culture plates (5 x 103 per well) and incubated for 48 hours with cisplatin at a final concentration of 2.5 µg/ml for 48 hours. After medium removal, cells were trypsinized, quantified and replated in the presence of AR2011 at 100 MOI for another 96 hours. When only cisplatin was used, cells were plated in the presence of cisplatin at 2.5 µg/ml for 48 hours followed by medium addition without virus for another 96 hours; for the control with virus alone, cells were plated only in medium for 48 hours followed by incubation in the presence of AR2011 (MOI 100) for another 96 hours as described above. At the end of the experiments cell viability was assessed with the MTS system.
4.6. Assessment of CD40L and 4-1BBL expression
For assessment of hCD40-L expression by flow cytometry, A549 and SKOV-3 cells were infected with AR2011(h404). At the end, cells were harvested in 0.5 mM EDTA, washed and resuspended in PBS containing 0.5 % BSA at a concentration of 5x106 cells/ml followed by incubation with phycoerythrin (PE) - conjugated hCD40L monoclonal antibody (eBioscience, CA, USA). A PE-conjugated mouse IgG1 kappa isotype (clone P3.6.2.8.1, eBioscience, CA, USA) was used as a matched control antibody (eBioscience, CA, USA). The cells were washed again and resuspended in 0.4% paraformaldehyde in PBS prior to analysis with a FACS Calibur flow cytometer (Becton Dickinson, Oxford, United Kinngdom). Ten thousand cells were analysed in each case.
For western blot assessment of h4-1BBL in cell lines, SKOV3 and A549 cells were seeded at 1 x 105/well in a 6 multiplate well plate. The next day cells were infected with AR2011(h404) and incubated at 37 °C for 30 hours. Cells were then harvested, and total protein extracts were prepared in lysis buffer containing 10 mM Tris (pH 7.5), 1 mM EDTA, 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS and a protease inhibitor cocktail. Total protein extracts were separated in 10% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad Laboratories, CA, USA). The membranes were probed with anti-human 4-1BBL antibody (ab68185, Abcam, MA, USA) and anti–β-actin antibody (A4700; Sigma, USA). HRP-Goat anti Rabbit (111035144, Jackson, NE, USA) and HRP Goat anti Mouse (115035003: Jackson, NE, USA) were used as secondary antibodies. Enhanced chemiluminescence (ECL) reagents were used to detect the signals following the manufacturer’s instructions (Amersham, USA). Antibody signals were digitized by Image Quant LAS 4000 (GE-Cytiva MA, USA). Anti β-actin antibody (A4700; Sigma, USA) and anti-αTubulin Ab (12g10 DSHB) were used as a loading control. HRP-Goat anti Rabbit (111035144: Jackson NE, USA and HRP Goat anti Mouse (115035003: Jackson NE, USA) were used as secondary antibodies. Enhanced chemiluminescence reagents were used to detect the signals following manufacturer’s instructions (Amersham, USA). Antibody signals were digitized by Image Quant LAS 4000 (GE-Cytiva MA, USA).
For assessment of hCD40-L and h4-1BBL expression in tumors, the tumor samples extracts were prepared using lysis buffer containing 50mM Tris (pH 7.3), 150 mM NaCl and 0.1% Tween 20 plus Halt protease inhibitor cocktail (8775, Thermo, IL USA). One hundred μg of protein extract was separated in 12% SDS-PAGE and transferred into nitrocellulose membranes. The membranes were probed with anti-h4-1BBL (ab68185, Abcam, MA, USA), and anti-hCD40L (ab2391, Abcam, MA, USA). Anti β-actin antibody (A4700; Sigma, USA) and anti-α Tubulin (12g10 DSHB, IA, USA) were used as a loading control. HRP-Goat anti Rabbit (111035144, Jackson NE, USA) and HRP Goat anti Mouse (115035003, Jackson NE, USA) were used as secondary antibodies. Enhanced chemiluminescence reagents were used to detect the signals following manufacturer’s instructions (Amersham, USA). Antibody signals were digitized by Image Quant LAS 4000 (GE-Cytiva MA, USA).
4.7. In vivo studies with nude mice.
Six to eight weeks-old female nude mice were obtained from the animal facility of the University of La Plata. After an acclimation period in Instituto Leloir animal facility, mice were injected with 4.5 x 106 SKOV3 cells in the flank. Tumor volumes were followed with caliper measurement every 2-3 days. Once tumors reached an average volume of 100 mm3, mice were injected intratumorally with 1 x 1010 v.p. of AR2011 or AR2011(h404) in 30 μl (as well as an equivalent volume of PBS for the control group). Viral or PBS administration was repeated 2 and 4 days later. Mice were followed with daily observations on general health until the control group (PBS) needed to be sacrificed due to animal distress following the approved protocol of the Institutional Animal Care and Use Committee of Instituto Leloir. At the end of the study remaining tumors were excised and weighed. For the in vivo assessment of hCD40L and h4-1BBL expression, mice were injected with 4.5 x 106 SKOV3 cells in the flank. When tumors reached 100 mm3 mice were administered i.t. with 5 x 1010 v.p. of AR2011, AR2011(h404) or PBS. Seventy-two hours later mice were sacrificed, the tumor area was removed, and a protein extract was prepared for western blot analyses as described above.
For intraperitoneally injected tumors, mice were injected with 6 x 10
6 SKOV3 cells. Five days later, mice were injected i.p. with 5 x10
10 vp in 400 μl of AR2011, AR2011(h404) or PBS. Injection was repeated 2 and 4 days later. Three mice per group were sacrificed 3 days after the last viral administration for assessment of E4 levels as a surrogate marker of viral particles [
18]. Mice were followed as described above. The remaining mice were sacrificed at day 54 of the first viral administration following institutional guidelines and remaining i.p. tumors were photographed
in situ, excised, weighed and a sample used for assessment of E4 levels.
4.8. In vivo studies with syngeneic models
Balb/c mice (6-8 week old male) were obtained from the animal facility of the University of La Plata. After an acclimation period in Instituto Leloir animal facility, mice were injected in both flanks with 5 x 105 syngeneic CT26 colorectal carcinoma cells. The tumorigenic inoculum (with 100% tumor take) was selected from a pilot study where mice were injected with 3 x 105, 5 x 105 and 1 x 106 cells in 100 μl of PBS (data not shown). Once tumors reached a volume of 75-100 mm3, mice were injected in the left tumor with 7.5 x 1010 vp of either AR2011(H3) or AR2011(m404) in a final volume of 50 μl PBS. Control mice were injected with 50 μl PBS. Tumors were measured bi-weekly in two dimensions with a caliper. The mice were followed until they need to be sacrificed due to a tumor size that exceeded 2000 mm3. In a second type of experiment Balb/c mice were injected in the left flank with 5 x 105 CT26 cells. Once tumors reached an average volume of 100 mm3 mice were injected in the contralateral right flank with 50 μl containing 5 x 105 CT26 cells pre-infected overnight with 3 x 104 MOI of either AR2011(H3) or AR2011(m404). Control CT26 cells were pretreated with PBS. Mice tumors were assessed with digital calipers and the volume was obtained with the following formula: volume=0.52 x (width)2 x length.
4.9. Statistical analysis
For figures 1B to E, 2B, 4A to C, 5C and D the statistical difference between groups was determined by a t-test and the F test (H. J. Motulsky, GraphPad Statistics Guide.
http://www.graphpad.com/guides/prism/7/statistics/index.htm). The survival curve in
Figure 5E was analyzed with Log-rank (Mantel-Cox) test. A
P-value of <0.05 was considered statistically significant. Data analysis was performed with the GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA). Bliss independence model [
21] was used to analyze drug combination data. The Bliss method compares the observed combination response (Y(O)) with the predicted combination response (Y(P)), which was obtained based on the assumption that there is no effect from drug-drug interactions. Typically, the combination effect is declared synergistic if Y(O) is greater than Y(P).
4.10. Ethics statement
All the experiments were approved by the Institutional Animal Care and Use Committee of the Fundación Instituto Leloir (Protocol #69OP). The Fundación Instituto Leloir has an approved Animal Welfare Assurance as a foreign institution with the Office of Laboratory Animal Welfare (NIH), Number A5168-01. All the patients at the Hospital Maria Curie signed an informed consent for samples’ use for research. All the samples reached Instituto Leloir in an anonymized code. The study was approved by the Ethics Committee of Hospital Maria Curie and by the Bioethics Committee of Fundación Instituto Leloir that also approved the use of human samples.
4.11. Patents
Part of the present data is included in the US patent application No.: 16/797,291 whose inventors are D.T.C., O.L.P. and M.V.L.
5. Conclusions
Based on previous evidence and the current study, it is highly likely that AR2011(404) will provide high lytic selectivity and cytokine expression specificity due to the combination of the triple hybrid SPARC-based promoter and the hTERT promoter. The fact that the virus expresses the pseudotyped fiber 5/3 also provides higher selectivity in terms of ovarian cancer cells targeting. The evidence that our virus showed efficacy in samples from additional gynecologic cancers makes this virus a reliable candidate to reach clinical trials.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Photographs from in vivo studies in nude mice harboring s.c. tumors; Figure S2: Western Blot of tumor samples obtained from in vivo studies in nude mice.; Figure S3 Photographs from in vivo studies in nude mice harboring intraperitoneal tumors.
Author Contributions
Conceptualization O.L.P. and M.V.L..; Methodology M.V.L., E.G.A.C., A.A.; M.G; A.N.C; C.M.; I.B; G.D. R; C.R; Data Analysis O.L.P., M.V.L., E.G.A.C., A.A.; Resources O.L.P., M.V.L., N.C., D.T.C.; Data Curation O.L.P., M.V.L.; Writing-original draft preparation O.L.P., M.V.L.; Writing-review and editing O.L.P., M.V.L.
Funding
This research was funded in part by grants from the National Agency for Promotion of Science, Technology and Innovation, The Instituto Nacional del Cáncer, the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Fundación Bunge y Born, all from Argentina. The study was supported in part by a sponsored research grant of Unleash Immuno Oncolytics. We are indebted to the continuous support of Amigos de la Fundación Instituto Leloir para la Investigación en Cancer (AFULIC), Argentina.
Institutional Review Board Statement
The study has been approved by the bioethical Committee of Fundacion Instituto Leloir. All the experiments were conducted in accordance with animal use guidelines and protocols approved by the Institutional Animal Care and Use Committee of Fundacion Instituto Leloir (IACUC protocol # 69).
Informed Consent Statement
Informed consent prepared by Hospital Maria Curie Ethical Committee was obtained from all subjects involved in the study.
Acknowledgments
We thank the technical assistance of Micaela Benlolo at the Laboratory of Molecular and Cellular Therapy. We are very grateful to the administrative support of Marcos Olivera and Julieta Portillo.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The authors declare no other conflict of interest.
References
- Wang, W.; Kryczek, I.; Dostal, L.; Lin, H.; Tan, L.; Zhao, L.; Lu, F.; Wei, S.; Maj, T.; Peng, D.; He, G.; Vatan, L.; Szeliga, W.; Kuick, R.; Kotarski, J.; Tarkowski, R.; Dou, Y.; Rattan, R.; Munkarah, A.; Liu, J. R.; Zou, W. , Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016, 165 (5), 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Au Yeung, C. L.; Co, N. N.; Tsuruga, T.; Yeung, T. L.; Kwan, S. Y.; Leung, C. S.; Li, Y.; Lu, E. S.; Kwan, K.; Wong, K. K.; Schmandt, R.; Lu, K. H.; Mok, S. C. , Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nature communications 2016, 7, 11150. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. , Ovarian cancer in the world: epidemiology and risk factors. International journal of women's health, 2019; 11, 287-299. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. , Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71(3), 209–249. [Google Scholar] [CrossRef]
- Miller, R. E.; Leary, A.; Scott, C. L.; Serra, V.; Lord, C. J.; Bowtell, D.; Chang, D. K.; Garsed, D. W.; Jonkers, J.; Ledermann, J. A.; Nik-Zainal, S.; Ray-Coquard, I.; Shah, S. P.; Matias-Guiu, X.; Swisher, E. M.; Yates, L. R. , ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol 2020, 31 (12), 1606–1622. [Google Scholar] [CrossRef]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. , Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomarkers in cancer 2019, 11, 1179299X19860815. [Google Scholar] [CrossRef] [PubMed]
- Lokadasan, R.; James, F. V.; Narayanan, G.; Prabhakaran, P. K. , Targeted agents in epithelial ovarian cancer: review on emerging therapies and future developments. Ecancermedicalscience 2016, 10, 626–626. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. , Ovarian cancer development and metastasis. Am J Pathol 2010, 177(3), 1053–64. [Google Scholar] [CrossRef]
- Alfano, A. L.; Nicola Candia, A.; Cuneo, N.; Guttlein, L. N.; Soderini, A.; Rotondaro, C.; Sganga, L.; Podhajcer, O. L.; Lopez, M. V. , Oncolytic Adenovirus-Loaded Menstrual Blood Stem Cells Overcome the Blockade of Viral Activity Exerted by Ovarian Cancer Ascites. Molecular Therapy Oncolytics 2017, 6, 31–44. [Google Scholar] [CrossRef]
- Lou, E.; Vogel, R. I.; Hoostal, S.; Klein, M.; Linden, M. A.; Teoh, D.; Geller, M. A. , Tumor-Stroma Proportion as a Predictive Biomarker of Resistance to Platinum-Based Chemotherapy in Patients With Ovarian Cancer. JAMA oncology 2019. [Google Scholar] [CrossRef]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. , Ovarian Cancer Immunotherapy and Personalized Medicine. International journal of molecular sciences, 2021; 22 (12), 12. [Google Scholar]
- Li, H.; Liu, Z. Y.; Wu, N.; Chen, Y. C.; Cheng, Q.; Wang, J. , PARP inhibitor resistance: the underlying mechanisms and clinical implications. Mol Cancer 2020, 19(1), 107. [Google Scholar] [CrossRef]
- Huang, X.; Li, X. Y.; Shan, W. L.; Chen, Y.; Zhu, Q.; Xia, B. R. , Targeted therapy and immunotherapy: Diamonds in the rough in the treatment of epithelial ovarian cancer. Front Pharmacol 2023, 14, 1131342. [Google Scholar] [CrossRef]
- Zamarin, D.; Burger, R. A.; Sill, M. W.; Powell, D. J., Jr.; Lankes, H. A.; Feldman, M. D.; Zivanovic, O.; Gunderson, C.; Ko, E.; Mathews, C.; Sharma, S.; Hagemann, A. R.; Khleif, S.; Aghajanian, C. , Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J Clin Oncol 2020, 38 (16), 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, B. R.; Zhang, Z. C.; Zhang, Y. J.; Lou, G.; Jin, W. L. , Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Frontiers in immunology 2020, 11, 577869. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y. A. , Mirvetuximab Soravtansine: First Approval. Drugs 2023, 83(3), 265–273. [Google Scholar] [CrossRef] [PubMed]
- Hajeri, P. B.; Sharma, N. S.; Yamamoto, M. , Oncolytic Adenoviruses: Strategies for Improved Targeting and Specificity. Cancers, 2020; 12(6), 18. [Google Scholar]
- Lopez, M. V.; Rivera, A. A.; Viale, D. L.; Benedetti, L.; Cuneo, N.; Kimball, K. J.; Wang, M.; Douglas, J. T.; Zhu, Z. B.; Bravo, A. I.; Gidekel, M.; Alvarez, R. D.; Curiel, D. T.; Podhajcer, O. L. , A Tumor-stroma Targeted Oncolytic Adenovirus Replicated in Human Ovary Cancer Samples and Inhibited Growth of Disseminated Solid Tumors in Mice. Mol Ther 2012. [Google Scholar] [CrossRef] [PubMed]
- Viale, D. L.; Cafferata, E. G.; Gould, D.; Rotondaro, C.; Chernajovsky, Y.; Curiel, D. T.; Podhajcer, O. L.; Lopez, M. V. , Therapeutic improvement of a stroma-targeted CRAd by incorporating motives responsive to the melanoma microenvironment. J Invest Dermatol 2013, 133 (11), 2576–84. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M. V.; Rivera, A. A.; Viale, D. L.; Benedetti, L.; Cuneo, N.; Kimball, K. J.; Wang, M.; Douglas, J. T.; Zhu, Z. B.; Bravo, A. I.; Gidekel, M.; Alvarez, R. D.; Curiel, D. T.; Podhajcer, O. L. , A tumor-stroma targeted oncolytic adenovirus replicated in human ovary cancer samples and inhibited growth of disseminated solid tumors in mice. Mol Ther 2012, 20(12), 2222–33. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, X.; Languino, L. R.; Altieri, D. C. , Evaluation of drug combination effect using a Bliss independence dose-response surface model. Statistics in biopharmaceutical research 2018, 10(2), 112–122. [Google Scholar] [CrossRef]
- Short, J. J.; Rivera, A. A.; Wu, H.; Walter, M. R.; Yamamoto, M.; Mathis, J. M.; Curiel, D. T. , Substitution of adenovirus serotype 3 hexon onto a serotype 5 oncolytic adenovirus reduces factor X binding, decreases liver tropism, and improves antitumor efficacy. Mol Cancer Ther 2010, 9 (9), 2536–44. [Google Scholar] [CrossRef]
- Young, A. M.; Archibald, K. M.; Tookman, L. A.; Pool, A.; Dudek, K.; Jones, C.; Williams, S. L.; Pirlo, K. J.; Willis, A. E.; Lockley, M.; McNeish, I. A. , Failure of translation of human adenovirus mRNA in murine cancer cells can be partially overcome by L4-100K expression in vitro and in vivo. Mol Ther 2012, 20(9), 1676–88. [Google Scholar] [CrossRef]
- Castells, M.; Thibault, B.; Delord, J. P.; Couderc, B. , Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. International journal of molecular sciences 2012, 13(8), 9545–71. [Google Scholar] [CrossRef] [PubMed]
- Tothill, R. W.; Tinker, A. V.; George, J.; Brown, R.; Fox, S. B.; Lade, S.; Johnson, D. S.; Trivett, M. K.; Etemadmoghadam, D.; Locandro, B.; Traficante, N.; Fereday, S.; Hung, J. A.; Chiew, Y. E.; Haviv, I.; Gertig, D.; DeFazio, A.; Bowtell, D. D. , Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res 2008, 14(16), 5198–208. [Google Scholar] [CrossRef]
- Wang, S.; Shu, J.; Chen, L.; Chen, X.; Zhao, J.; Li, S.; Mou, X.; Tong, X. , Synergistic suppression effect on tumor growth of ovarian cancer by combining cisplatin with a manganese superoxide dismutase-armed oncolytic adenovirus. OncoTargets and therapy 2016, 9, 6381–6388. [Google Scholar] [CrossRef]
- Nounamo, B.; Liem, J.; Cannon, M.; Liu, J. , Myxoma Virus Optimizes Cisplatin for the Treatment of Ovarian Cancer In Vitro and in a Syngeneic Murine Dissemination Model. Mol Ther Oncolytics 2017, 6, 90–99. [Google Scholar] [CrossRef] [PubMed]
- González-Pastor, R.; Ashshi, A. M.; El-Shemi, A. G.; Dmitriev, I. P.; Kashentseva, E. A.; Lu, Z. H.; Goedegebuure, S. P.; Podhajcer, O. L.; Curiel, D. T. , Defining a murine ovarian cancer model for the evaluation of conditionally-replicative adenovirus (CRAd) virotherapy agents. Journal of ovarian research 2019, 12 (1), 18. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Qiu, H.; Zhang, M.; Zhang, F.; Yang, H.; Yang, L.; Jia, L.; Qin, K.; Jia, L.; Dou, X.; Cheng, L.; Sang, M.; Zhang, C.; Shan, B.; Zhang, Z. , Soluble CD40 ligands sensitize the epithelial ovarian cancer cells to cisplatin treatment. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 2016; 79, 166–175. [Google Scholar]
- Yi, L.; Zhao, Y.; Wang, X.; Dai, M.; Hellström, K. E.; Hellström, I.; Zhang, H. , Human and Mouse CD137 Have Predominantly Different Binding CRDs to Their Respective Ligands. PLOS ONE 2014, 9 (1), e86337. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Sanmamed, M. F.; Wang, J.; Melero, I. , Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood 2018, 131(1), 49–57. [Google Scholar] [CrossRef]
- Guo, Z.; Cheng, D.; Xia, Z.; Luan, M.; Wu, L.; Wang, G.; Zhang, S. , Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J Transl Med 2013, 11, 215. [Google Scholar] [CrossRef]
- Galanis, E.; Hartmann, L. C.; Cliby, W. A.; Long, H. J.; Peethambaram, P. P.; Barrette, B. A.; Kaur, J. S.; Haluska, P. J., Jr.; Aderca, I.; Zollman, P. J.; Sloan, J. A.; Keeney, G.; Atherton, P. J.; Podratz, K. C.; Dowdy, S. C.; Stanhope, C. R.; Wilson, T. O.; Federspiel, M. J.; Peng, K. W.; Russell, S. J. , Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res 2010, 70(3), 875–82. [Google Scholar] [CrossRef]
- Hoare, J.; Campbell, N.; Carapuça, E. , Oncolytic virus immunotherapies in ovarian cancer: moving beyond adenoviruses. Porto biomedical journal 2018, 3(1), e7. [Google Scholar] [CrossRef]
- Vasey, P. A.; Shulman, L. N.; Campos, S.; Davis, J.; Gore, M.; Johnston, S.; Kirn, D. H.; O'Neill, V.; Siddiqui, N.; Seiden, M. V.; Kaye, S. B. , Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol 2002, 20(6), 1562–9. [Google Scholar]
- Kim, K. H.; Dmitriev, I. P.; Saddekni, S.; Kashentseva, E. A.; Harris, R. D.; Aurigemma, R.; Bae, S.; Singh, K. P.; Siegal, G. P.; Curiel, D. T.; Alvarez, R. D. , A phase I clinical trial of Ad5/3-Δ24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol Oncol 2013, 130(3), 518–24. [Google Scholar] [CrossRef]
- Cerullo, V.; Pesonen, S.; Diaconu, I.; Escutenaire, S.; Arstila, P. T.; Ugolini, M.; Nokisalmi, P.; Raki, M.; Laasonen, L.; Sarkioja, M.; Rajecki, M.; Kangasniemi, L.; Guse, K.; Helminen, A.; Ahtiainen, L.; Ristimaki, A.; Raisanen-Sokolowski, A.; Haavisto, E.; Oksanen, M.; Karli, E.; Karioja-Kallio, A.; Holm, S. L.; Kouri, M.; Joensuu, T.; Kanerva, A.; Hemminki, A. , Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res 2010, 70(11), 4297–309. [Google Scholar] [CrossRef] [PubMed]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; Jäger, E.; Karbach, J.; Wahle, C.; Kankainen, M.; Backman, C.; von Euler, M.; Haavisto, E.; Hakonen, T.; Heiskanen, R.; Jaderberg, M.; Juhila, J.; Priha, P.; Suoranta, L.; Vassilev, L.; Vuolanto, A.; Joensuu, T. , Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers. Journal for immunotherapy of cancer 2016, 4, 17. [Google Scholar] [CrossRef]
- Gonzalez-Pastor, R.; Goedegebuure, P. S.; Curiel, D. T. , Understanding and addressing barriers to successful adenovirus-based virotherapy for ovarian cancer. Cancer Gene Ther 2020. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T. , Multidisciplinary oncolytic virotherapy for gastrointestinal cancer. Annals of gastroenterological surgery 2019, 3(4), 396–404. [Google Scholar] [CrossRef]
- Wu, H.; Dmitriev, I.; Kashentseva, E.; Seki, T.; Wang, M.; Curiel, D. T. , Construction and Characterization of Adenovirus Serotype 5 Packaged by Serotype 3 Hexon. Journal of Virology 2002, 76(24), 12775–12782. [Google Scholar] [CrossRef]
- Lopez, M. V. n.; Viale, D. L.; Cafferata, E. G. A.; Bravo, A. I.; Carbone, C.; Gould, D.; Chernajovsky, Y.; Podhajcer, O. L. , Tumor Associated Stromal Cells Play a Critical Role on the Outcome of the Oncolytic Efficacy of Conditionally Replicative Adenoviruses. PLoS ONE 2009, 4(4), e5119. [Google Scholar] [CrossRef]
- Kirby, T. O.; Rivera, A.; Rein, D.; Wang, M.; Ulasov, I.; Breidenbach, M.; Kataram, M.; Contreras, J. L.; Krumdieck, C.; Yamamoto, M.; Rots, M. G.; Haisma, H. J.; Alvarez, R. D.; Mahasreshti, P. J.; Curiel, D. T. , A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin Cancer Res 2004, 10(24), 8697–703. [Google Scholar] [CrossRef]
Figure 1.
In vitro lytic activity of AR2011. (A) lytic effect of different MOIs of AR2011 on various human cervical cancer cell lines. (B to E) AR2011 replication on fresh explants obtained from human ovarian, cervix and uterus cancer samples and normal uterus. For further details and statistical analysis, see materials and methods. Statistical significance, *p<0.05, **p<0.01, ***p<0.001.
Figure 1.
In vitro lytic activity of AR2011. (A) lytic effect of different MOIs of AR2011 on various human cervical cancer cell lines. (B to E) AR2011 replication on fresh explants obtained from human ovarian, cervix and uterus cancer samples and normal uterus. For further details and statistical analysis, see materials and methods. Statistical significance, *p<0.05, **p<0.01, ***p<0.001.
Figure 2.
In vitro lytic activity of AR2011 on malignant cells obtained from human ovarian cancer ascites. (A) Lytic effect of AR2011 at different MOIs on three different samples of OC-AF. (B) lytic effect of AR2011 at MOI 100 on different samples of OC-AF combined or not with cisplatin. *p<0.05, **p<0.01, ***p<0.001. For further details, see materials and methods.
Figure 2.
In vitro lytic activity of AR2011 on malignant cells obtained from human ovarian cancer ascites. (A) Lytic effect of AR2011 at different MOIs on three different samples of OC-AF. (B) lytic effect of AR2011 at MOI 100 on different samples of OC-AF combined or not with cisplatin. *p<0.05, **p<0.01, ***p<0.001. For further details, see materials and methods.
Figure 3.
Cytokines expression and lytic activity of AR2011(h404). (A) Scheme of AR2011(h404) genome structure showing that the cytokines cassette under hTERT regulation was cloned in the 3’-5’ orientation in the AR2011(h404) genome. (B) Cell surface expression of hCD40L in SKOV-3 (left) and A549 (right) cells: Light grey: Isotype control, Grey: AR2011(h404) MOI 100; Dark grey: AR2011(h404) MOI 1000. (C) Western Blot for the detection of h4-1BBL expression. The blot shows that both cell lines expressed endogenous 4-1BBL. Data for cytokines’ expression was obtained 30 hr after infection since after 48 hr cells were completely lysed by the OAdVs. (D) In vitro lytic activity of AR2011(h404) in different human malignant cell lines. Percent of surviving cells was assessed by the MTS assay. AR2011 was used as a comparator.
Figure 3.
Cytokines expression and lytic activity of AR2011(h404). (A) Scheme of AR2011(h404) genome structure showing that the cytokines cassette under hTERT regulation was cloned in the 3’-5’ orientation in the AR2011(h404) genome. (B) Cell surface expression of hCD40L in SKOV-3 (left) and A549 (right) cells: Light grey: Isotype control, Grey: AR2011(h404) MOI 100; Dark grey: AR2011(h404) MOI 1000. (C) Western Blot for the detection of h4-1BBL expression. The blot shows that both cell lines expressed endogenous 4-1BBL. Data for cytokines’ expression was obtained 30 hr after infection since after 48 hr cells were completely lysed by the OAdVs. (D) In vitro lytic activity of AR2011(h404) in different human malignant cell lines. Percent of surviving cells was assessed by the MTS assay. AR2011 was used as a comparator.
Figure 4.
In vivo efficacy of the OAdVs. (A) Nude mice harboring established SKOV-3 tumors were injected with PBS, AR2011 or AR2011(h404) at the indicated times (see arrows). Tumor growth was followed with digital calipers. At day 56 the experiment was finalized. (B) Mice harboring intraperitoneal 5 days-old SKOV-3 tumors were i.p. injected either with PBS, AR2011 or AR2011(h404) as described in Methods. At the end of the study tumors were removed, photographed, and weighted. (C) Levels of viral E4 in tumor samples as a surrogate marker of virion numbers. **p<0.01, ***p<0.001. For statistical analysis, see materials and methods.
Figure 4.
In vivo efficacy of the OAdVs. (A) Nude mice harboring established SKOV-3 tumors were injected with PBS, AR2011 or AR2011(h404) at the indicated times (see arrows). Tumor growth was followed with digital calipers. At day 56 the experiment was finalized. (B) Mice harboring intraperitoneal 5 days-old SKOV-3 tumors were i.p. injected either with PBS, AR2011 or AR2011(h404) as described in Methods. At the end of the study tumors were removed, photographed, and weighted. (C) Levels of viral E4 in tumor samples as a surrogate marker of virion numbers. **p<0.01, ***p<0.001. For statistical analysis, see materials and methods.
Figure 5.
In vivo studies in syngeneic mice models. (A) and (B) In vitro lytic activity of AR2011(H3) and AR2011(m404) on ID8 and CT26 cells. Surviving cells were assessed with the MTS assay. (C) and (D) Mice harboring established CT26 tumors in both flanks were injected in the left flank either with PBS, AR2011(H3) or AR2011(m404) and followed as described in the text. The arrows indicate the days of AdOV or PBS injection (E) Kaplan-Meier survival curve of mice harbouring established CT26 tumors in the left flank injected in the right flank either with CT-PBS, CT-AR or CT-AR404 cells. Statistical analysis: comparison of CT-PBS vs CT-AR404, p = 0.0017 (**) and CT-AR vs CT-AR404, p = 0.0009 (***) using the Long –Rank (Mantel-Cox) test. For further details, see materials and methods.
Figure 5.
In vivo studies in syngeneic mice models. (A) and (B) In vitro lytic activity of AR2011(H3) and AR2011(m404) on ID8 and CT26 cells. Surviving cells were assessed with the MTS assay. (C) and (D) Mice harboring established CT26 tumors in both flanks were injected in the left flank either with PBS, AR2011(H3) or AR2011(m404) and followed as described in the text. The arrows indicate the days of AdOV or PBS injection (E) Kaplan-Meier survival curve of mice harbouring established CT26 tumors in the left flank injected in the right flank either with CT-PBS, CT-AR or CT-AR404 cells. Statistical analysis: comparison of CT-PBS vs CT-AR404, p = 0.0017 (**) and CT-AR vs CT-AR404, p = 0.0009 (***) using the Long –Rank (Mantel-Cox) test. For further details, see materials and methods.
Table 1.
Characteristics of the human fresh explants.
Table 1.
Characteristics of the human fresh explants.
| Sample |
Pathology |
Observation |
| 1 |
Epithelial ovarian cancer |
First cytoreduction |
| 2 |
Epithelial ovarian cancer |
Relapse |
| 3 |
Epithelial ovarian cancer |
Bilateral tumor Neoadjuvant chemotherapy C, P and Bevacizumab |
| 4 |
Epithelial ovarian cancer |
Neoadjuvant chemotherapy |
| 5 |
Krukenberg tumor |
First cytoreduction |
| 6 |
Cervical cancer |
Conization |
| 7 |
Cervical cancer |
Conization |
| 8 |
Cervical cancer |
Simple hysterectomy |
| 9 |
Cervical cancer |
Simple hysterectomy |
| 10 |
Cervical cancer |
Conization |
| 11 |
Cervical cancer |
Simple hysterectomy |
| 12 |
Uterus cancer |
Radical Hysterectomy |
| 13 |
Uterus cancer |
Normal uterus tissue after radical hysterectomy* |
| 14 |
Uterus cancer |
Normal uterus tissue obtained after radical hysterectomy* |
| 15 |
Uterus cancer |
Hysterectomy |
| 16 |
Uterus cancer |
Hysterectomy |
Table 2.
Analysis of the synergistic interaction of AR2011 and cisplatin combination on OC-AF samples.
Table 2.
Analysis of the synergistic interaction of AR2011 and cisplatin combination on OC-AF samples.
| OC-AF |
Y(P)1 |
Y(O)1 |
| 8 |
0.30 |
0.60 |
| 10 |
0.71 |
0.81 |
| 14 |
0.66 |
0.64 |
| 15 |
0.66 |
0.70 |
| 18 |
0.81 |
0.77 |
| 19 |
0.57 |
0.66 |
| 20 |
0.80 |
0.85 |
| 21 |
0.88 |
0.87 |
| 24 |
0.78 |
0.80 |
| 28 |
0.80 |
0.87 |
| 30 |
0.88 |
0.83 |
| 38 |
0.77 |
0.74 |
| 39 |
0.61 |
0.72 |
| 40 |
0.90 |
0.92 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).