Submitted:
26 April 2023
Posted:
03 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background
1.2. Born polarity vs. Onsager polarity: What’s the difference?
2. Results
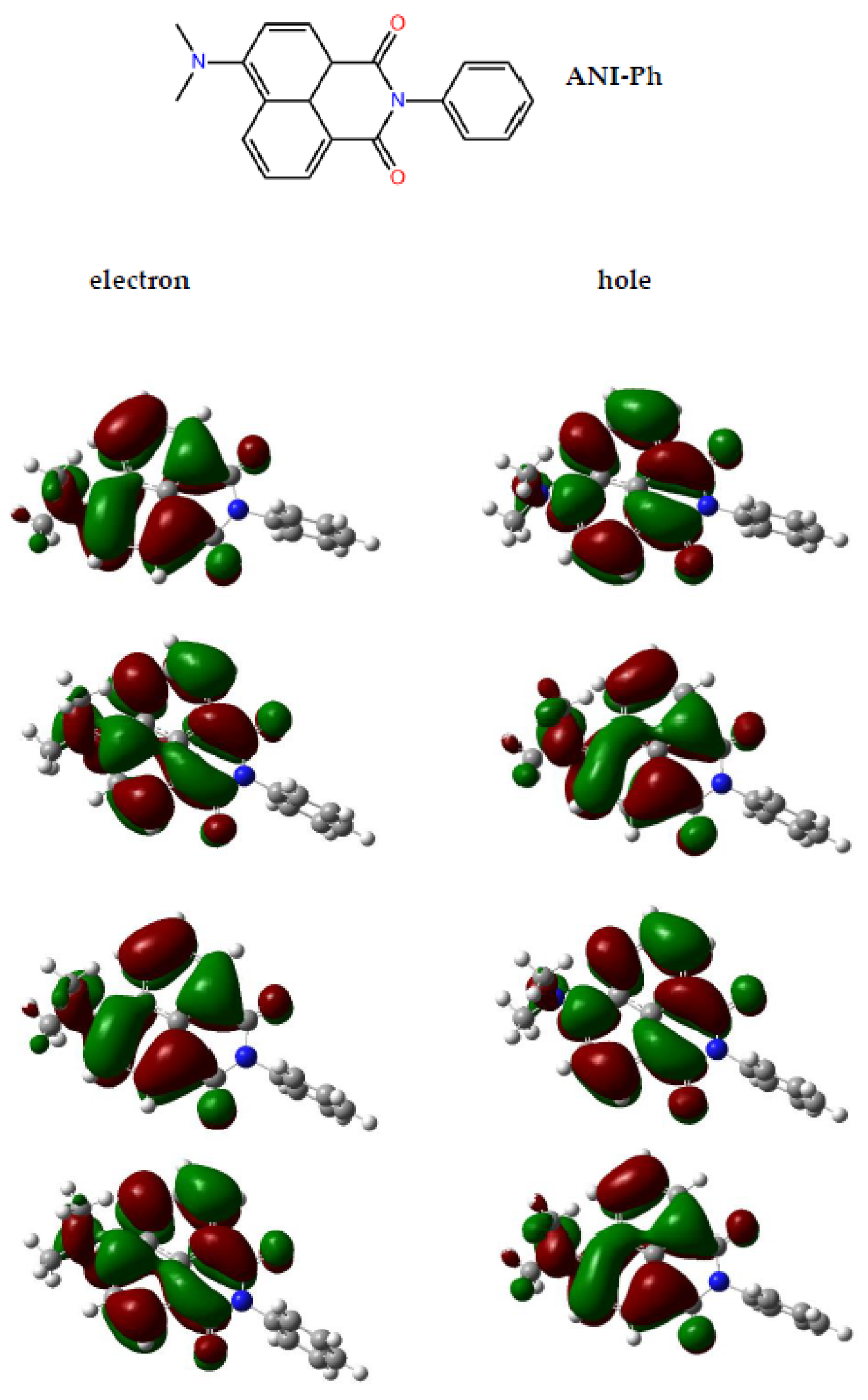
2.1. Selection of a Photoprobe
2.2. Onsager Polarity of Electrolyte Solutions
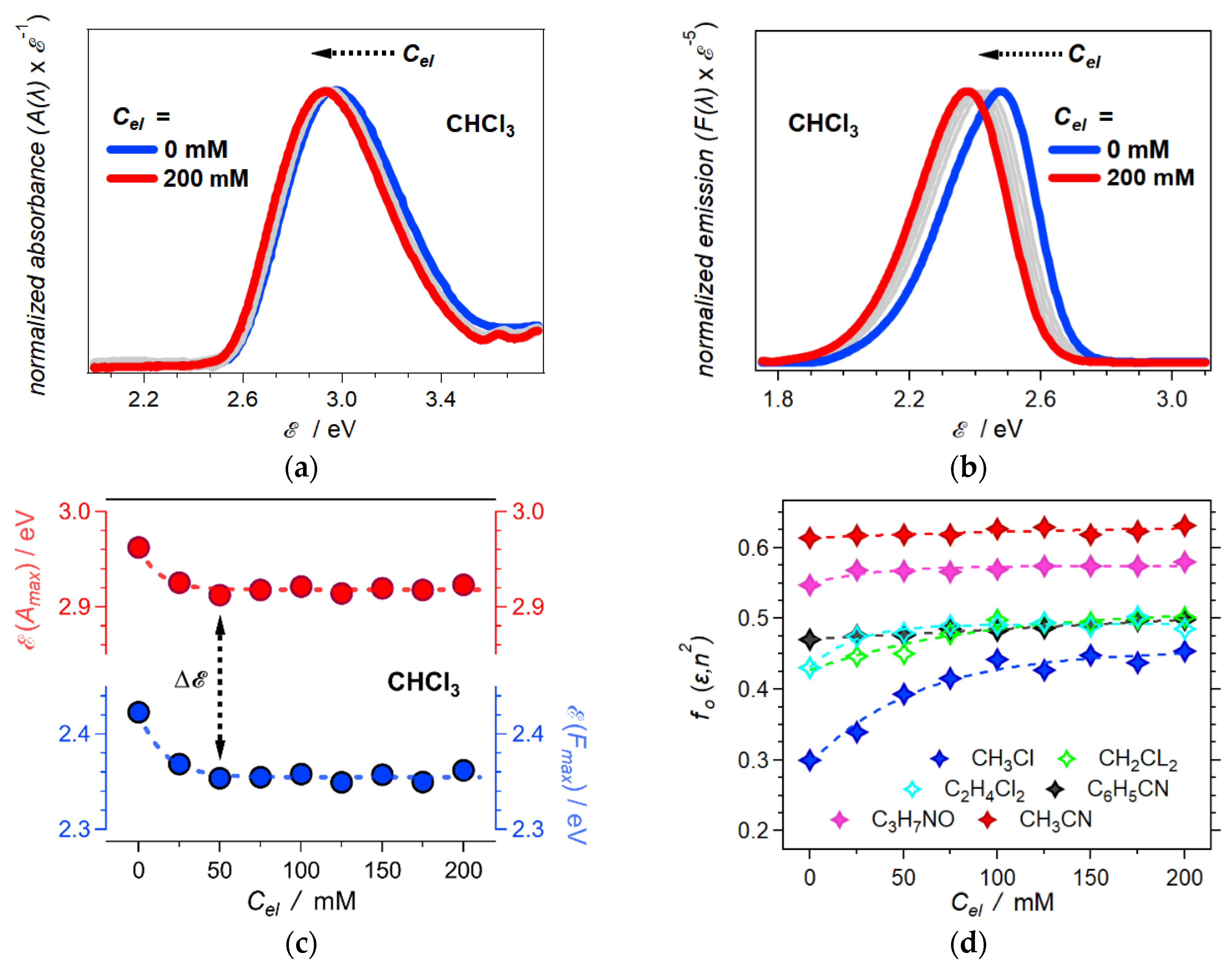
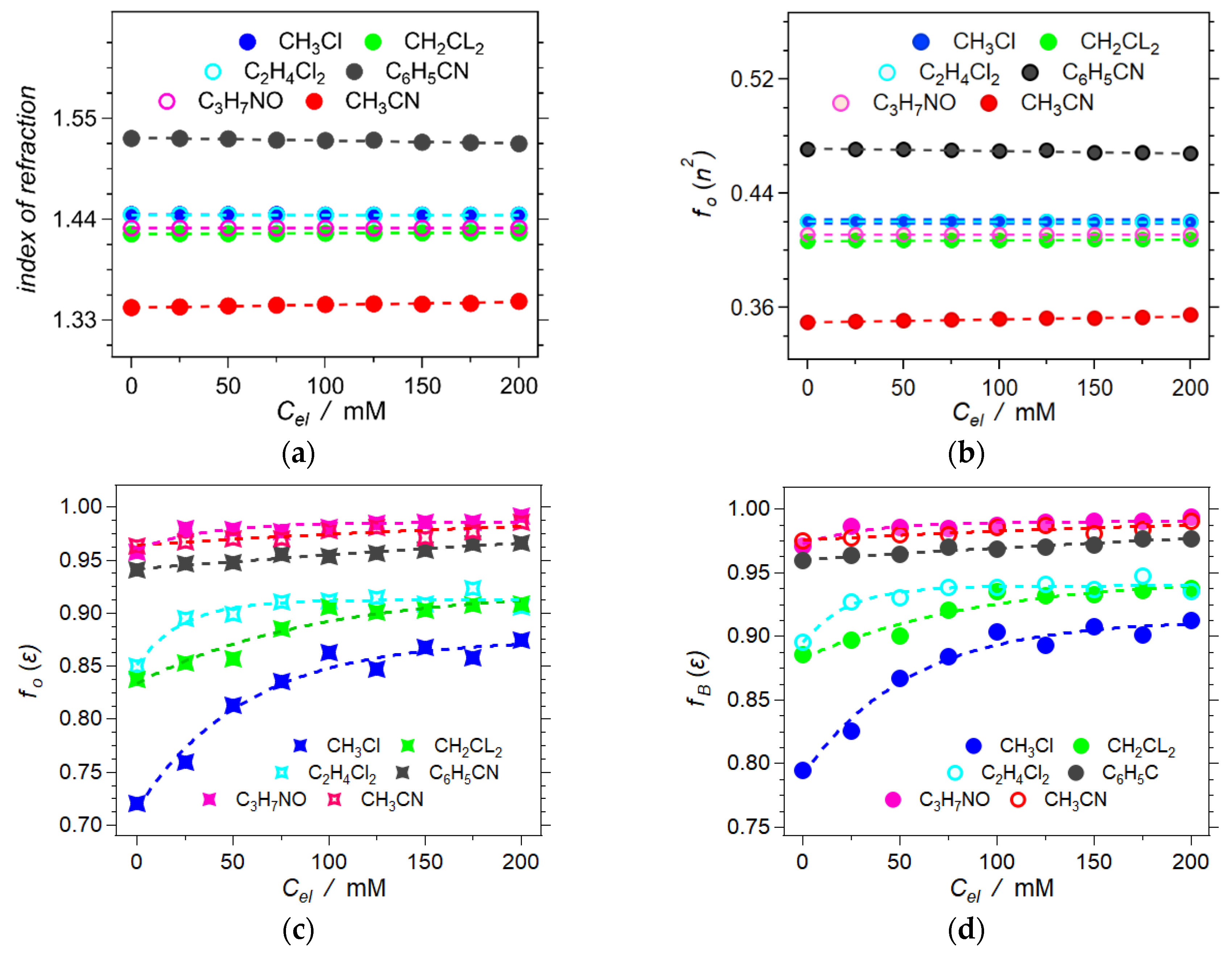
2.3. Born Polarity of Electrolyte Solutions
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Derr, J.B.; Tamayo, J.; Clark, J.A.; Morales, M.; Mayther, M.F.; Espinoza, E.M.; Rybicka-Jasinska, K.; Vullev, V.I. Multifaceted aspects of charge transfer. Phys. Chem. Chem. Phys. 2020, 22, 21583–21629. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Fara, D.C.; Yang, H.; Tämm, K.; Tamm, T.; Karelson, M. Quantitative Measures of Solvent Polarity. Chem. Rev. 2004, 104, 175–198. [Google Scholar] [CrossRef]
- Spange, S.; Weiß, N.; Schmidt, C.H.; Schreiter, K. Reappraisal of Empirical Solvent Polarity Scales for Organic Solvents. Chemistry–Methods 2021, 1, 42–60. [Google Scholar] [CrossRef]
- Schein, C.H. Solubility as a Function of Protein-Structure and Solvent Components. Bio-Technol. 1990, 8, 308–315. [Google Scholar] [CrossRef]
- Tedder, J.M. Which Factors Determine the Reactivity and Regioselectivity of Free-Radical Substitution and Addition-Reactions. Angew. Chem. Int. Edit. 1982, 21, 401–410. [Google Scholar] [CrossRef]
- Espinoza, E.M.; Clark, J.A.; Soliman, J.; Derr, J.B.; Morales, M.; Vullev, V.I. Practical Aspects of Cyclic Voltammetry: How to Estimate Reduction Potentials When Irreversibility Prevails. J. Electrochem. Soc. 2019, 166, H3175–H3187. [Google Scholar] [CrossRef]
- Karmakar, N.K.; Pandey, S.; Pandey, R.K.; Shukla, S.S. Solvatochromism: a tool for solvent discretion for UV-Vis spectroscopic studies. Appl. Spectrosc. Rev. 2021, 56, 513–529. [Google Scholar] [CrossRef]
- Ryu, H.G.; Mayther, M.F.; Tamayo, J.; Azarias, C.; Espinoza, E.M.; Banasiewicz, M.; Lukasiewicz, L.G.; Poronik, Y.M.; Jezewski, A.; Clark, J. , et al. Bidirectional Solvatofluorochromism of a Pyrrolo[3,2-b]pyrrole-Diketopyrrolopyrrole Hybrid. J. Phys. Chem. C 2018, 122, 13424–13434. [Google Scholar] [CrossRef]
- Jones, G., II; Vullev, V.I. Medium Effects on the Stability of Terbium(III) Complexes with Pyridine-2,6-dicarboxylate. J. Phys. Chem. A 2002, 106, 8213–8222. [Google Scholar] [CrossRef]
- Larsen, J.M.; Espinoza, E.M.; Hartman, J.D.; Lin, C.-K.; Wurch, M.; Maheshwari, P.; Kaushal, R.K.; Marsella, M.J.; Beran, G.J.O.; Vullev, V.I. Building blocks for bioinspired electrets: molecular-level approach to materials for energy and electronics. Pure Appl. Chem. 2015, 87, 779–792. [Google Scholar] [CrossRef]
- Born, M. Volumes and heats of hydration of ions. Z. Phys. 1920, 1, 45–48. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron Transfers in Chemistry and Biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar] [CrossRef]
- O'Mari, O.; Vullev, V.I. Electrochemical analysis in charge-transfer science: The devil in the details. Curr. Opin. Electrochem. 2022, 31, 100862. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Millare, B.; Xia, W.; Steyer, B.G.; Gerasimenko, A.A.; Ferreira, A.; Contreras, A.; Vullev, V.I. Electrochemical Oxidation of Ferrocene: A Strong Dependence on the Concentration of the Supporting Electrolyte for Nonpolar Solvents. J. Phys. Chem. A 2009, 113, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Ramu, S.; Contreras, A.; Upadhyayula, S.; Vasquez, J.M.; Beran, G.; Vullev, V.I. Electrochemical Reduction of Quinones: Interfacing Experiment and Theory for Defining Effective Radii of Redox Moieties. J. Phys. Chem. B 2010, 114, 14467–14479. [Google Scholar] [CrossRef]
- Asaki, M.L.T.; Redondo, A.; Zawodzinski, T.A.; Taylor, A.J. Dielectric relaxation of electrolyte solutions using terahertz transmission spectroscopy. J. Chem. Phys. 2002, 116, 8469–8482. [Google Scholar] [CrossRef]
- Gavish, N.; Promislow, K. Dependence of the dielectric constant of electrolyte solutions on ionic concentration: A microfield approach. Phys. Rev. E 2016, 94. [Google Scholar] [CrossRef]
- Paljk, Š.; Klofutar, C.; Lubej, M. Dielectric studies of some tri-n-alkylammonium nitrates and perchlorates in benzene solutions at 298.15 K. J. Chem. Soc., Faraday Trans. 1 1984, 80, 1957–1964. [Google Scholar] [CrossRef]
- Gestblom, B.; Svorstol, I.; Songstad, J. Dielectric-Properties of Solutions of Some Onium Salts in Dichloromethane. J. Phys. Chem. 1986, 90, 4684–4686. [Google Scholar] [CrossRef]
- Wang, P.M.; Anderko, A. Computation of dielectric constants of solvent mixtures and electrolyte solutions. Fluid Phase Equilibr. 2001, 186, 103–122. [Google Scholar] [CrossRef]
- Sigvartsen, T.; Songstad, J.; Gestblom, B.; Noreland, E. Dielectric-Properties of Solutions of Tetra-Iso-Pentylammonium Nitrate in Dioxane-Water Mixtures. J. Solution Chem. 1991, 20, 565–582. [Google Scholar] [CrossRef]
- Sigvartsen, T.; Gestblom, B.; Noreland, E.; Songstad, J. Conductometric and Dielectric Behavior of Solutions of Tetrabutylammonium Perchlorate in Solvents of Low and Medium Permittivity. Acta Chem. Scand. 1989, 43, 103–115. [Google Scholar] [CrossRef]
- Cachet, H. From the bulk electrolyte solution to the electrochemical interface. Condens. Matter Phys. 2017, 20, 33701. [Google Scholar] [CrossRef]
- Breitung, E.M.; Vaughan, W.E.; McMahon, R.J. Measurement of solute dipole moments in dilute solution: A simple three-terminal cell. Rev. Sci. Instrum. 2000, 71, 224–227. [Google Scholar] [CrossRef]
- Hu, J.; Xia, B.; Bao, D.; Ferreira, A.; Wan, J.; Jones, G.; Vullev, V.I. Long-Lived Photogenerated States of α-Oligothiophene-Acridinium Dyads Have Triplet Character. J. Phys. Chem. A 2009, 113, 3096–3107. [Google Scholar] [CrossRef] [PubMed]
- Onsager, L. Electric moments of molecules in liquids. J. Am. Chem. Soc. 1936, 58, 1486–1493. [Google Scholar] [CrossRef]
- Lippert, E. Dipole moment and electronic structure of excited molecules. Z. Naturforsch. 1955, 10a, 541–545. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent Effects Upon Fluorescence Spectra and the Dipolemoments of Excited Molecules. B. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
- Kakitani, T.; Mataga, N. New Energy-Gap Laws for the Charge Separation Process in the Fluorescence Quenching Reaction and the Charge Recombination Process of Ion-Pairs Produced in Polar-Solvents. J. Phys. Chem. 1985, 89, 8–10. [Google Scholar] [CrossRef]
- Ooshika, Y. Absorption Spectra of Dyes in Solution. J. Phys. Soc. Jpn. 1954, 9, 594–602. [Google Scholar] [CrossRef]
- Varghese, A.; Akshaya, K.B. Application of Fluorescence in Solvatochromic Studies of Organic Compounds. In Reviews in Fluorescence 2017, Geddes, C.D., Ed. Springer International Publishing: Cham, 2018; pp. 99-121. [CrossRef]
- Poronik, Y.M.; Baryshnikov, G.V.; Deperasinska, I.; Espinoza, E.M.; Clark, J.A.; Agren, H.; Gryko, D.T.; Vullev, V.I. Deciphering the unusual fluorescence in weakly coupled bis-nitro-pyrrolo[3,2-b]pyrroles. Commun. Chem. 2020, 3, 190. [Google Scholar] [CrossRef]
- Derr, J.B.; Tamayo, J.; Espinoza, E.M.; Clark, J.A.; Vullev, V.I. Dipole-induced effects on charge transfer and charge transport. Why do molecular electrets matter? Can. J. Chem. 2018, 96, 843–858. [Google Scholar] [CrossRef]
- Mayther, M.F.; O’Mari, O.; Flacke, P.; Bhatt, D.; Andrews, S.; Vullev, V.I. How Do Liquid-Junction Potentials and Medium Polarity at Electrode Surfaces Affect Electrochemical Analyses for Charge-Transfer Systems? J. Phys. Chem. B 2023, 127, 1443–1458. [Google Scholar] [CrossRef]
- Upadhyayula, S.; Bao, D.; Millare, B.; Sylvia, S.S.; Habib, K.M.M.; Ashraf, K.; Ferreira, A.; Bishop, S.; Bonderer, R.; Baqai, S. , et al. Permanent Electric Dipole Moments of Carboxyamides in Condensed Media: What Are the Limitations of Theory and Experiment? J. Phys. Chem. B 2011, 115, 9473–9490. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Israel J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Pekar, S.I. Local quantum states of the electron in an ideal ionic crystal. Zh. Eksp. Teor. Fiz. 1946, 16, 341–348. [Google Scholar]
- Vlassiouk, I.; Smirnov, S. Electric Polarization of Dilute Polar Solutions: Revised Treatment for Arbitrary Shaped Molecules. J. Phys. Chem. A 2003, 107, 7561–7566. [Google Scholar] [CrossRef]
- Kirkwood, J.G. The Dielectric Polarization of Polar Liquids. J. Chem. Phys. 2004, 7, 911–919. [Google Scholar] [CrossRef]
- Song, H.; Wang, K.; Kuang, Z.; Zhao, Y.S.; Guo, Q.; Xia, A. Solvent modulated excited state processes of push–pull molecule with hybridized local excitation and intramolecular charge transfer character. Phys. Chem. Chem. Phys. 2019, 21, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Angulo, G.; Grampp, G.; Rosspeintner, A. Recalling the appropriate representation of electronic spectra. Spectrochim. Acta A 2006, 65, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Daniels, I.N.; Wang, Z.X.; Laird, B.B. Dielectric Properties of Organic Solvents in an Electric Field. J. Phys. Chem. C 2017, 121, 1025–1031. [Google Scholar] [CrossRef]
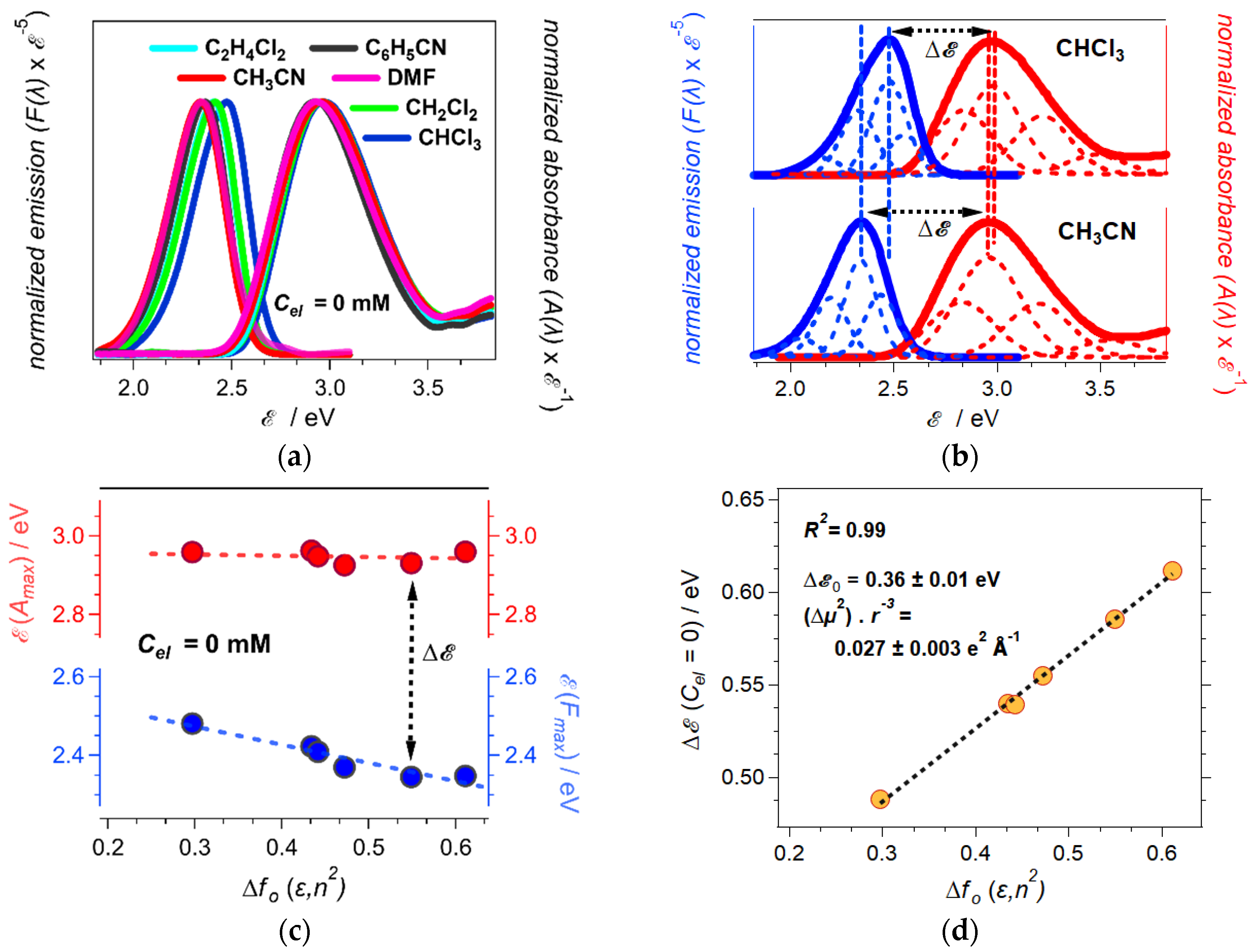

| solvent | fO(ε, n2) a | |μ0| / D b | |μ*| / D b | α / deg c | Δμ / D d | |Δμ| / D e | |μ0| / D b |
|---|---|---|---|---|---|---|---|
| CHCl3 | 0.29 | 6.67 | 9.97 | 2.7 | 3.30 | 3.32 | 0.29 |
| CH2Cl2 | 0.43 | 7.07 | 10.41 | 2.7 | 3.34 | 3.36 | 0.43 |
| (CH2Cl)2 | 0.44 | 7.11 | 10.49 | 2.7 | 3.38 | 3.40 | 0.44 |
| C6H5CN | 0.47 | 7.29 | 10.87 | 2.7 | 3.58 | 3.60 | 0.47 |
| DMF | 0.55 | 7.33 | 10.95 | 2.7 | 3.62 | 3.64 | 0.55 |
| CH3CN | 0.61 | 7.33 | 10.93 | 2.7 | 3.60 | 3.62 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





