1. Introduction
The taxonomic status of the cosmopolitan genus
Protospirura Seurat, 1914 (Spiruridae) has been confusing for a long time, particularly due to the morphological similitudes with the genus
Mastophorus Diesing, 1853 (Spirocercidae). According to several authors [
1,
2], this confusion is due to the consideration of inappropriate characters. In this sense, Chitwood [
1] established the differential characteristics between the genera
Protospirura and
Mastophorus: the number of teeth in the pseudolabia (two or four in
Protospirura vs. three, five, seven or nine in
Mastophorus), the morphology of the pharynx (laterally compressed in
Protospirura vs. cylindrical in
Mastophorus), the morphology of cloacal papillae in males (sessile in
Protospirura vs. pedunculated in
Mastophorus), the tail length in males (short in
Protospirura vs. long in
Mastophorus) and the position of the vulva in females (postequatorial in
Protospirura vs. preequatorial in
Mastophorus). However, some of these characteristics are not present in all the currently accepted species of the genus
Protospirura. In fact, some
Protospirura species have pedunculated cloacal papillae [
3,
4,
6,
10,
16] or the vulva is located anteriorly to mid-body [
5,
11,
16]. Also, the morphology of pseudolabia in the genera
Protospirura and
Mastophorus is quite different. In
Protospirura the four submedian lobes are clearly less developed than the lateral lobes whereas in
Mastophorus the oral opening is hexagonal and the submedian lobes of pseudolabia are well developed and quadrangular [
17].
Currently, the genus
Protospirura comprises 13 species parasites of mammals included in five orders and in 18 families: Artiodactyla (Bovidae), Carnivora (Canidae, Felidae and Viverridae), Eulipotyphla (Erinaceidae and Talpidae), Primates (Aotidae, Atelidae, Cebidae, Cercopythecidae, Hominidae and Lorisidae) and Rodentia (Bathyergidae, Cricetidae, Heteromyidae, Muridae, Nesomyidae and Sciuridae) [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
18,
19]. Except for
P. muricola, which has a worldwide distribution including Africa, the Caribbean region, Central and South America, Southeast Asia and Europe [
14], the remaining species of this genus have a limited geographical distribution. With respect to the parasitized hosts, only seven species of
Protospirura have been recorded in Muridae rodents. These are
P. armeniana,
P. chabaudi,
P. kaindiensis,
P. munimuniensis,
P. muricola,
P. okinavensis and
P. siamensis [
8,
9,
13,
14,
15,
16,
18,
19]. Other species described as belonging to the genus
Protospirura, namely
P. ascaroidea,
P. bestiarum,
P. columbiana,
P. glareoli,
P. gracilis,
P. labiodenta and
P. marsupialis were considered synonyms of
M. muris by Chitwood [
1]. These synonymies were further confirmed by Wertheim [
2]. Additionally, other species described posteriorly, namely
P. chanchanensis,
P. paucidentata and
P. srivastavai should be included in the Spirocercidae because their pharynx is not laterally compressed [
8]. Finally,
P. bonnei was considered synonym of
P. muricola and
P. congolense was transferred to the genus
Mastophorus by Quentin [
20].
In the present study, we describe a new species, Protospirura canariensis n. sp., parasitizing the black rat (Rattus rattus) in El Hierro Island (Canary Archipelago, Spain). Additionally, the sequence of the mitochondrial cytochrome c oxidase subunit I gene (cox1) is provided and compared with data of related species.
3. Results
3.1. Taxonomic summary
Family Spiruridae Oerley, 1885
Genus Protospirura Seurat, 1914
Type host: Rattus rattus Linnaeus, 1758 (Rodentia: Muridae).
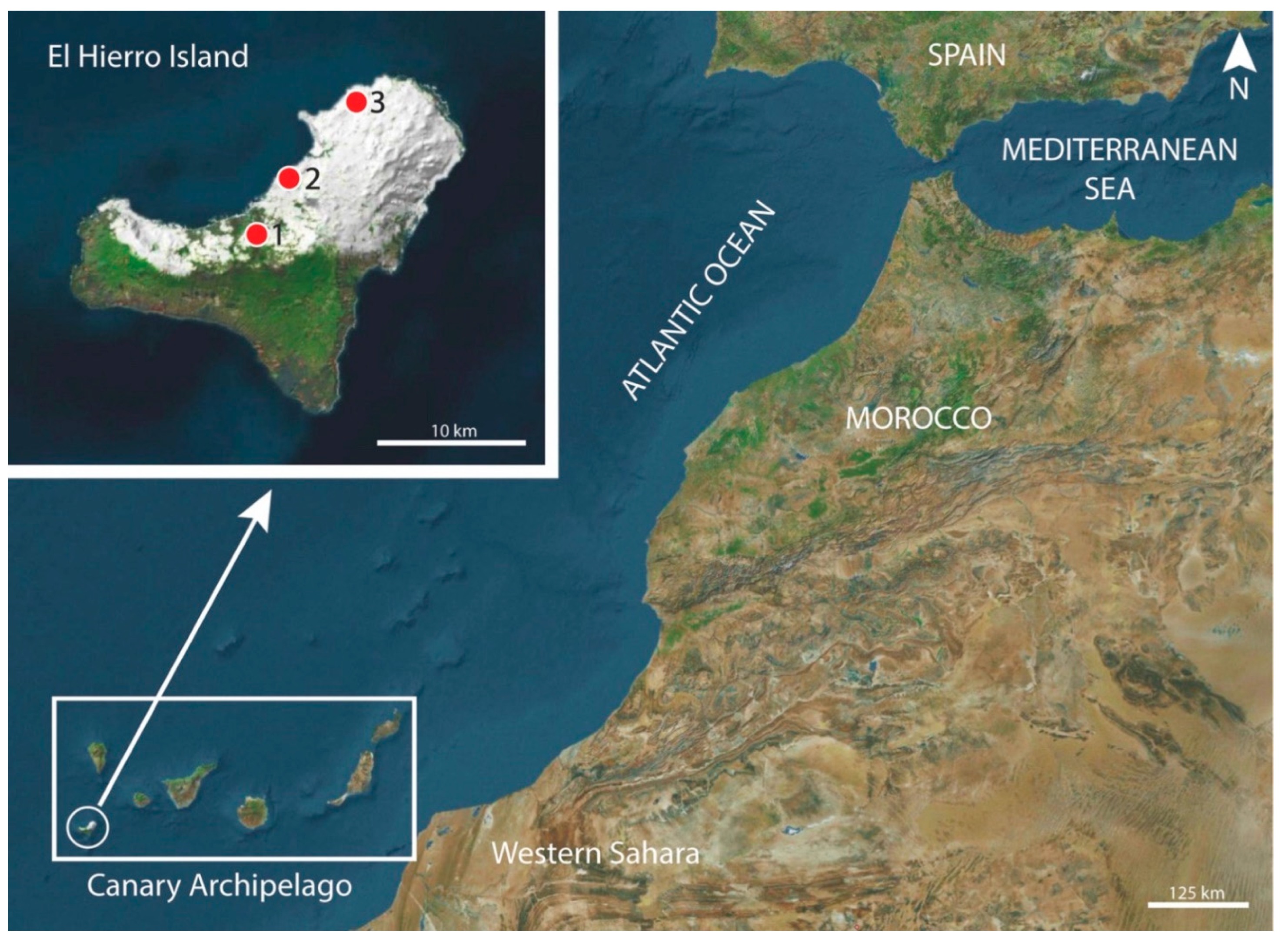
Type locality: Lagartario-Frontera (El Hierro Island, Canary Archipelago, Spain) (27° 46' 29.9" N, 17° 59' 55.59" W) (
Figure 1).
Other localities: Camino-Frontera (El Hierro Island, Canary Archipelago, Spain) (27° 44' 38.07" N, 18° 2' 25.4" W), Túnel-Valverde (El Hierro Island, Canary Archipelago, Spain) (27° 49' 12.41" N, 17° 57' 49.27" W) (
Figure 1).
Site of infection: stomach.
Type specimens: deposited in the "Muséum National d'Histoire Naturelle" (Paris, France) under the following accession numbers:
- holotype,

No. 1, MNHN HEL1905.
- allotype,

No. 1, MNHN HEL1906.
- 19 paratypes:
- 7 males: ♂︎No. 2, MNHN HEL1907; ♂︎No. 4, MNHN HEL1908; ♂︎No. 5, MNHN HEL1909; ♂︎No. 7, MNHN HEL1910; ♂︎No. 8, MNHN HEL1911; ♂︎No. 10, MNHN HEL1912 and ♂︎No. 11, MNHN HEL1913.
- 12 females: ♀︎No. 2, MNHN HEL1914; ♀︎No. 3, MNHN HEL1915; ♀︎No. 4, MNHN HEL1916; ♀︎No. 5, MNHN HEL1917; ♀︎No. 6, MNHN HEL1918; ♀︎No. 7, MNHN HEL1919; ♀︎No. 8, MNHN HEL1920; ♀︎No. 9, MNHN HEL1921; ♀︎No. 10, MNHN HEL1922; ♀︎No. 11, MNHN HEL1923; ♀︎No. 12, MNHN HEL1924 and ♀︎No. 13, MNHN HEL1925.
Mitochondrial cytochrome c oxidase subunit I gene (cox1) sequence: a fragment of 650-bp was obtained for the cox1. Four fragments of 409-bp were successfully sequenced and submitted to the GenBank database; accession numbers: OQ799521, OQ799522, OQ799523 and OQ799524.
Etymology: the specific name of this nematode refers to its geographical distribution.
3.2. Description
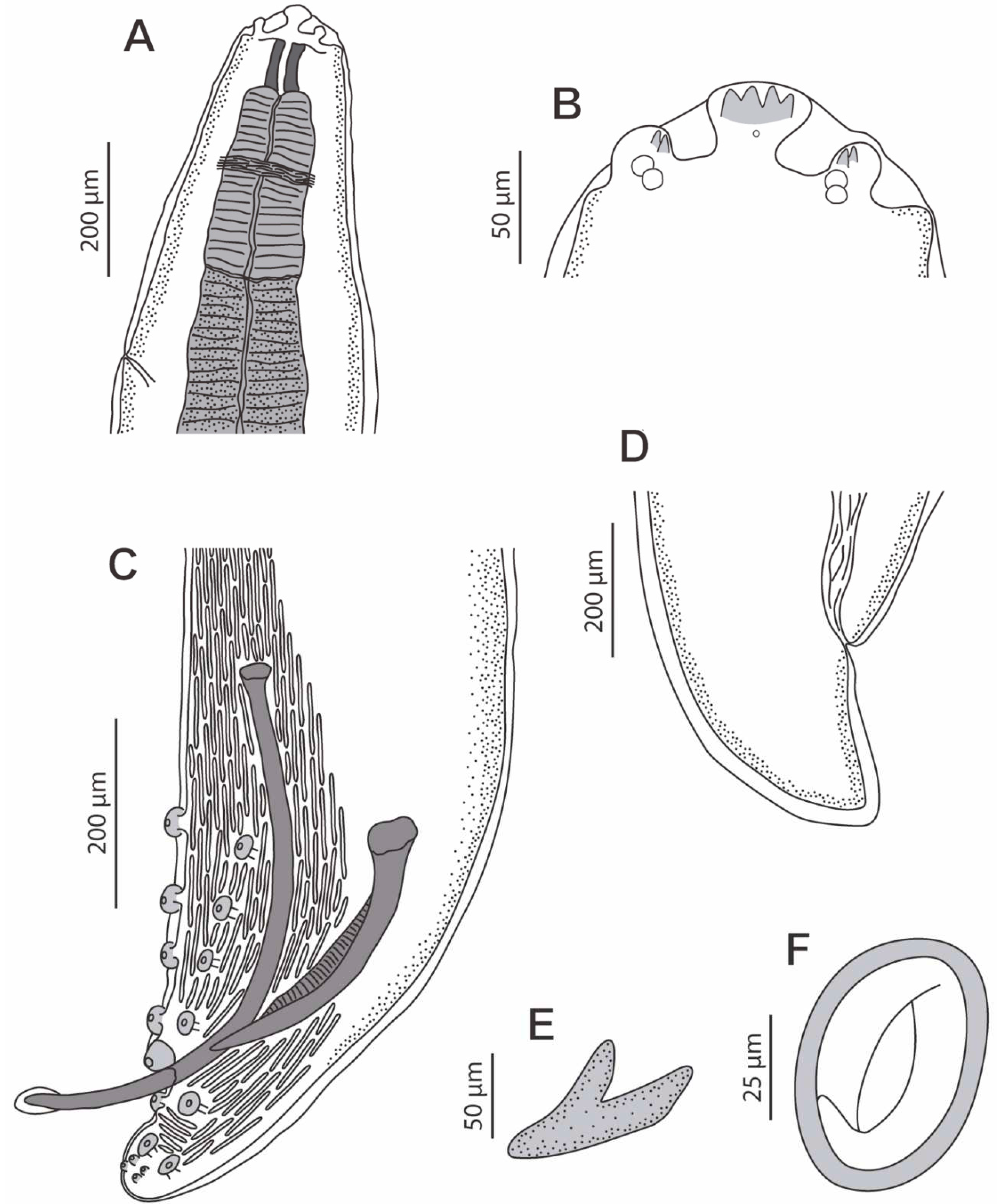
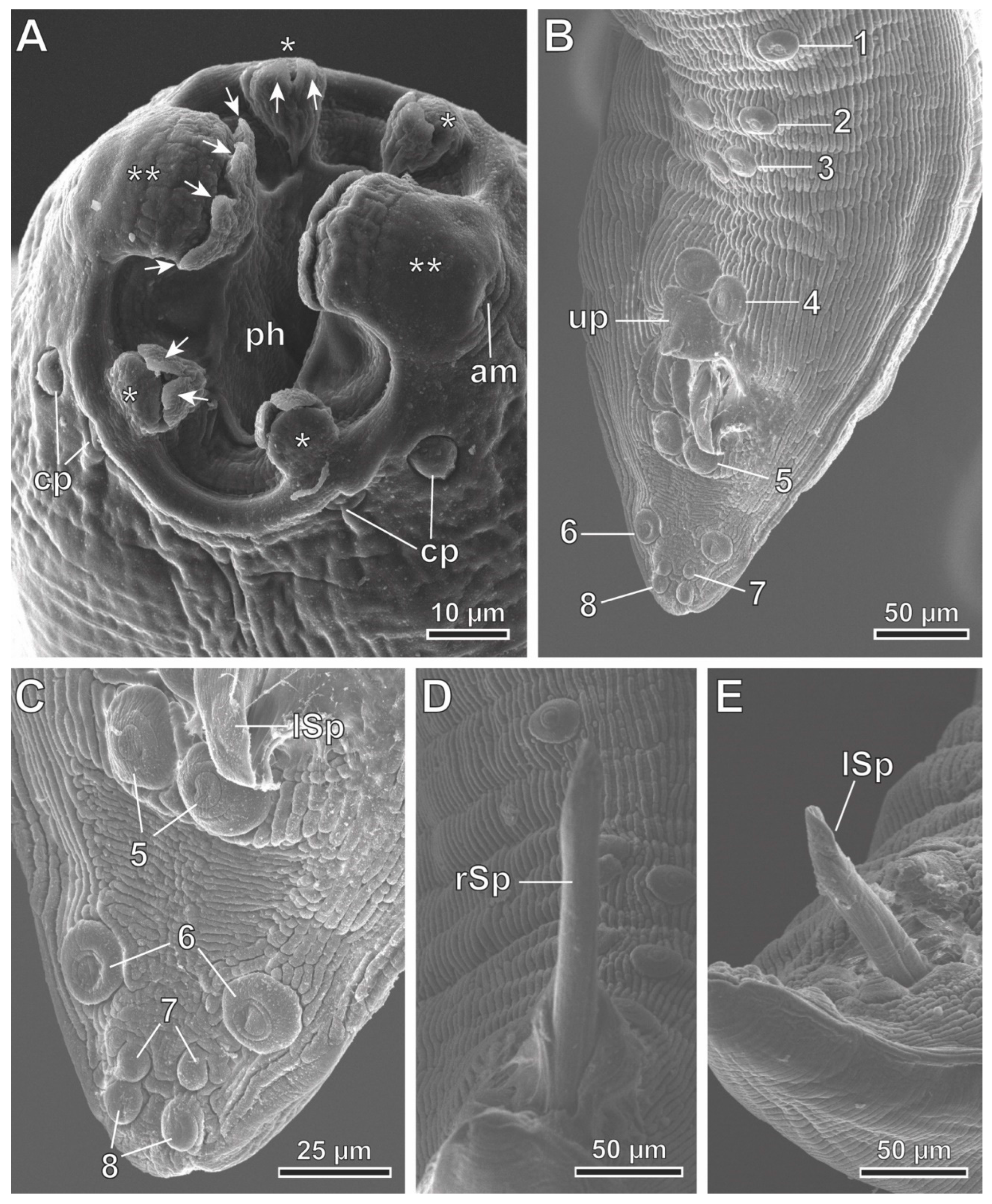
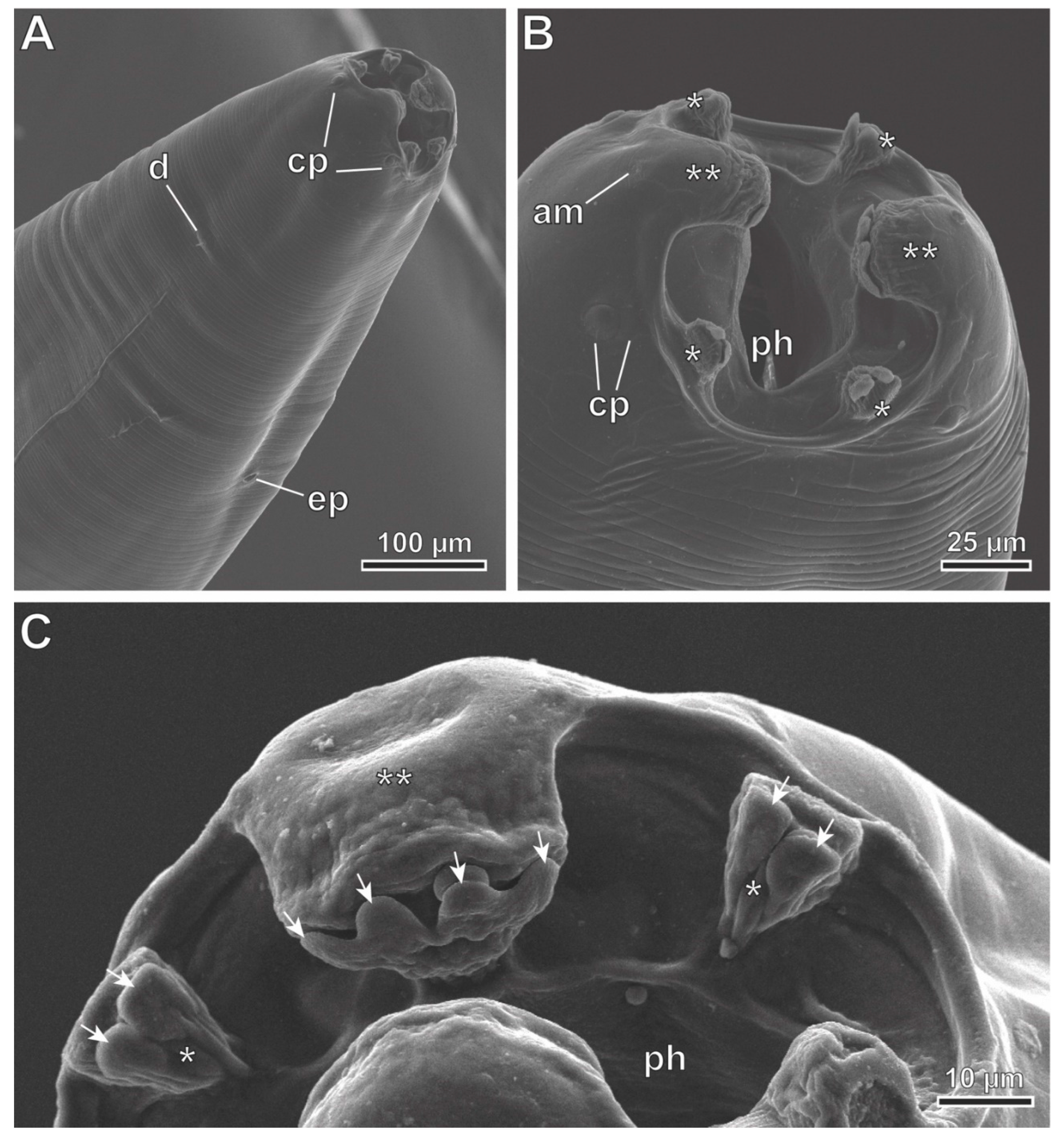
General: Large stout worms. Cuticle thick, with transverse striations. Anterior extremity with two highly developed pseudolabia raised above the mouth opening (
Figure 3 3A,B,
Figure 5A and
Figure 6A–C). Each pseudolabium formed by a well-developed lateral lobe and two smaller submedian lobes (
Figure 3B,
Figure 5A and
Figure 6A–C). Each lateral lobe has four tooth-like prominences in its internal side (
Figure 3B,
Figure 5A and
Figure 7C). Each submedian lobe has two tooth-like prominences in its internal side (
Figure 3B,
Figure 5A and
Figure 7C). Four pairs of submedian cephalic papillae at the base of the submedian lobes and two amphids in the lateral lobes (
Figure 3B,
Figure 5A and
Figure 7A,B). Pharynx thick-walled, laterally compressed (
Figure 5A and
Figure 6B). Oesophagus divided into anterior muscular and posterior glandular portions (
Figure 3A). Nerve ring surrounding oesophagus at the level of its muscular portion (
Figure 3A). Excretory pore slightly posterior to nerve ring (
Figure 3A and
Figure 7A). Deirids placed between nerve ring and excretory pore (
Figure 7A). Phasmids not seen.
Male (11 specimens measured, range, mean in parentheses, holotype measurements in brackets) (
Figure 3C,E,
Figure 4A–D and
Figure 5A–E) (all measurements are given in micrometres, except where indicated): Oral opening surrounded by two tri-lobed pseudolabia (
Figure 5A). Presence of four tooth-like prominences in the lateral lobes and two tooth-like prominences in the submedian lobes (
Figure 5A). Body length 13.67–23.37 mm (18.43 mm) [15.02 mm]; width at the level of the oesophagus basis 330–526 (430) [382]. Pharynx length 39–69 (56) [>57]. Muscular oesophagus length 219–340 (280) [219]; width at base 75–103 (82) [75]. Glandular oesophagus length 2.95–4.67 mm (3.97 mm) [3.75 mm]; width at base 123–206 (167) [165]. Nerve ring located at 123–273 (217) [123] from the cephalic extremity. Deirids located at 213–308 (250) [231] from the cephalic extremity. Excretory pore located at 371–567 (474) [351] from the cephalic extremity. Posterior end of body strongly curved ventrally. The pericloacal surface is ornamented with cuticular markings (
Figure 3C,
Figure 4A and
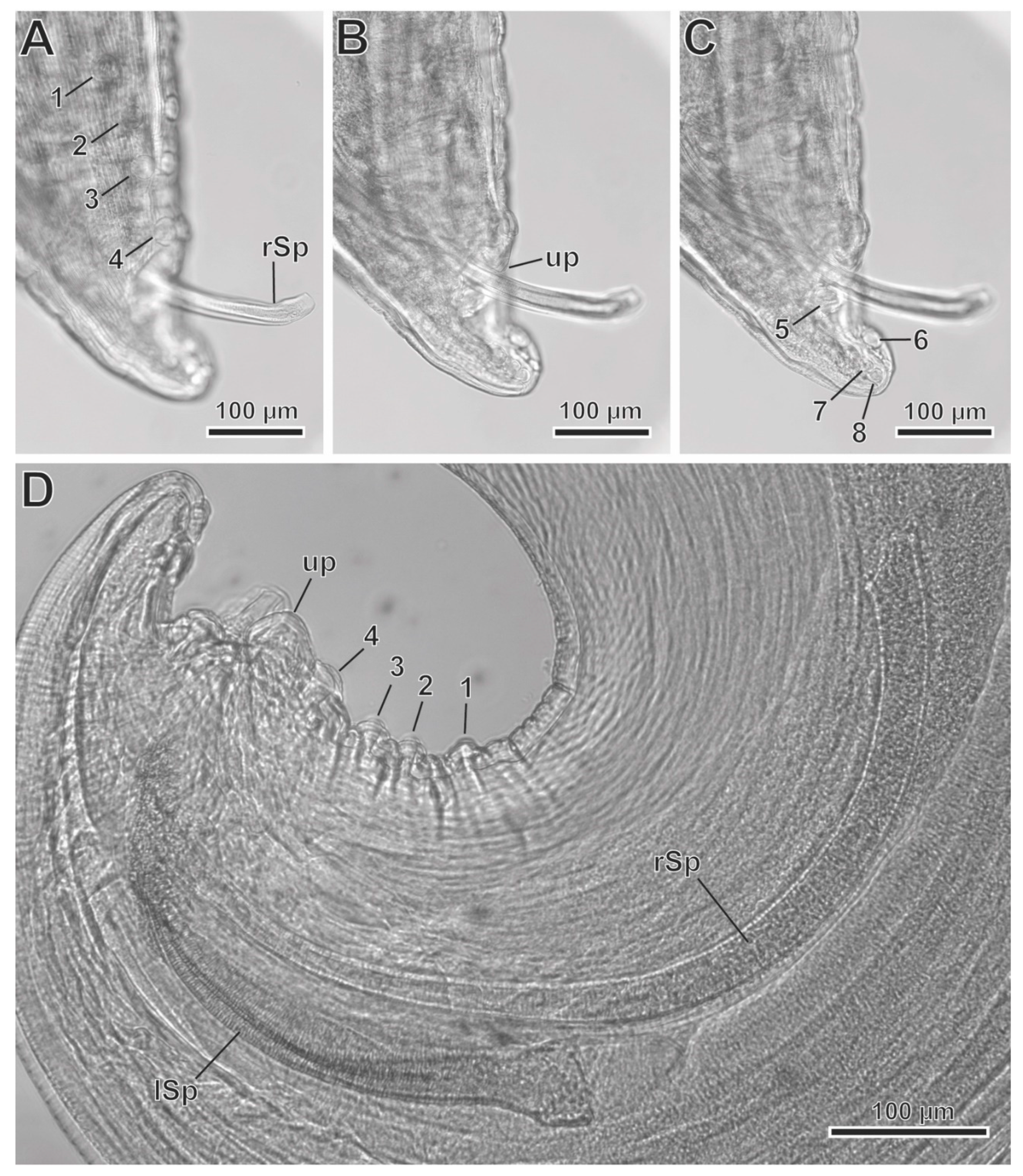
Figure 5B–E). Total of 17 caudal papillae; four pairs of large and pedunculated precloacal papillae (pairs 1 to 4) (
Figure 3C,
Figure 4A and
Figure 5B), one large unpaired precloacal papilla (
Figure 3C,
Figure 4B and
Figure 5B) and four pairs of postcloacal papillae (pairs 5 to 8) (
Figure 3C,
Figure 4C and
Figure 5B,C). The postcloacal papillae in pair 5 are large, pedunculated and placed near the posterior edge of the cloaca (
Figure 3C,
Figure 4C and
Figure 5B,C). The remaining three pairs of postcloacal papillae (pairs 6 to 8) are grouped and placed near the posterior tip (
Figure 3C,
Figure 4C and
Figure 5B,C). The papillae of pair 6 are large and pedunculated (
Figure 3C,
Figure 4C and
Figure 5B,C). The papillae of pairs 7 and 8 are smaller and apparently sessile (
Figure 3C,
Figure 4C and
Figure 5B,C). Phasmids were not observed. Spicules unequal in size and shape; right spicule longer and slender 643–715 (675) [694] (
Figure 3C and
Figure 4D); left spicule shorter, stout and alate 309–412 (368) [360] (
Figure 3C and
Figure 4D). Gubernaculum, V-shaped, 116–159 (141) [123] (
Figure 3D).
Female (13 specimens measured, range, mean in parentheses, allotype measurements in brackets) (
Figure 3A,B,D,F and
Figure 6A–C) (all measurements are given in micrometres, except where indicated): Oral opening surrounded by two tri-lobed pseudolabia (
Figure 3A,B and
Figure 6A–C). Presence of four tooth-like prominences in the lateral lobes and two tooth-like prominences in the submedian lobes (
Figure 6C). Body length 25.52–41.99 mm (32.51 mm) [27.73 mm]; width at the level of the vulva 795–1455 (1137) [815]. Pharynx length 57–90 (74) [87]. Muscular oesophagus length 273–464 (346) [310]; width at base 108–175 (128) [113]. Glandular oesophagus length 3.49–5.79 mm (4.52 mm) [3.84 mm]; width at base 165–310 (230) [185]. Nerve ring located at 234–311 (278) [311] from the cephalic extremity (
Figure 3A). Deirids located at 273–360 (317) [309] from the cephalic extremity. Excretory pore located at 464–660 (577) [309] from the cephalic extremity (
Figure 3A). Vulva slightly preequatorial, located at a 11.92–19.43 mm (15.22 mm) [13.33 mm] distance from the cephalic extremity. Tail 258–423 (327) [289] (
Figure 3D). Eggs, embryonated, 48.9–56.7 x 33.4–41.1 (51.8 x 38.2) [51.4 x 38.6] (
Figure 3F).
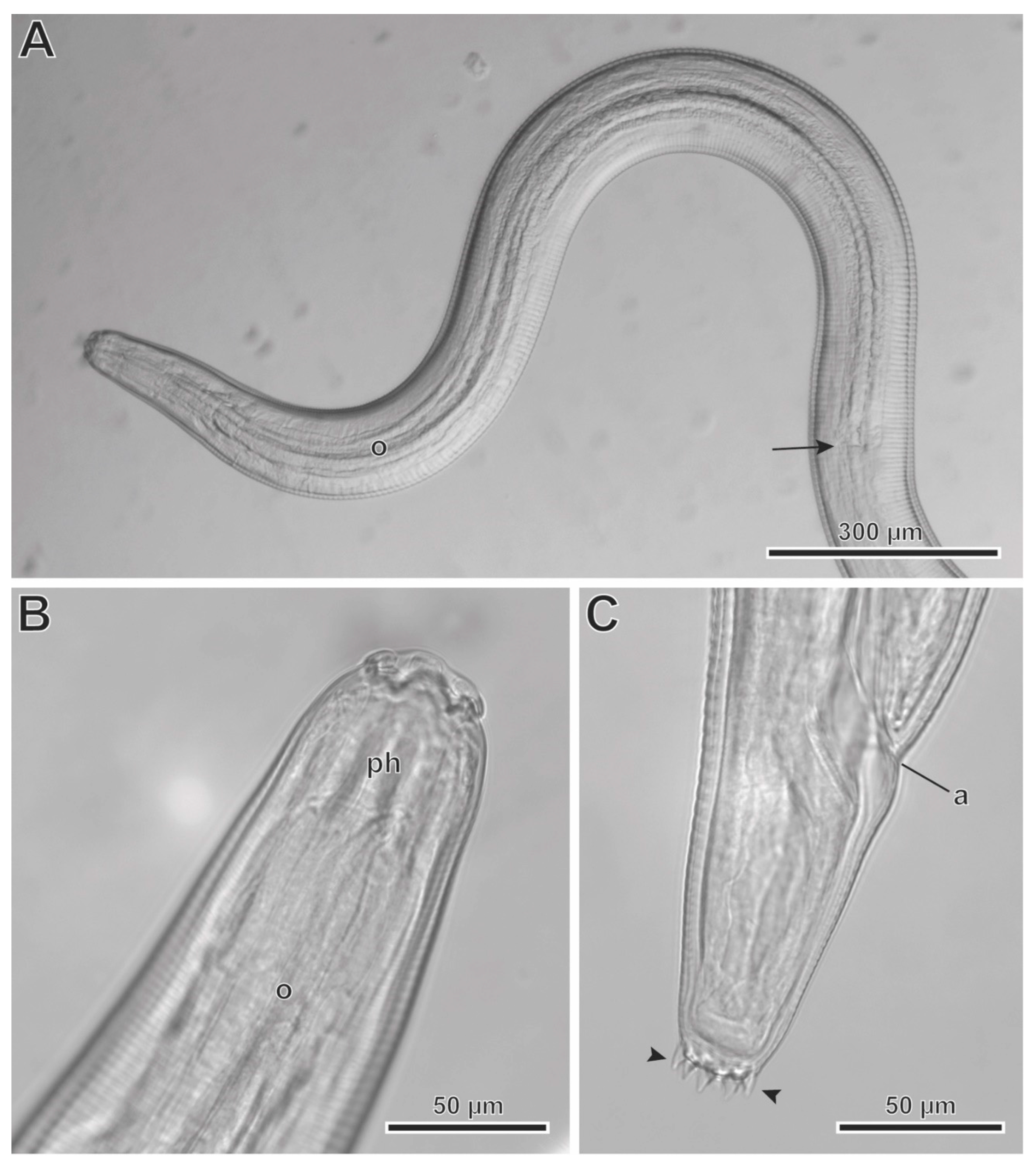
L3 larva (1 specimen measured) (
Figure 7A–C) (all measurements are given in micrometres, except where indicated): Body length 5.91 mm; width at the level of the oesophagus basis 162. Pharynx length 33. Muscular oesophagus length 159; width at base 39. Glandular oesophagus length 1.62 mm; width at base 67. Nerve ring located at 121 from the cephalic extremity. Deirids not observed. Excretory pore located at 270 from the cephalic extremity. Anus at 495 from the posterior extremity. Tail notched, with 10 pointed prominences arranged circularly in the same plan (
Figure 7C).
3.3. Molecular analyses
All genomic DNA samples were amplified to the expected size (650-bp). Four of these amplicons were selected for sequencing as all the fragments had the same host species (R. rattus) and had the same origin (Frontera, El Hierro, Canary Islands, Spain), this was done in such a way for resource optimization. Four 409-bp fragments were successfully sequenced and sent to the GenBank database with accession numbers OQ799521, OQ799522, OQ799523 and OQ799524.
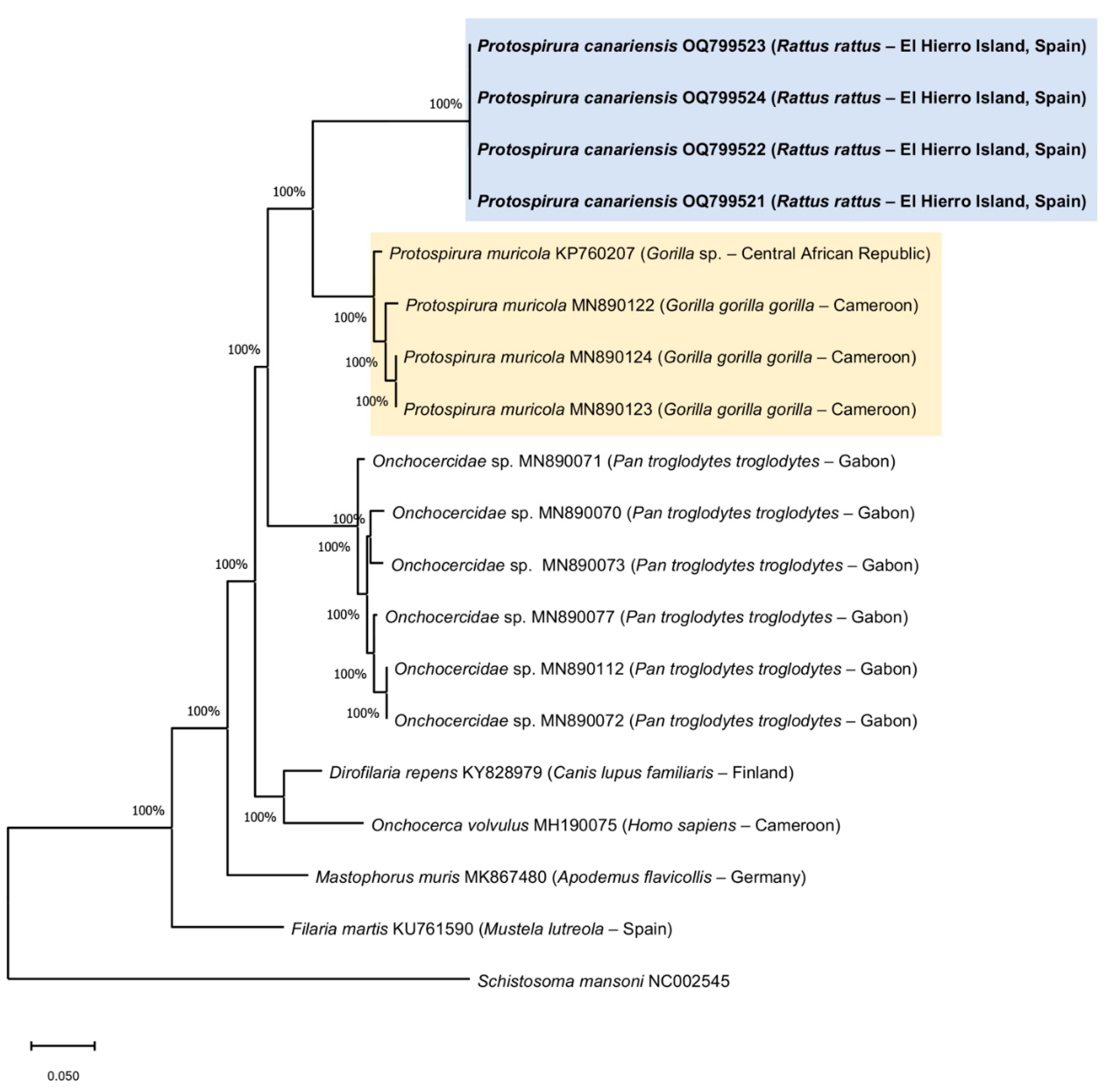
Phylogenetic analyses of the cox1 gene showed a tree composed of six monophyletic groups (
Figure 2). We observe in the upper part a clade composed of the specimens of Protospirura canariensis n. sp. contributed by this study. Below is a clade made up of the species of Protospirura muricola with a robustness of 100%. Both clades are clearly separated from the rest of the included sequences.
4. Discussion
One of the most useful morphological characteristics in the recognition and differentiation of
Protospirura species concerns the morphology of pseudolabia and their associated structures. The oral opening is surrounded by two pseudolabia, each with three lobes (a larger lateral lobe and two smaller submedian lobes). In the internal face of these lobes there are tooth-like prominences variable in number (usually two or four).
Protospirura canariensis n. sp. has four tooth-like prominences in each lateral lobe and two tooth-like prominences in each submedian lobe. Only
P. peromysci exhibit a similar arrangement of tooth-like prominences in pseudolabia lobes [
3]. However, there are some discrepancies among earlier works that described several
Protospirura species, including
P. peromysci (see
Table 1). Thus, Babero and Mathias [
3] in the original description of the species mention the presence of four flat denticles in the lateral lobes and two triangular denticles in submedian lobes, while Smales [
9] considers this species to have four teeth in both lateral and submedian lobes. In male specimens,
P. canariensis n. sp. can be differentiated from
P. peromysci in the size of the right spicule (respectively 643–715 µm vs. 820–1200 µm) and in the number of cloacal papillae (17 in
P. canariensis n. sp. vs. 23 in
P. peromysci) [
3].
In male specimens, the size of spicules is another diagnostic character when the species of
Protospirura are compared. Considering the size of both right and left spicules
P. pseudomuris and
P. siamensis present similar sized spicules: right spicule measures 643–715 µm in
P. canariensis n. sp., 540–790 µm in
P. pseudomuris and 469–701 µm in
P. siamensis; left spicule measures 309–412 µm in
P. canariensis n. sp., 270–380 µm in
P. pseudomuris and 348–380 µm in
P. siamensis [
7,
13]. Both species can be differentiated from the new species in the number of tooth-like prominences in lobes of pseudolabia (see
Table 1). Additionally, the number of cloacal papillae also distinguish the new species from
P. pseudomuris (17 vs. 21, respectively). In the remaining
Protospirura species, spicule sizes are out of the range of those of
P. canariensis n. sp. (see
Table 1).
The number, morphology and disposition of cloacal papillae are other differential characters between
P. canariensis n. sp. and the remaining species of the genus
Protospirura. Thus,
P. canariensis n. sp. has a total of 17 cloacal papillae, 4 pedunculated precloacal pairs, an unpaired precloacal papilla located in the front edge of cloaca and 4 postcloacal pairs (1 pedunculated pair near the cloaca and 1 pedunculated and 2 sessile pairs near the posterior tip). Only two species have a similar number of cloacal papillae. The first of them is
P. mexicana, which has 17 cloacal papillae with a similar arrangement, although Falcón & Sanabria [
4] described some variability in the arrangement, particularly for the postcloacal papillae. The second one is
P. siamensis, with males that have 4 or 5 precloacal pairs of cloacal papillae and having a total of 17 or 19 cloacal papillae [
13]. Both species clearly differ from
P. canariensis n. sp. in the number of tooth-like prominences present in the submedian lobes of the pseudolabia (1 in
P. mexicana, 4 in
P. siamensis and 2 in
P. canariensis n. sp.) and in the size of spicules, particularly the right spicule (340-465 µm in
P. mexicana, 469-701µm in
P. siamensis and 643-715 µm in
P. canariensis n. sp.). The remaining species of
Protospirura have a higher number of cloacal papillae, comprised between 18 and 25 papillae (see
Table 1).
In females of
P. canariensis n. sp., the vulva is located slightly before mid-body. Other
Protospirura species, namely
P. kaindiensis,
P. okinavensis and
P. pseudomuris also present a preequatorial placement of the vulva [
7,
8,
9]. Females of the new species differ from those of these three species in the number of tooth-like prominences present in lateral and submedian lobes of pseudolabia (see
Table 1).
Within the
Protospirura genus, the life cycles of
P. muricola and
P. numidica criceticola have been elucidated [
12,
20,
26]. Quentin et al. [
12] described the life cycle of
P. numidica criceticola with an L3 larva characterized by a notched posterior extremity having 12 to 15 pointed prominences. Posteriorly, Quentin [
20] elucidated the life cycle of
P. muricola and described a L3 larva presenting nine pointed prominences arranged in the same plan in its notched posterior extremity. In the present study, the single recovered L3 larva of
P. canariensis n. sp. has 10 pointed prominences arranged circularly in the same plan. This morphological trait differs clearly from the two above-mentioned L3 larva of
Protospirura [
12,
20] and is an additional feature differentiating these three species.
The parasitized host and the geographical distribution are additional criteria to distinguish
P. canariensis n. sp. from the remaining species of
Protospirura. There are seven
Protospirura species parasites of Muridae rodents, namely
P. armeniana,
P. chabaudi,
P. kaindiensis,
P. munimuniensis,
P. muricola,
P. okinavensis and
P. siamensis [
6,
8,
9,
13,
14,
15,
16,
18,
19].
P. armeniana was found parasitizing the house mouse
Mus musculus in Mongolia and Armenia [
6,
16,
19].
Protospirura chabaudi was found parasitizing
R. rattus in the Democratic Republic of Congo [
18]. Both
P. kaindiensis and
P. munimuniensis were found in Papua New Guinea parasitizing
Pseudohydromys murinus and
Chiruromys lamia, respectively [
9,
15].
Protospirura okinavensis was reported in Japan parasitizing
Mus caroli [
8].
Protospirura muricola is the species with the widest geographical distribution parasitizing Muridae rodents, including numerous African and Asian countries and also with the largest host range, being recorded in diverse genera of murids such as
Arvicanthis,
Gerbilliscus,
Hybomys,
Lemniscomys,
Malacomys,
Mastomys,
Mus,
Praomys,
Rattus and
Uranomys [
25,
27,
28,
29,
30,
31]. Finally,
P. siamensis was described in Thailand parasitizing several murids of genera
Bandicota,
Berylmys,
Mus and
Rattus [
13], and more recently found in rats and mice from Pakistan [
32]. However, these authors do not specify the species of murids parasitized by
P. siamensis [
32]. Thus, to our knowledge, apart from
P. canariensis n. sp. presently recorded at the Canary Archipelago, only two species of
Protospirura, namely
P. chabaudi and
P. muricola have been cited parasitizing the black rat
R. rattus and reported in Democratic Republic of Congo, Nigeria, China and Taiwan [
18,
25,
28,
29].
The molecular results confirm the morphological observations showing that the specimens here described as a new species are clearly separated from the genus Mastophorus and cluster with P. muricola. The remaining species of the genus Protospirura should be reevaluated by integrating morphologic discriminant characters and molecular information.
In conclusion, the morphologic characteristics such as pseudolabia and the number of tooth-like prominences present in their lateral and submedian lobes, the pharynx, the particular characters of males (number and arrangement of cloacal papillae, and size of spicules) and females (position of the vulva), the molecular data, and the geographical distribution and parasitized host identify the discovered nematode as a new species of the genus Protospirura.
Author Contributions
Conceptualization, J.M., C.F. and P.F.; Methodology, J.M., N.M.-C., A.R. and P.F.; Investigation, J.M., N.M.-C., A.R., S.S.-V., C.F. and P.F.; Resources, J.M., N.M.-C. and P.F.; Writing—Original Draft Preparation, J.M., N.M.-C. and P.F.; Writing—Review & Editing, J.M., C.F. and P.F.; Supervision, J.M., C.F. and P.F.; Project Administration, C.F. and P.F.; Funding Acquisition, P.F. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Localities where Rattus rattus parasitized by Protospirura canariensis n. sp. were captured on El Hierro Island (Canary Archipelago, Spain). (1) Camino-Frontera (27° 44' 38.07" N, 18° 2' 25.4" W); (2) Lagartario-Frontera (type locality) (27° 46' 29.9" N, 17° 59' 55.59" W); (3) Túnel-Valverde (27° 49' 12.41" N, 17° 57' 49.27" W).
Figure 1.
Localities where Rattus rattus parasitized by Protospirura canariensis n. sp. were captured on El Hierro Island (Canary Archipelago, Spain). (1) Camino-Frontera (27° 44' 38.07" N, 18° 2' 25.4" W); (2) Lagartario-Frontera (type locality) (27° 46' 29.9" N, 17° 59' 55.59" W); (3) Túnel-Valverde (27° 49' 12.41" N, 17° 57' 49.27" W).
Figure 2.
Phylogenetic analysis using the Maximum Likelihood method with p-distance and 1,000 bootstrap replications based on the mitochondrial cytochrome c oxidase subunit I gene (cox1) sequence. Sequences obtained in the present study are shown in bold. Schistosoma mansoni was used as the outgroup.
Figure 2.
Phylogenetic analysis using the Maximum Likelihood method with p-distance and 1,000 bootstrap replications based on the mitochondrial cytochrome c oxidase subunit I gene (cox1) sequence. Sequences obtained in the present study are shown in bold. Schistosoma mansoni was used as the outgroup.
Figure 3.
Protospirura canariensis n. sp., male and female. A Cephalic extremity of female, lateral view. B Detail of submedian and lateral lobes of one of the pseudolabia of female, lateral view. C Caudal extremity of male showing the two spicules and the cloacal papillae, lateral view. D Tail of female, lateral view. E V-shaped gubernaculum. F Embryonated egg.
Figure 3.
Protospirura canariensis n. sp., male and female. A Cephalic extremity of female, lateral view. B Detail of submedian and lateral lobes of one of the pseudolabia of female, lateral view. C Caudal extremity of male showing the two spicules and the cloacal papillae, lateral view. D Tail of female, lateral view. E V-shaped gubernaculum. F Embryonated egg.
Figure 4.
Protospirura canariensis n. sp., male, LM. A Caudal extremity showing the four pairs of precloacal papillae (1 to 4). B Caudal extremity showing the unpaired precloacal papilla (up). C Caudal extremity showing the four pairs of postcloacal papillae (5 to 8). D Detail of right (rSp) and left spicule (lSp).
Figure 4.
Protospirura canariensis n. sp., male, LM. A Caudal extremity showing the four pairs of precloacal papillae (1 to 4). B Caudal extremity showing the unpaired precloacal papilla (up). C Caudal extremity showing the four pairs of postcloacal papillae (5 to 8). D Detail of right (rSp) and left spicule (lSp).
Figure 5.
Protospirura canariensis n. sp., male, SEM. A Apical view of the cephalic extremity showing the two tri-lobed pseudolabia. B Caudal extremity illustrating the distribution of cloacal papillae. C Postcloacal pairs of papillae. D Detail of right spicule (rSp). E Detail of left spicule (lSp). (arrows) tooth-like prominences; (*) submedian lobes; (**) lateral lobes; (1–4) pairs of precloacal papillae; (5–8) pairs of postcloacal papillae; (am) amphid; (cp) cephalic papillae; (ph) pharynx; (up) unpaired precloacal papilla.
Figure 5.
Protospirura canariensis n. sp., male, SEM. A Apical view of the cephalic extremity showing the two tri-lobed pseudolabia. B Caudal extremity illustrating the distribution of cloacal papillae. C Postcloacal pairs of papillae. D Detail of right spicule (rSp). E Detail of left spicule (lSp). (arrows) tooth-like prominences; (*) submedian lobes; (**) lateral lobes; (1–4) pairs of precloacal papillae; (5–8) pairs of postcloacal papillae; (am) amphid; (cp) cephalic papillae; (ph) pharynx; (up) unpaired precloacal papilla.
Figure 6.
Protospirura canariensis n. sp., female, SEM. A Lateroventral view of the cephalic extremity showing the right deirid (d) and the excretory pore (ep). B Apical view of the cephalic extremity showing the two tri-lobed pseudolabia. C Detail of one of the pseudolabia showing tooth-like prominences (arrows). (*) submedian lobes; (**) lateral lobes; (am) amphid; (cp) cephalic papillae; (ph) pharynx.
Figure 6.
Protospirura canariensis n. sp., female, SEM. A Lateroventral view of the cephalic extremity showing the right deirid (d) and the excretory pore (ep). B Apical view of the cephalic extremity showing the two tri-lobed pseudolabia. C Detail of one of the pseudolabia showing tooth-like prominences (arrows). (*) submedian lobes; (**) lateral lobes; (am) amphid; (cp) cephalic papillae; (ph) pharynx.
Figure 7.
Protospirura canariensis n. sp., L3 larva, LM. A Cephalic extremity showing the entire oesophagus (o). B Detail of cephalic extremity showing the pharynx (ph). C Detail of the notched posterior extremity showing caudal pointed prominences (arrowheads). (arrow) posterior extremity of the oesophagus; (a) anus.
Figure 7.
Protospirura canariensis n. sp., L3 larva, LM. A Cephalic extremity showing the entire oesophagus (o). B Detail of cephalic extremity showing the pharynx (ph). C Detail of the notched posterior extremity showing caudal pointed prominences (arrowheads). (arrow) posterior extremity of the oesophagus; (a) anus.
Table 1.
Some differential characteristics of Protospirura species.
Table 1.
Some differential characteristics of Protospirura species.
| Species |
Pseudolabia |
Spicules |
Cloacal papillae |
Vulva |
References |
| |
subm |
lat |
right |
left |
|
|
|
| Protospirura anopla |
01
42
|
01
42
|
287 |
632 |
4 pre, 5 post
(18) |
posteq |
[6,9] |
| Protospirura armeniana |
21
42
|
21
42
|
620–639 |
370–411 |
4 pre, upre, 7 post, upost
(24) |
posteq |
[6,9] |
|
Protospirura canariensis n. sp. |
2 |
4 |
643–715
(675) |
309–412
(368) |
4 pre, upre, 4 post
(17) |
preeq |
Present study |
| Protospirura chabaudi |
23
42
|
03
42
|
980 |
420 |
4 pre, 5 post
(18) |
posteq |
[9,18] |
| Protospirura kaindiensis |
2 |
2 |
450–480 |
310–330 |
4 pre, upre, 5 post
(19) |
preeq |
[9] |
| Protospirura mexicana |
1 |
0 |
340–465
(404) |
420–527
(481) |
4 pre, upre, 4 post
(17) |
posteq |
[4] |
| Protospirura munimuniensis |
2 |
2 |
602–603 |
430–455 |
4 pre, upre, 6 post
(21) |
? |
[15] |
| Protospirura muricola |
2 |
2 |
268–430
(352) |
290–501
(411) |
4 pre, upre, 6 post
(21) |
posteq |
[14,25] |
| Protospirura numidica |
34
42
|
34
45
42
|
830 |
420 |
4 pre, upre, 5 post
(19) |
posteq |
[5,9,12] |
| Protospirura numidica criceticola |
? |
4 |
1250 |
470 |
4 pre, upre, 7 post
(23) |
posteq |
[12] |
| Protospirura okinavensis |
4 |
4 |
600–650
(620) |
320–350
(320) |
5–6 pre, upre, 4 post
(19 or 21) |
preeq |
[8] |
| Protospirura peromysci |
26
42
|
46
42
|
820–1200 |
330–380 |
4 pre, upre, 7 post
(23) |
posteq |
[3,9] |
| Protospirura pseudomuris |
17
22
|
17
22
|
540–790
(680) |
270–380
(330) |
4 pre, upre, 6 post
(21) |
preeq |
[7,8,9] |
| Protospirura siamensis |
4 |
4 |
469–701
(572.6) |
348–380
(368.7) |
4–5 pre, upre, 4 post
(17 or 19) |
posteq |
[13] |
| Protospirura suslica |
21
42
|
21
42
|
315 |
873 |
5 pre, upre, 6 post
(23) |
posteq |
[6,9] |
 No. 1, MNHN HEL1905.
No. 1, MNHN HEL1905. No. 1, MNHN HEL1906.
No. 1, MNHN HEL1906.