Submitted:
27 April 2023
Posted:
28 April 2023
You are already at the latest version
Abstract
Keywords:
1. Biopolymer-Based Microgels/Nanogels as A Drug Delivery System
2. Advantages of Biopolymer-Based Nanogels Over Other Drug Delivery Systems
3. Synthesis of Nanogels
3.1. Physical Self-Assembling of Interacting Polymer Chains
3.2. Chemical Cross-Linking of Preformed Polymers
3.3. Polymerization of Monomers in A Homogeneous Phase or A Micro- And/Or Nano-Heterogeneous Phase
3.4. Template-Assisted Nanofabrication
4. Nanogels of Natural Polymers
4.1. Polysaccharide-Based Nanogels
4.1.1. Advantages of Polysaccharide Nanogels
4.1.2. Different Types of Natural Polysaccharides Used in Nanogels
- Chitosan
- Dextran
- Heparin
- Pectin
- Hyaluronic Acid (HA)
- Alginate
- Pullulan
- Chondroitin sulfate
- Carrageenan
- Cyclodextrins
4.2. Protein-Based Nanogels
4.2.1. Advantages of Protein Nanogels
4.2.2. Different Types of Natural Proteins Used in Nanogels
- Elastin
- Collagen
- Gelatin
- Silk Fibroin
- Soy Protein
5. Drug Release Mechanisms of Nanogels
5.1. Diffusion
5.2. Erosion of the nanogel matrices
5.3. Ionic exchange with the environment
5.4. Stimuli responsiveness
5.4.1. pH sensitive release
5.4.2. Thermo-sensitive triggered release
5.4.3. Magnetic field responsive release
5.4.4. Photo-sensitive release
5.4.5. Redox-responsive release
6. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duceac, I.A.; Coseri, S. Biopolymers and their derivatives: Key components of advanced biomedical technologies. Biotechnol. Adv. 2022, 61, 108056, . [CrossRef]
- Franzesi, G.T.; Ni, B.; Ling, Y.; Khademhosseini, A. A Controlled-Release Strategy for the Generation of Cross-Linked Hydrogel Microstructures. J. Am. Chem. Soc. 2006, 128, 15064–15065, . [CrossRef]
- Yang, Z.; Xu, K.; Wang, L.; Gu, H.; Wei, H.; Zhang, M.; Xu, B. Self-assembly of small molecules affords multifunctional supramolecular hydrogels for topically treating simulated uranium wounds. Chem. Commun. 2005, 4414–4416, . [CrossRef]
- Vinogradov, S.V. Colloidal Microgels in Drug Delivery Applications. Curr. Pharm. Des. 2006, 12, 4703–4712, . [CrossRef]
- Uthaman, S.; Maya, S.; Jayakumar, R.; Cho, C.-S.; Park, I.-K. Carbohydrate-based nanogels as drug and gene delivery systems.. J. Nanosci. Nanotechnol. 2014, 14, 694–704, . [CrossRef]
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268, . [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18, . [CrossRef]
- Soni, G.; Yadav, K.S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm. J. 2016, 24, 133–139, . [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126, . [CrossRef]
- Sindhu, R.K.; Gupta, R.; Wadhera, G.; Kumar, P. Modern Herbal Nanogels: Formulation, Delivery Methods, and Applications. Gels 2022, 8, 97, . [CrossRef]
- Diouf, S.I., et al., Multi-stimuli responsive tetra-PPO 60-PEO 20 ethylene diamine block copolymer enables pH, temperature, and solvent regulation of Au nanoparticle composite plasmonic response. Polymer Chemistry, 2019. 10(47): p. 6456-6472.
- Sharmin, E., Medical Applications of Nanogels. Nanogels for Biomedical Applications, 2017. 30: p. 29.
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649, . [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429, . [CrossRef]
- Sahiner, N.; Godbey, W.T.; McPherson, G.L.; John, V.T. Microgel, nanogel and hydrogel–hydrogel semi-IPN composites for biomedical applications: synthesis and characterization. Colloid Polym. Sci. 2006, 284, 1121–1129, . [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29, . [CrossRef]
- Pelton, R.H.; Chibante, P. Preparation of aqueous latices with N-isopropylacrylamide. Colloids Surf. 1986, 20, 247–256, . [CrossRef]
- Wu, X.; Pelton, R.H.; Hamielec, A.E.; Woods, D.R.; McPhee, W. The kinetics of poly(N-isopropylacrylamide) microgel latex formation. Colloid Polym. Sci. 1994, 272, 467–477, . [CrossRef]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-Assembled Hydrogel Nanoparticles for Drug Delivery Applications. Materials 2010, 3, 1420–1460, . [CrossRef]
- Mackiewicz, M.; Romanski, J.; Krug, P.; Mazur, M.; Stojek, Z.; Karbarz, M. Tunable environmental sensitivity and degradability of nanogels based on derivatives of cystine and poly(ethylene glycols) of various length for biocompatible drug carrier. Eur. Polym. J. 2019, 118, 606–613, . [CrossRef]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255, . [CrossRef]
- Li, D.; van Nostrum, C.F.; Mastrobattista, E.; Vermonden, T.; Hennink, W.E. Nanogels for intracellular delivery of biotherapeutics. J. Control. Release 2017, 259, 16–28, . [CrossRef]
- Yallapu, M.M., M.K. Reddy, and V. Labhasetwar, Nanogels: chemistry to drug delivery. Biomedical applications of nanotechnology, 2007: p. 131-171.
- Farazi, S., et al., Real time monitoring of peptide delivery in vitro using high payload pH responsive nanogels. Polymer Chemistry, 2020. 11(2): p. 425-432.
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Accounts Chem. Res. 2017, 50, 131–140, . [CrossRef]
- Vashist, A., et al., Nanogels for biomedical applications. 2017: Royal Society of Chemistry.
- Sultana, F.; Manirujjaman, M.; Haque, I.U.; Arafat, M.; Sharmin, S. An Overview of Nanogel Drug Delivery System. J. Appl. Pharm. Sci. 2013, 3,, S95–S105, . [CrossRef]
- Xu, D.; Hong, J.; Sheng, K.; Dong, L.; Yao, S. Preparation of polyethyleneimine nanogels via photo-Fenton reaction. Radiat. Phys. Chem. 2007, 76, 1606–1611, . [CrossRef]
- Baipaywad, P.; Udomluck, N.; Pyo, S.-G.; Park, H.H.; Park, H. Fabrication of Nanogels for Delivery of Molecules. J. Nanosci. Nanotechnol. 2014, 14, 7363–7373, . [CrossRef]
- Sharma, A.; Garg, T.; Aman, A.; Panchal, K.; Sharma, R.; Kumar, S.; Markandeywar, T. Nanogel—an advanced drug delivery tool: Current and future. Artif. Cells, Nanomedicine, Biotechnol. 2016, 44, 165–177, . [CrossRef]
- Eckmann, D.M.; Composto, R.J.; Tsourkas, A.; Muzykantov, V.R. Nanogel carrier design for targeted drug delivery. J. Mater. Chem. B 2014, 2, 8085–8097, . [CrossRef]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376, . [CrossRef]
- Tahara, Y.; Akiyoshi, K. Current advances in self-assembled nanogel delivery systems for immunotherapy. Adv. Drug Deliv. Rev. 2015, 95, 65–76, . [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today 2011, 16, 457–463, . [CrossRef]
- Kousalová, J.; Etrych, T. Polymeric Nanogels as Drug Delivery Systems. Physiol. Res. 2018, 67, S305–S317, . [CrossRef]
- Setia, A. and P. Ahuja, Nanohydrogels: Emerging trend for drug delivery. Organic Materials as Smart Nanocarriers for Drug Delivery, 2018: p. 293-368.
- Pulickal, M.A., et al., Injectable Nanogels in Drug Delivery, in Nanogels for Biomedical Applications. 2017. p. 181-209.
- Akiyoshi, K.; Sasaki, Y.; Sunamoto, J. Molecular Chaperone-Like Activity of Hydrogel Nanoparticles of Hydrophobized Pullulan: Thermal Stabilization with Refolding of Carbonic Anhydrase B. Bioconjugate Chem. 1999, 10, 321–324, . [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851, . [CrossRef]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282, . [CrossRef]
- Li, X.; Yin, C.; Liu, B.; Zou, L.; Xu, Q.; Li, C.M. Glycerol-compressed self-assembly nanogel based on ovomucin and chito-oligosaccharide: A novel green strategy for curcumin delivery. Food Hydrocoll. 2023, 134, . [CrossRef]
- Atallah, M.A.; Sallam, M.A.; Abdelmoneem, M.A.; Teleb, M.; Elkhodairy, K.A.; Bekhit, A.A.; Khafaga, A.F.; Noreldin, A.E.; Elzoghby, A.O.; Khattab, S.N. Green self-assembled lactoferrin carboxymethyl cellulose nanogels for synergistic chemo/herbal breast cancer therapy. Colloids Surfaces B: Biointerfaces 2022, 217, 112657, . [CrossRef]
- Labhasetwar, V. and D.L. Leslie-Pelecky, Biomedical applications of nanotechnology. 2007.
- Murakami, S.; Aoki, N. Bio-Based Hydrogels Prepared by Cross-Linking of Microbial Poly(γ-glutamic acid) with Various Saccharides. Biomacromolecules 2006, 7, 2122–2127, . [CrossRef]
- Mariconti, M.; Morel, M.; Baigl, D.; Rudiuk, S. Enzymatically Active DNA-Protein Nanogels with Tunable Cross-Linking Density. Biomacromolecules 2021, 22, 3431–3439, . [CrossRef]
- Du, X., et al., Temperature/pH-responsive carmofur-loaded nanogels rapidly prepared via one-pot laser-induced emulsion polymerization. Colloids and Surfaces B: Biointerfaces, 2022. 217: p. 112611.
- Klier, J., et al., Properties and applications of microemulsions. Advanced Materials, 2000. 12(23): p. 1751-1757.
- Mousaviasl, S.; Saleh, T.; Shojaosadati, S.A.; Boddohi, S. Synthesis and characterization of schizophyllan nanogels via inverse emulsion using biobased materials. Int. J. Biol. Macromol. 2018, 120, 468–474, . [CrossRef]
- Gratton, S.E.; Pohlhaus, P.D.; Lee, J.; Guo, J.; Cho, M.J.; DeSimone, J.M. Nanofabricated particles for engineered drug therapies: A preliminary biodistribution study of PRINT™ nanoparticles. J. Control. Release 2007, 121, 10–18, . [CrossRef]
- Sansone, F.; Casnati, A. Multivalent glycocalixarenes for recognition of biological macromolecules: glycocalyx mimics capable of multitasking. Chem. Soc. Rev. 2013, 42, 4623–4639, . [CrossRef]
- Fasolin, L.H., et al., Emergent food proteins–Towards sustainability, health and innovation. Food Research International, 2019. 125: p. 108586.
- Silva, M.P.; Fabi, J.P. Food biopolymers-derived nanogels for encapsulation and delivery of biologically active compounds: A perspective review. Food Hydrocoll. Heal. 2022, 100079., . [CrossRef]
- Zhang, N.; Wardwell, P.R.; Bader, R.A. Polysaccharide-Based Micelles for Drug Delivery. Pharmaceutics 2013, 5, 329–352, doi:10.3390/pharmaceutics5020329.
- Leung, M.; Liu, C.; Koon, J.; Fung, K. Polysaccharide biological response modifiers. Immunol. Lett. 2006, 105, 101–114, . [CrossRef]
- Aspinall, G.O., The polysaccharides. 2014: Academic press.
- Miller, T.; Goude, M.C.; McDevitt, T.C.; Temenoff, J.S. Molecular engineering of glycosaminoglycan chemistry for biomolecule delivery. Acta Biomater. 2014, 10, 1705–1719, . [CrossRef]
- Drogoz, A.; David, L.; Rochas, C.; Domard, A.; Delair, T. Polyelectrolyte Complexes from Polysaccharides: Formation and Stoichiometry Monitoring. Langmuir 2007, 23, 10950–10958, . [CrossRef]
- Posocco, B.; Dreussi, E.; de Santa, J.; Toffoli, G.; Abrami, M.; Musiani, F.; Grassi, M.; Farra, R.; Tonon, F.; Grassi, G.; et al. Polysaccharides for the Delivery of Antitumor Drugs. Materials 2015, 8, 2569–2615, . [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981, . [CrossRef]
- sterberg, E., et al., Protein-rejecting ability of surface-bound dextran in end-on and side-on configurations: Comparison to PEG. Journal of biomedical materials research, 1995. 29(6): p. 741-747.
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Seaweed Polysaccharide-Based Nanoparticles: Preparation and Applications for Drug Delivery. Polymers 2016, 8, 30, . [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430, . [CrossRef]
- Elgadir, M.; Uddin, M.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629, . [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Agar/Chitosan IPN Thin Hydrogel Films with Antimicrobial and Antioxidant Properties for Potential Dressing Applications. Curr. Appl. Polym. Sci. 2017, 1, 1–1, . [CrossRef]
- Kamat, V.; Marathe, I.; Ghormade, V.; Bodas, D.; Paknikar, K. Synthesis of Monodisperse Chitosan Nanoparticles and in Situ Drug Loading Using Active Microreactor. ACS Appl. Mater. Interfaces 2015, 7, 22839–22847, . [CrossRef]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902, . [CrossRef]
- Debele, T.A.; Peng, S.; Tsai, H.-C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136, . [CrossRef]
- Sinha, V.; Singla, A.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33, . [CrossRef]
- Makhlouf, A.S.H. and N.Y. Abu-Thabit, Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications: Volume 1: Types and Triggers. 2018: Woodhead Publishing.
- Baxter, R.M.; Dai, T.; Kimball, J.; Wang, E.; Hamblin, M.R.; Wiesmann, W.P.; McCarthy, S.J.; Baker, S.M. Chitosan dressing promotes healing in third degree burns in mice: Gene expression analysis shows biphasic effects for rapid tissue regeneration and decreased fibrotic signaling. J. Biomed. Mater. Res. Part A 2012, 101A, 340–348, . [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426, . [CrossRef]
- Grenha, A. Chitosan nanoparticles: a survey of preparation methods. J. Drug Target. 2012, 20, 291–300, . [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37, . [CrossRef]
- Li, X.-M.; Wu, Z.-Z.; Zhang, B.; Pan, Y.; Meng, R.; Chen, H.-Q. Fabrication of chitosan hydrochloride and carboxymethyl starch complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2019, 293, 197–203, . [CrossRef]
- Lu, D.-Q.; Liu, D.; Liu, J.; Li, W.-X.; Ai, Y.; Wang, J.; Guan, D. Facile synthesis of chitosan-based nanogels through photo-crosslinking for doxorubicin delivery. Int. J. Biol. Macromol. 2022, 218, 335–345, . [CrossRef]
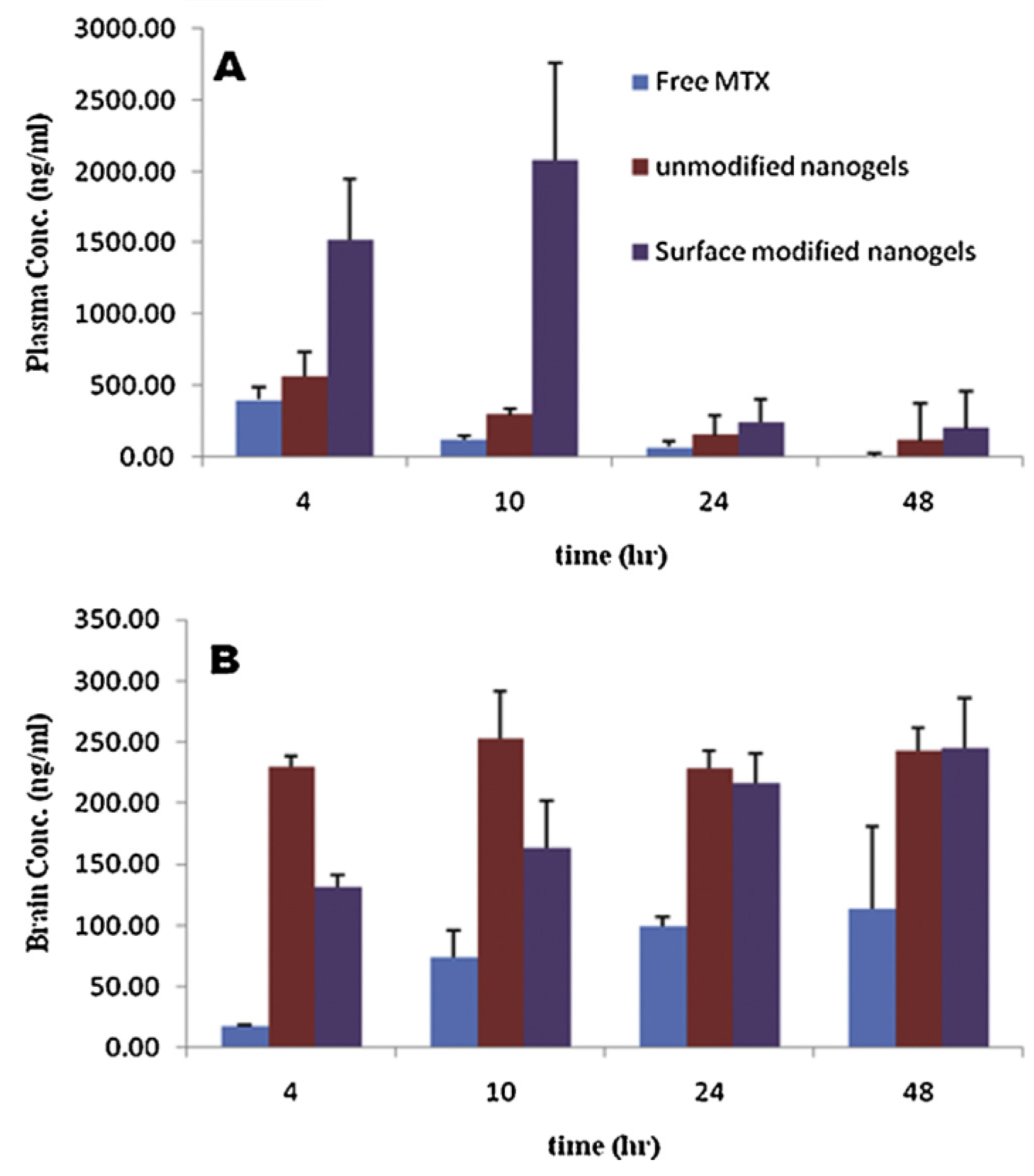
- Azadi, A.; Hamidi, M.; Rouini, M.-R. Methotrexate-loaded chitosan nanogels as ‘Trojan Horses’ for drug delivery to brain: Preparation and in vitro/in vivo characterization. Int. J. Biol. Macromol. 2013, 62, 523–530, . [CrossRef]
- Lindblad, M.S., et al., Hydrogels from polysaccharides for biomedical applications. 2007, ACS Publications.
- Bhavani, A.L. and J. Nisha, Dextran—the polysaccharide with versatile uses. Int J Pharm Biol Sci, 2010. 1(4): p. 569-573.
- Xu, W.; Ding, J.; Li, L.; Xiao, C.; Zhuang, X.; Chen, X. Acid-labile boronate-bridged dextran–bortezomib conjugate with up-regulated hypoxic tumor suppression. Chem. Commun. 2015, 51, 6812–6815, . [CrossRef]
- Xu, W.; Ding, J.; Xiao, C.; Li, L.; Zhuang, X.; Chen, X. Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials 2015, 54, 72–86, . [CrossRef]
- Cui, L.; Cohen, J.L.; Chu, C.K.; Wich, P.R.; Kierstead, P.H.; Fréchet, J.M.J. Conjugation Chemistry through Acetals toward a Dextran-Based Delivery System for Controlled Release of siRNA. J. Am. Chem. Soc. 2012, 134, 15840–15848, . [CrossRef]
- Zhang, Q.; Yue, W.; Zhao, D.; Chen, L.; Xu, Z.; Lin, D.; Qin, W. Preparation and characterization of soybean protein isolate-dextran conjugate-based nanogels. Food Chem. 2022, 384, 132556, . [CrossRef]
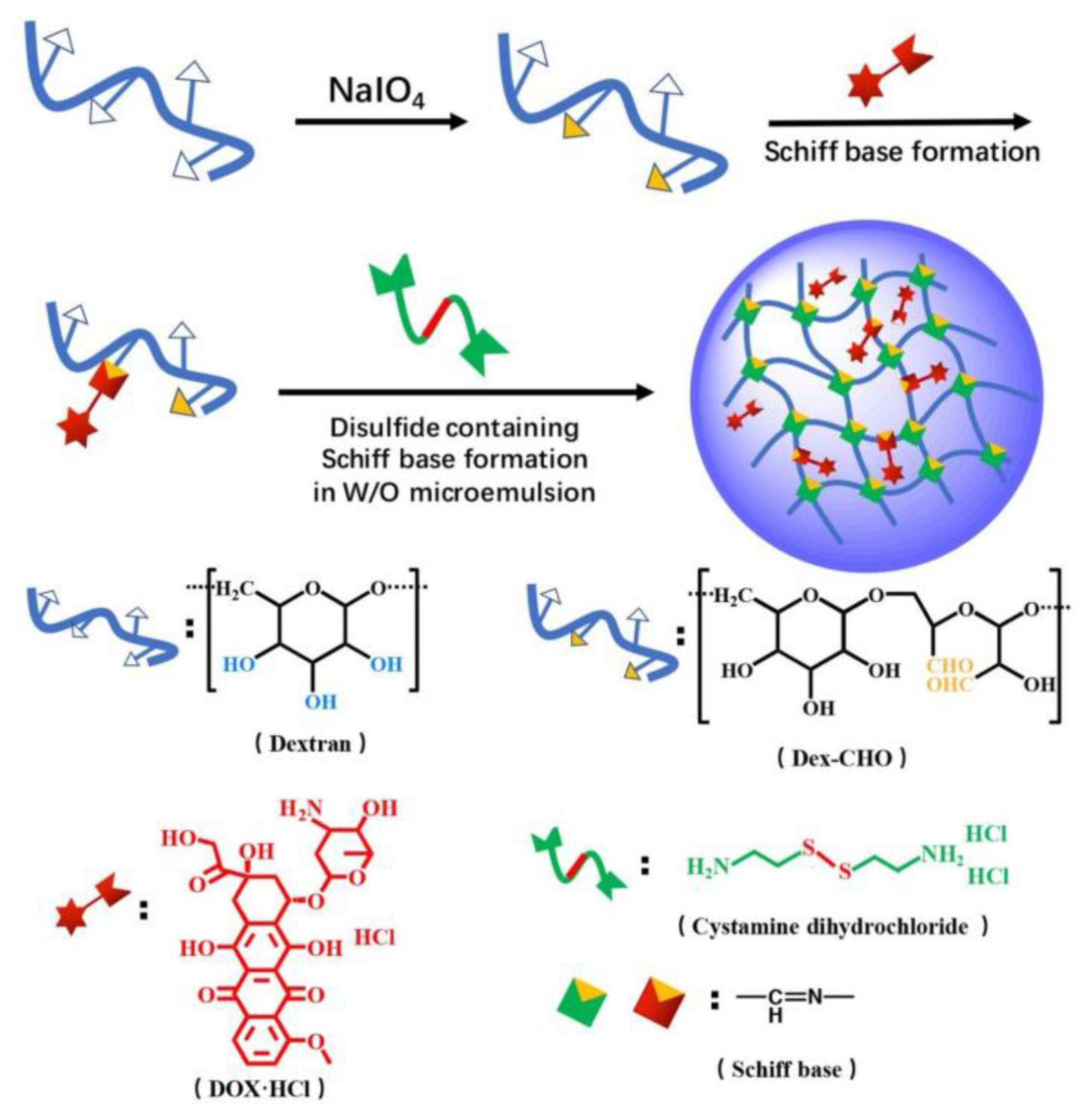
- Yu, K.; Yang, X.; He, L.; Zheng, R.; Min, J.; Su, H.; Shan, S.; Jia, Q. Facile preparation of pH/reduction dual-stimuli responsive dextran nanogel as environment-sensitive carrier of doxorubicin. Polymer 2020, 200, 122585, . [CrossRef]
- Nagahama, K.; Sano, Y.; Kumano, T. Anticancer drug-based multifunctional nanogels through self-assembly of dextran–curcumin conjugates toward cancer theranostics. Bioorganic Med. Chem. Lett. 2015, 25, 2519–2522, . [CrossRef]
- Naeye, B.; Raemdonck, K.; Remaut, K.; Sproat, B.; Demeester, J.; De Smedt, S. PEGylation of biodegradable dextran nanogels for siRNA delivery. Eur. J. Pharm. Sci. 2010, 40, 342–351, . [CrossRef]
- Naeye, B.; Deschout, H.; Röding, M.; Rudemo, M.; Delanghe, J.; Devreese, K.; Demeester, J.; Braeckmans, K.; De Smedt, S.C.; Raemdonck, K. Hemocompatibility of siRNA loaded dextran nanogels. Biomaterials 2011, 32, 9120–9127, . [CrossRef]
- Li, D.; Kordalivand, N.; Fransen, M.F.; Ossendorp, F.; Raemdonck, K.; Vermonden, T.; Hennink, W.E.; van Nostrum, C.F. Reduction-Sensitive Dextran Nanogels Aimed for Intracellular Delivery of Antigens. Adv. Funct. Mater. 2015, 25, 2993–3003, . [CrossRef]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-based and heparin-inspired hydrogels: size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208, . [CrossRef]
- Baldwin, A.D.; Robinson, K.G.; Militar, J.L.; Derby, C.D.; Kiick, K.L.; Akins, R.E. In situ crosslinkable heparin-containing poly(ethylene glycol) hydrogels for sustained anticoagulant release. J. Biomed. Mater. Res. Part A 2012, 100A, 2106–2118, . [CrossRef]
- Bae, K.H.; Mok, H.; Park, T.G. Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials 2008, 29, 3376–3383, . [CrossRef]
- Sasisekharan, R.; Shriver, Z.; Venkataraman, G.; Narayanasami, U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat. Rev. Cancer 2002, 2, 521–528, . [CrossRef]
- Lee, J.-H.; Lee, H.; Joung, Y.K.; Jung, K.H.; Choi, J.-H.; Lee, D.-H.; Park, K.D.; Hong, S.-S. The use of low molecular weight heparin–pluronic nanogels to impede liver fibrosis by inhibition the TGF-β/Smad signaling pathway. Biomaterials 2011, 32, 1438–1445, . [CrossRef]
- Sriamornsak, P., Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn University International Journal, 2003. 3(1-2): p. 206-228.
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275, . [CrossRef]
- Yapo, B.M.; Lerouge, P.; Thibault, J.-F.; Ralet, M.-C. Pectins from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydr. Polym. 2007, 69, 426–435, . [CrossRef]
- Bhatia, M.S.; Deshmukh, R.; Choudhari, P.; Bhatia, N.M. Chemical Modification of Pectins, Characterization and Evaluation for Drug Delivery. Sci. Pharm. 2008, 76, 775–784, . [CrossRef]
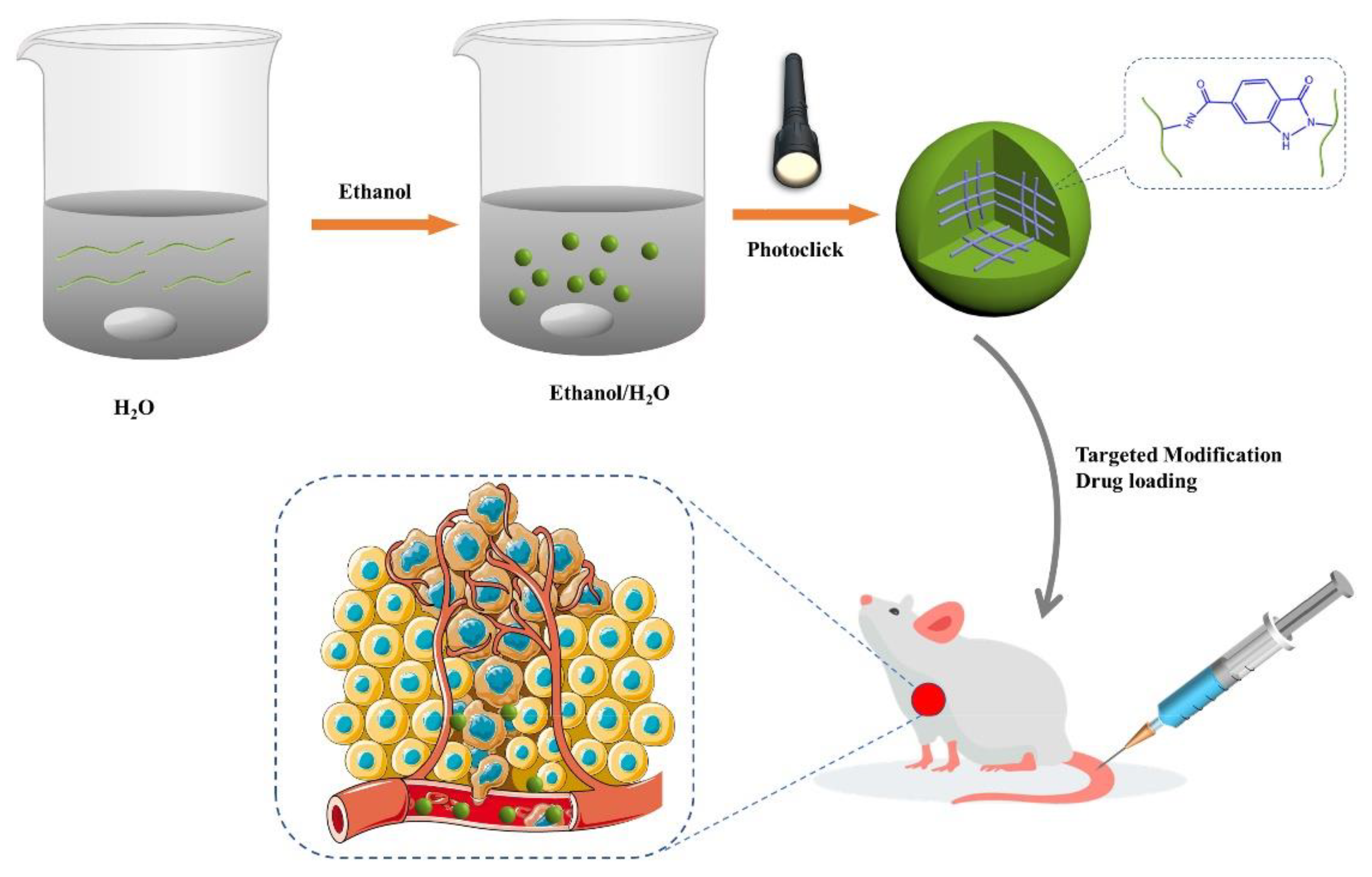
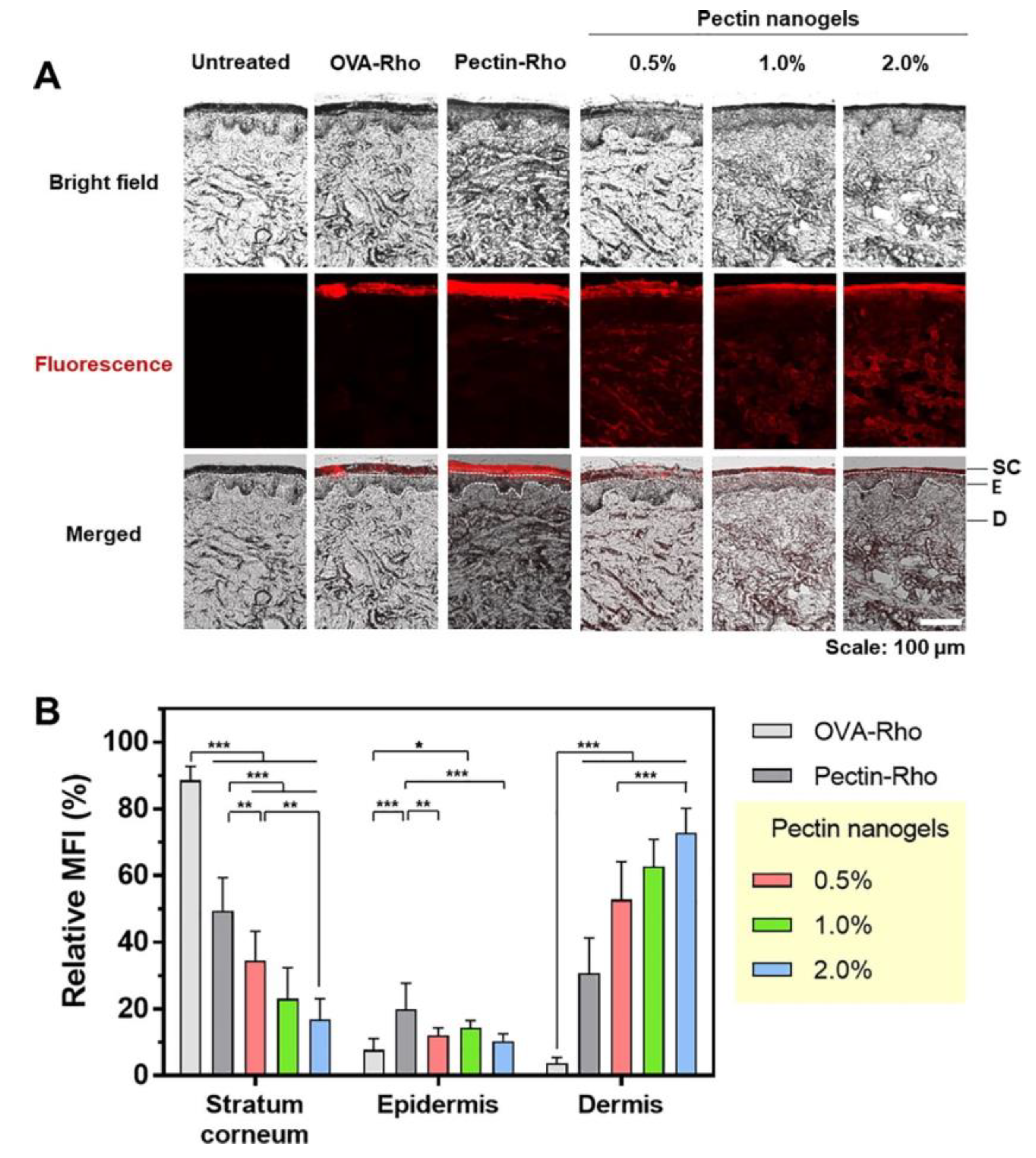
- Lee, S.; Woo, C.; Ki, C.S. Pectin nanogel formation via thiol-norbornene photo-click chemistry for transcutaneous antigen delivery. J. Ind. Eng. Chem. 2022, 108, 159–169, . [CrossRef]
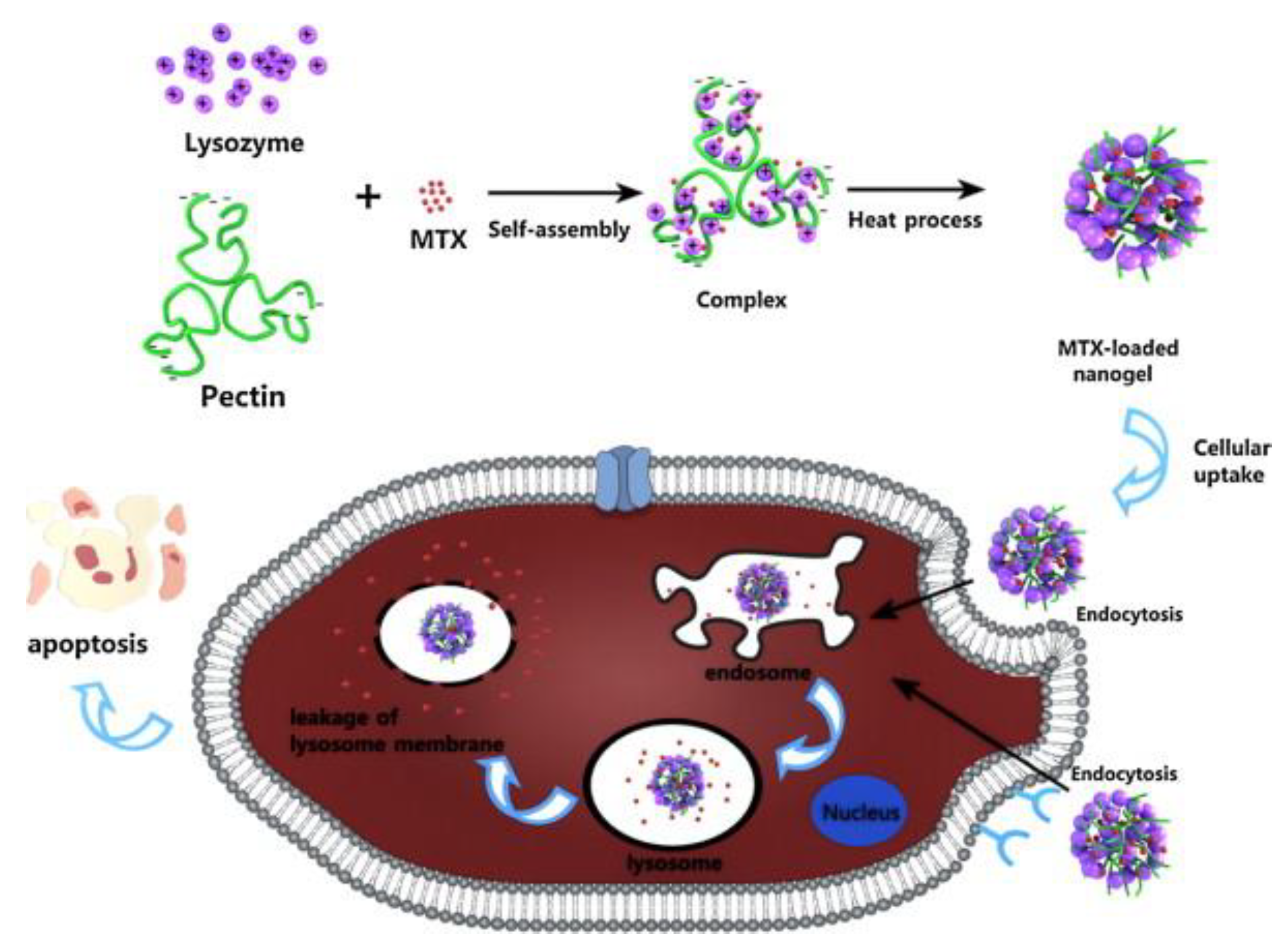
- Lin, L., et al., Construction of pH-sensitive lysozyme/pectin nanogel for tumor methotrexate delivery. Colloids and Surfaces B: Biointerfaces, 2015. 126: p. 459-466.
- Ilgin, P.; Avci, G.; Silan, C.; Ekici, S.; Aktas, N.; Ayyala, R.S.; John, V.T.; Sahiner, N. Colloidal drug carries from (sub)micron hyaluronic acid hydrogel particles with tunable properties for biomedical applications. Carbohydr. Polym. 2010, 82, 997–1003, . [CrossRef]
- Sahiner, N.; Silan, C.; Sagbas, S.; Ilgin, P.; Butun, S.; Erdugan, H.; Ayyala, R.S. Porous and modified HA particles as potential drug delivery systems. Microporous Mesoporous Mater. 2012, 155, 124–130, . [CrossRef]
- Ekici, S.; Ilgin, P.; Yilmaz, S.; Aktas, N.; Sahiner, N. Temperature and magnetic field responsive hyaluronic acid particles with tunable physical and chemical properties. Appl. Surf. Sci. 2011, 257, 2669–2676, . [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Ayyala, R.S. Polyethyleneimine modified poly(Hyaluronic acid) particles with controllable antimicrobial and anticancer effects. Carbohydr. Polym. 2017, 159, 29–38, . [CrossRef]
- Sahiner, N.; Jha, A.K.; Nguyen, D.; Jia, X. Fabrication and characterization of cross-linkable hydrogel particles based on hyaluronic acid: potential application in vocal fold regeneration. J. Biomater. Sci. Polym. Ed. 2008, 19, 223–243, . [CrossRef]
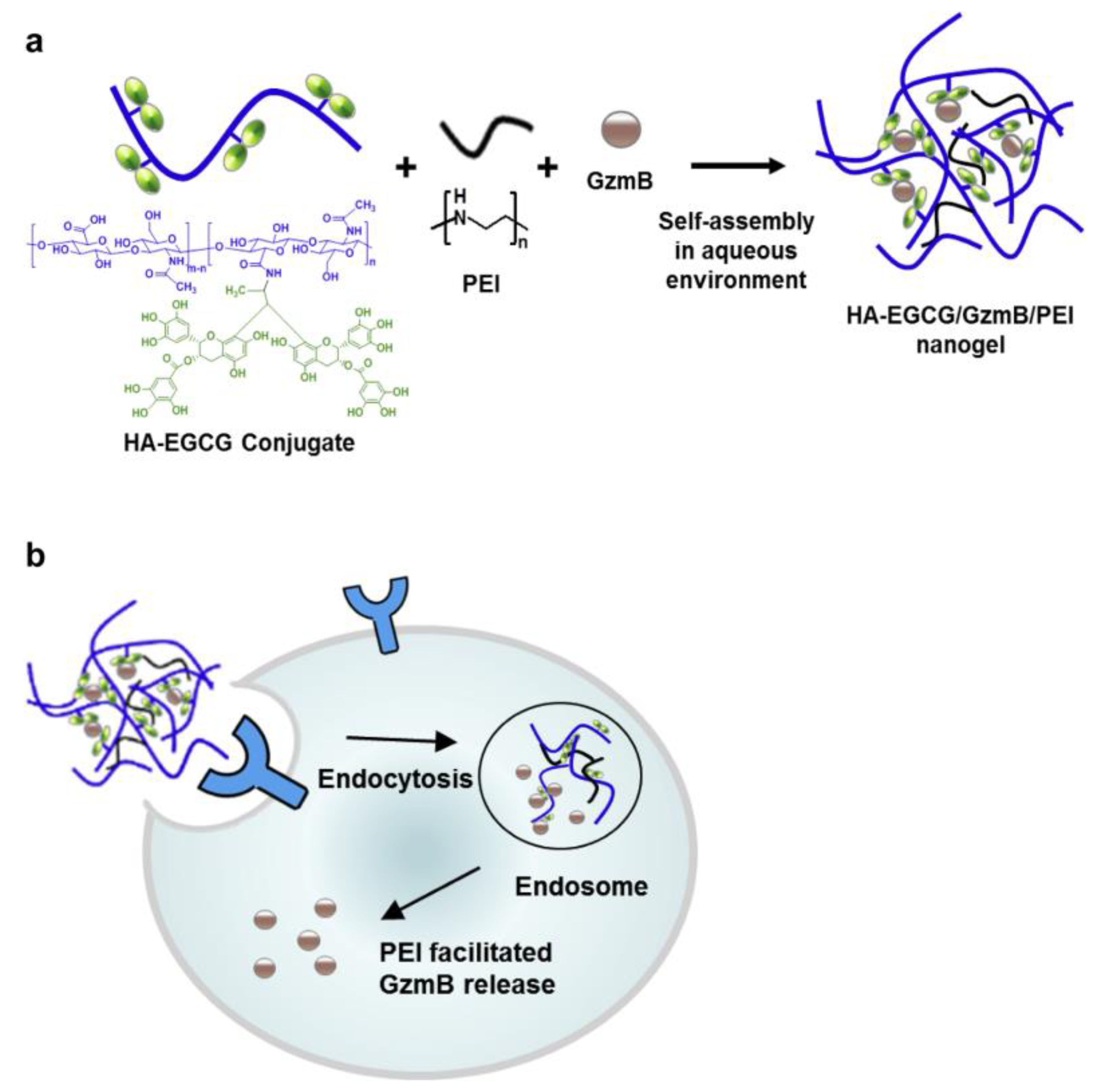
- Liang, K.; Ng, S.; Lee, F.; Lim, J.; Chung, J.E.; Lee, S.S.; Kurisawa, M. Targeted intracellular protein delivery based on hyaluronic acid–green tea catechin nanogels. Acta Biomater. 2016, 33, 142–152, . [CrossRef]
- Coninx, S.; Kalot, G.; Godard, A.; Bodio, E.; Goze, C.; Sancey, L.; Auzély-Velty, R. Tailored hyaluronic acid-based nanogels as theranostic boron delivery systems for boron neutron cancer therapy. Int. J. Pharm. X 2022, 4, . [CrossRef]
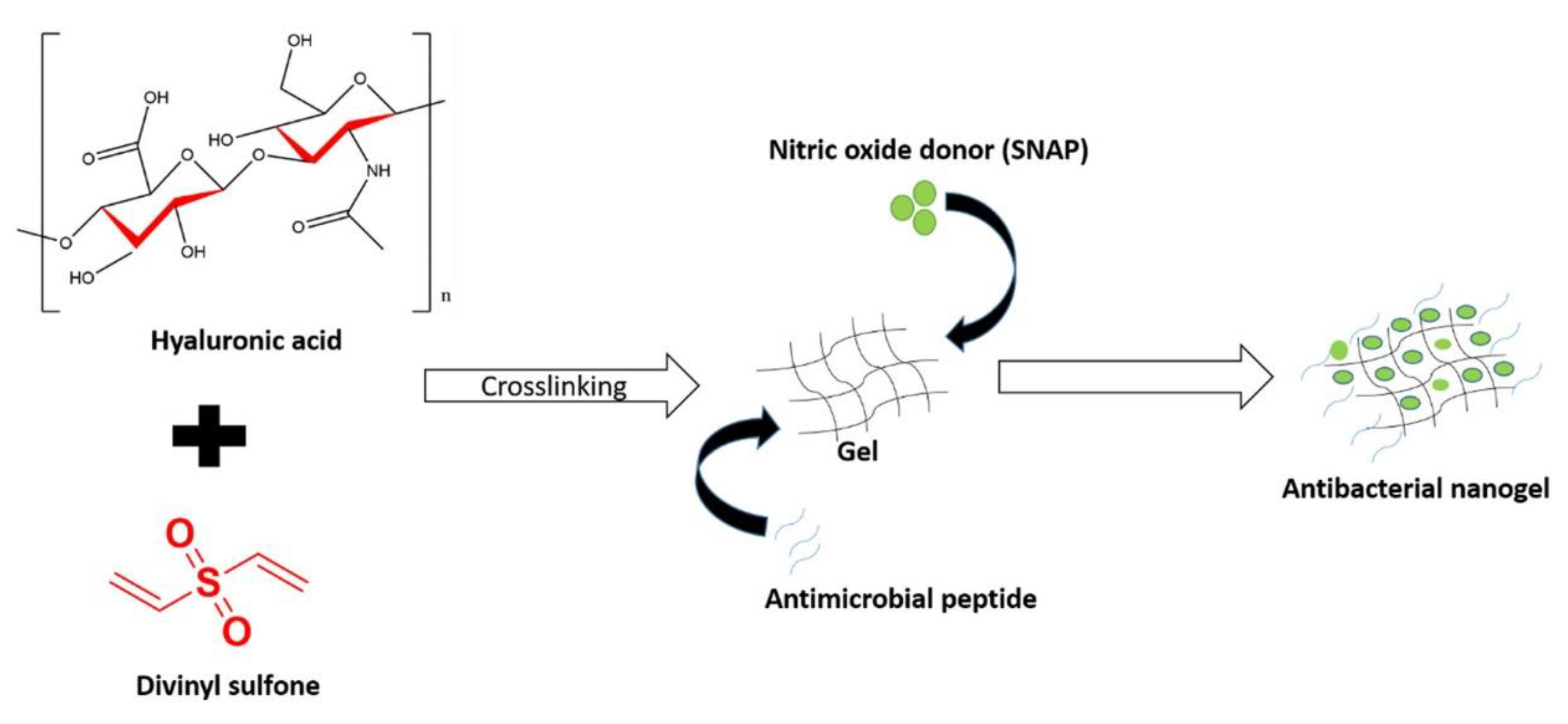
- Fasiku, V.O.; Omolo, C.A.; Kiruri, L.W.; Devnarain, N.; Faya, M.; Mocktar, C.; Govender, T. A hyaluronic acid-based nanogel for the co-delivery of nitric oxide (NO) and a novel antimicrobial peptide (AMP) against bacterial biofilms. Int. J. Biol. Macromol. 2022, 206, 381–397, . [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305, . [CrossRef]
- Valentino, C.; Vigani, B.; Fedeli, I.; Miele, D.; Marrubini, G.; Malavasi, L.; Ferrari, F.; Sandri, G.; Rossi, S. Development of alginate-spermidine micro/nanogels as potential antioxidant and anti-inflammatory tool in peripheral nerve injuries. Formulation studies and physico-chemical characterization. Int. J. Pharm. 2022, 626, 122168, . [CrossRef]
- Gaur, R., et al., Aureobasidium pullulans, an economically important polymorphic yeast with special reference to pullulan. African journal of biotechnology, 2010. 9(47): p. 7989-7997.
- Miyahara, T.; Nyan, M.; Shimoda, A.; Yamamoto, Y.; Kuroda, S.; Shiota, M.; Akiyoshi, K.; Kasugai, S. Exploitation of a novel polysaccharide nanogel cross-linking membrane for guided bone regeneration (GBR). J. Tissue Eng. Regen. Med. 2011, 6, 666–672, . [CrossRef]
- Zhang, T.; Yang, R.; Yang, S.; Guan, J.; Zhang, D.; Ma, Y.; Liu, H. Research progress of self-assembled nanogel and hybrid hydrogel systems based on pullulan derivatives. Drug Deliv. 2018, 25, 278–292, . [CrossRef]
- Nakahashi-Ouchida, R.; Yuki, Y.; Kiyono, H. Development of a nanogel-based nasal vaccine as a novel antigen delivery system. Expert Rev. Vaccines 2017, 16, 1231–1240, . [CrossRef]
- Quazi, M.Z.; Park, N. Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. Int. J. Mol. Sci. 2022, 23, 1943, . [CrossRef]
- Muraoka, D.; Harada, N.; Shiku, H.; Akiyoshi, K. Self-assembled polysaccharide nanogel delivery system for overcoming tumor immune resistance. J. Control. Release 2022, 347, 175–182, . [CrossRef]
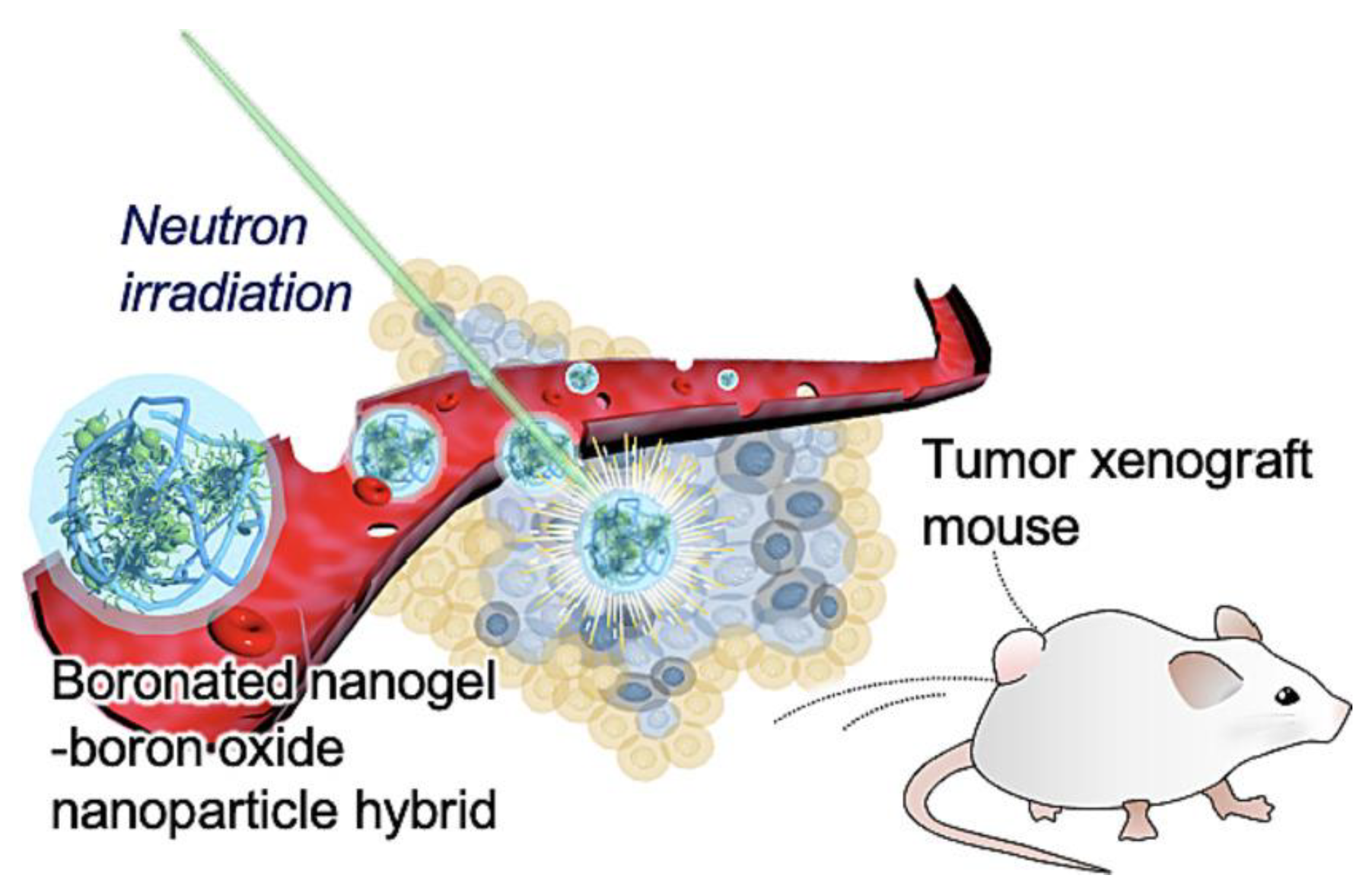
- Kawasaki, R.; Hirano, H.; Yamana, K.; Isozaki, H.; Kawamura, S.; Sanada, Y.; Bando, K.; Tabata, A.; Yoshikawa, K.; Azuma, H.; et al. Carborane bearing pullulan nanogel-boron oxide nanoparticle hybrid for boron neutron capture therapy. Nanomedicine: Nanotechnology, Biol. Med. 2023, 49, 102659, . [CrossRef]
- Igarashi, N.; Takeguchi, A.; Sakai, S.; Akiyama, H.; Higashi, K.; Toida, T. Effect of Molecular Sizes of Chondroitin Sulfate on Interaction with L-Selectin. Int. J. Carbohydr. Chem. 2013, 2013, 1–9, . [CrossRef]
- Egea, J.; Garcia, A.G.; Verges, J.; Montell, E.; Lopez, M.G. Antioxidant, antiinflammatory and neuroprotective actions of chondroitin sulfate and proteoglycans. Osteoarthr. Cartil. 2010, 18 (Suppl. 1), S24–S27, . [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr. Polym. 2020, 230, 115650, . [CrossRef]
- Ghaeini-Hesaroeiye, S.; Boddohi, S.; Vasheghani-Farahani, E. Dual responsive chondroitin sulfate based nanogel for antimicrobial peptide delivery. Int. J. Biol. Macromol. 2019, 143, 297–304, . [CrossRef]
- Lee, J.-Y.; Chung, S.-J.; Cho, H.-J.; Kim, D.-D. Bile acid-conjugated chondroitin sulfate A-based nanoparticles for tumor-targeted anticancer drug delivery. Eur. J. Pharm. Biopharm. 2015, 94, 532–541, . [CrossRef]
- Tayeferad, M.; Boddohi, S.; Bakhshi, B. Dual-responsive nisin loaded chondroitin sulfate nanogel for treatment of bacterial infection in soft tissues. Int. J. Biol. Macromol. 2021, 193, 166–172, . [CrossRef]
- Setayesh, A.; Bagheri, F.; Boddohi, S. Self-assembled formation of chondroitin sulfate-based micellar nanogel for curcumin delivery to breast cancer cells. Int. J. Biol. Macromol. 2020, 161, 771–778, . [CrossRef]
- Aga, M.B.; Dar, A.H.; Nayik, G.A.; Panesar, P.S.; Allai, F.; Khan, S.A.; Shams, R.; Kennedy, J.F.; Altaf, A. Recent insights into carrageenan-based bio-nanocomposite polymers in food applications: A review. Int. J. Biol. Macromol. 2021, 192, 197–209, . [CrossRef]
- Liang, W.; Mao, X.; Peng, X.; Tang, S. Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohydr. Polym. 2014, 101, 776–785. doi:10.1016/j.carbpol.2013.10.010.
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.-I.; Kim, C.W.; Lee, H.-R.; Kim, M. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 1–12, . [CrossRef]
- Madruga, L.Y.; Sabino, R.M.; Santos, E.C.; Popat, K.C.; Balaban, R.d.C.; Kipper, M.J. Carboxymethyl-kappa-carrageenan: A study of biocompatibility, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2020, 152, 483–491, . [CrossRef]
- Cicinskas, E.; Kalitnik, A.A.; Karetin, Y.A.; Ram, M.S.G.M.; Achary, A.; Kravchenko, A.O. Immunomodulating Properties of Carrageenan from Tichocarpus crinitus. Inflammation 2020, 43, 1387–1396, . [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256, . [CrossRef]
- Thrimawithana, T.R.; Young, S.; Dunstan, D.E.; Alany, R.G. Texture and rheological characterization of kappa and iota carrageenan in the presence of counter ions. Carbohydr. Polym. 2010, 82, 69–77, doi:10.1016/j.carbpol.2010.04.024.
- Moritaka, H.; Nishinari, K.; Nakahama, N.; Fukuba, H. Effects of Potassium Chloride and Sodium Chloride on the Thermal Properties of Gellan Gum Gels. Biosci. Biotechnol. Biochem. 1992, 56, 595–599, . [CrossRef]
- Daniel-Da-Silva, A.L.; Ferreira, L.; Gil, A.M.; Trindade, T. Synthesis and swelling behavior of temperature responsive κ-carrageenan nanogels. J. Colloid Interface Sci. 2010, 355, 512–517, . [CrossRef]
- van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175, . [CrossRef]
- Pamfil, D. and C. Vasile, Nanogels of natural polymers. Polymer Gels: Perspectives and Applications, 2018: p. 71-110.
- Layre, A.-M.; Volet, G.; Wintgens, V.; Amiel, C. Associative Network Based on Cyclodextrin Polymer: A Model System for Drug Delivery. Biomacromolecules 2009, 10, 3283–3289, . [CrossRef]
- Moya-Ortega, M.D., et al., Cross-linked hydroxypropyl-β-cyclodextrin and γ-cyclodextrin nanogels for drug delivery: Physicochemical and loading/release properties. Carbohydrate polymers, 2012. 87(3): p. 2344-2351.
- Jin, L.; Liu, Q.; Sun, Z.; Ni, X.; Wei, M. Preparation of 5-Fluorouracil/β-Cyclodextrin Complex Intercalated in Layered Double Hydroxide and the Controlled Drug Release Properties. Ind. Eng. Chem. Res. 2010, 49, 11176–11181, . [CrossRef]
- Blanco-Fernandez, B.; Lopez-Viota, M.; Concheiro, A.; Alvarez-Lorenzo, C. Synergistic performance of cyclodextrin–agar hydrogels for ciprofloxacin delivery and antimicrobial effect. Carbohydr. Polym. 2011, 85, 765–774, . [CrossRef]
- Oktay, A.N.; Celebi, N.; Ilbasmis-Tamer, S.; Kaplanoğlu, G.T. Cyclodextrin-based nanogel of flurbiprofen for dermal application: In vitro studies and in vivo skin irritation evaluation. J. Drug Deliv. Sci. Technol. 2023, 79, . [CrossRef]
- Snyders, R.; Shingel, K.I.; Zabeida, O.; Roberge, C.; Faure, M.-P.; Martinu, L.; Klemberg-Sapieha, J.E. Mechanical and microstructural properties of hybrid poly(ethylene glycol)–soy protein hydrogels for wound dressing applications. J. Biomed. Mater. Res. Part A 2007, 83A, 88–97, . [CrossRef]
- Panahi, R. and M. Baghban-Salehi, Protein-based hydrogels, in Cellulose-based superabsorbent hydrogels. 2019, Springer. p. 1561-1600.
- Ren, C., et al., A novel H 2 O 2 responsive supramolecular hydrogel for controllable drug release. RSC advances, 2017. 7(3): p. 1313-1317.
- Zhang, P.; Cheetham, A.G.; Lock, L.L.; Cui, H. Cellular Uptake and Cytotoxicity of Drug–Peptide Conjugates Regulated by Conjugation Site. Bioconjugate Chem. 2013, 24, 604–613, . [CrossRef]
- Cai, Y., et al., Supramolecular “Trojan Horse” for nuclear delivery of dual anticancer drugs. Journal of the American Chemical Society, 2017. 139(8): p. 2876-2879.
- Wang, K.; Liu, M.; Mo, R. Polysaccharide-Based Biomaterials for Protein Delivery. Med. Drug Discov. 2020, 7, 100031, . [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657, . [CrossRef]
- Rosa, E.; Diaferia, C.; Gallo, E.; Morelli, G.; Accardo, A. Stable Formulations of Peptide-Based Nanogels. Molecules 2020, 25, 3455, . [CrossRef]
- Cao, S.-J.; Xu, S.; Wang, H.-M.; Ling, Y.; Dong, J.; Xia, R.-D.; Sun, X.-H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 190, doi:10.1208/s12249-019-1325-z.
- Slastnikova, T.A.; Ulasov, A.V.; Rosenkranz, A.A.; Sobolev, A.S. Targeted Intracellular Delivery of Antibodies: The State of the Art. Front. Pharmacol. 2018, 9, 1208, doi:10.3389/fphar.2018.01208.
- Posey, N.D.; Hango, C.R.; Minter, L.M.; Tew, G.N. The Role of Cargo Binding Strength in Polymer-Mediated Intracellular Protein Delivery. Bioconjugate Chem. 2018, 29, 2679–2690, . [CrossRef]
- Kimchi-Sarfaty, C.; Schiller, T.; Hamasaki-Katagiri, N.; Khan, M.A.; Yanover, C.; Sauna, Z.E. Building better drugs: developing and regulating engineered therapeutic proteins. Trends Pharmacol. Sci. 2013, 34, 534–548, . [CrossRef]
- Lv, J.; Fan, Q.; Wang, H.; Cheng, Y. Polymers for cytosolic protein delivery. Biomaterials 2019, 218, 119358, . [CrossRef]
- Fu, A.; Tang, R.; Hardie, J.; Farkas, M.E.; Rotello, V.M. Promises and Pitfalls of Intracellular Delivery of Proteins. Bioconjugate Chem. 2014, 25, 1602–1608, . [CrossRef]
- Raemdonck, K.; Demeester, J.; De Smedt, S. Advanced nanogel engineering for drug delivery. Soft Matter 2008, 5, 707–715, . [CrossRef]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608, . [CrossRef]
- Zhang, X.; Malhotra, S.; Molina, M.; Haag, R. Micro- and nanogels with labile crosslinks – from synthesis to biomedical applications. Chem. Soc. Rev. 2015, 44, 1948–1973, . [CrossRef]
- Berti, C.; Boarino, A.; Graciotti, M.; Bader, L.P.E.; Kandalaft, L.E.; Klok, H.-A. Reduction-Sensitive Protein Nanogels Enhance Uptake of Model and Tumor Lysate Antigens In Vitro by Mouse- and Human-Derived Dendritic Cells. ACS Appl. Bio Mater. 2021, 4, 8291–8300, . [CrossRef]
- Froimchuk, E.; Carey, S.T.; Edwards, C.; Jewell, C.M. Self-Assembly as a Molecular Strategy to Improve Immunotherapy. Accounts Chem. Res. 2020, 53, 2534–2545, . [CrossRef]
- Tsai, S.J.; Amerman, A.; Jewell, C.M. Altering Antigen Charge to Control Self-Assembly and Processing of Immune Signals During Cancer Vaccination. Front. Immunol. 2021, 11, 3340. [CrossRef]
- Pei, M.; Xu, R.; Zhang, C.; Wang, X.; Li, C.; Hu, Y. Mannose-functionalized antigen nanoparticles for targeted dendritic cells, accelerated endosomal escape and enhanced MHC-I antigen presentation. Colloids Surfaces B: Biointerfaces 2021, 197, 111378, . [CrossRef]
- Ding, Y.-F.; Wei, J.; Li, S.; Pan, Y.-T.; Wang, L.-H.; Wang, R. Host–Guest Interactions Initiated Supramolecular Chitosan Nanogels for Selective Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 28665–28670, . [CrossRef]
- Wei, L.; Zhao, Y.; Hu, X.; Tang, L. Redox-Responsive Polycondensate Neoepitope for Enhanced Personalized Cancer Vaccine. ACS Central Sci. 2020, 6, 404–412, . [CrossRef]
- Wang, K.; Yang, Y.; Xue, W.; Liu, Z. Cell Penetrating Peptide-Based Redox-Sensitive Vaccine Delivery System for Subcutaneous Vaccination. Mol. Pharm. 2018, 15, 975–984, . [CrossRef]
- Wang, K., et al., “Minimalist” nanovaccine constituted from near whole antigen for cancer immunotherapy. ACS nano, 2018. 12(7): p. 6398-6409.
- Matsumoto, N.M., et al., Synthesis of nanogel–protein conjugates. Polymer chemistry, 2013. 4(8): p. 2464-2469.
- Mondal, M.I.H., Cellulose-based superabsorbent hydrogels. 2019: Springer Berlin Heidelberg.
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and Protein-Based Hydrogels. Chem. Mater. 2012, 24, 759–773, . [CrossRef]
- Chander, S.; Kulkarni, G.T.; Dhiman, N.; Kharkwal, H. Protein-Based Nanohydrogels for Bioactive Delivery. Front. Chem. 2021, 9, 573748, . [CrossRef]
- Lakshmanan, V.-K.; Kim, B.; Ojha, S.; Al-Abd, A.M.; Shin, M.G.; Jung, Y.D. Preparation and characterization of an elastin nanogel with enhanced biocompatibility and improved entrapment efficiency in prostate cancer cells. Mater. Express 2021, 11, 16–27, . [CrossRef]
- Jia, X.; Kiick, K.L. Hybrid Multicomponent Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 140–156, . [CrossRef]
- Masuda, T.; Furue, M.; Matsuda, T. Photocured, Styrenated Gelatin-Based Microspheres for de Novo Adipogenesis through Corelease of Basic Fibroblast Growth Factor, Insulin, and Insulin-Like Growth Factor I. Tissue Eng. 2004, 10, 523–535, . [CrossRef]
- Khan, R.; Khan, M.H. Use of collagen as a biomaterial: An update. J. Indian Soc. Periodontol. 2013, 17, 539–42, . [CrossRef]
- Pathan, I.B.; Munde, S.J.; Shelke, S.; Ambekar, W.; Setty, C.M. Curcumin loaded fish scale collagen-HPMC nanogel for wound healing application: Ex-vivo and In-vivo evaluation. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 165–174, . [CrossRef]
- Saraogi, G.K.; Gupta, P.; Gupta, U.D.; Jain, N.K.; Agrawal, G.P. Gelatin nanocarriers as potential vectors for effective management of tuberculosis. Int. J. Pharm. 2010, 385, 143–149, . [CrossRef]
- Said, M.I. Role and function of gelatin in the development of the food and non-food industry: A review. IOP Conf. Series: Earth Environ. Sci. 2020, 492, 012086, . [CrossRef]
- Kang, M.G.; Lee, M.Y.; Cha, J.M.; Lee, J.K.; Lee, S.C.; Kim, J.; Hwang, Y.-S.; Bae, H. Nanogels Derived from Fish Gelatin: Application to Drug Delivery System. Mar. Drugs 2019, 17, 246, . [CrossRef]
- Zhang, S.; Shah, S.A.-U.; Basharat, K.; Qamar, S.A.; Raza, A.; Mohamed, A.; Bilal, M.; Iqbal, H.M. Silk-based nano-hydrogels for futuristic biomedical applications. J. Drug Deliv. Sci. Technol. 2022, 103385. [CrossRef]
- Gong, Z.; Yang, Y.; Huang, L.; Chen, X.; Shao, Z. Formation kinetics and fractal characteristics of regenerated silk fibroin alcogel developed from nanofibrillar network. Soft Matter 2010, 6, 1217–1223, . [CrossRef]
- Hofmann, S.; Foo, C.W.P.; Rossetti, F.; Textor, M.; Vunjak-Novakovic, G.; Kaplan, D.; Merkle, H.; Meinel, L. Silk fibroin as an organic polymer for controlled drug delivery. J. Control. Release 2006, 111, 219–227, . [CrossRef]
- Vepari, C. and D.L. Kaplan, Silk as a biomaterial. Progress in polymer science, 2007. 32(8-9): p. 991-1007.
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and Properties of Silk Hydrogels. Biomacromolecules 2004, 5, 786–792, . [CrossRef]
- Wongkrongsak, S., et al., Radiation-processed silk fibroin micro-/nano-gels as promising antioxidants: Electron beam treatment and physicochemical characterization. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022. 653: p. 129892.
- Tang, C.-H. Assembly of food proteins for nano- encapsulation and delivery of nutraceuticals (a mini-review). Food Hydrocoll. 2021, 117, 106710. [CrossRef]
- Khatkar, A.B.; Kaur, A.; Khatkar, S.K. Restructuring of soy protein employing ultrasound: Effect on hydration, gelation, thermal, in-vitro protein digestibility and structural attributes. LWT 2020, 132, 109781, . [CrossRef]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Influence of soy protein’s structural modifications on their microencapsulation properties: α-Tocopherol microparticle preparation. Food Res. Int. 2012, 48, 387–396, . [CrossRef]
- Tang, C.-H. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives (a review). Food Hydrocoll. 2019, 91, 92–116, . [CrossRef]
- Chen, F.-P.; Li, B.-S.; Tang, C.-H. Nanocomplexation between Curcumin and Soy Protein Isolate: Influence on Curcumin Stability/Bioaccessibility and in Vitro Protein Digestibility. J. Agric. Food Chem. 2015, 63, 3559–3569, . [CrossRef]
- Ding, X.; Yao, P. Soy Protein/Soy Polysaccharide Complex Nanogels: Folic Acid Loading, Protection, and Controlled Delivery. Langmuir 2013, 29, 8636–8644, . [CrossRef]
- Cheng, X.; Zeng, X.; Li, D.; Wang, X.; Sun, M.; He, L.; Tang, R. TPGS-grafted and acid-responsive soy protein nanogels for efficient intracellular drug release, accumulation, penetration in 3D tumor spheroids of drug-resistant cancer cells. Mater. Sci. Eng. C 2019, 102, 863–875, . [CrossRef]
- Shah, S.; Rangaraj, N.; Laxmikeshav, K.; Sampathi, S. “Nanogels as drug carriers – Introduction, chemical aspects, release mechanisms and potential applications”. Int. J. Pharm. 2020, 581, 119268, . [CrossRef]
- Ahmed, S.; Alhareth, K.; Mignet, N. Advancement in nanogel formulations provides controlled drug release. Int. J. Pharm. 2020, 584, 119435, . [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071, doi:10.1038/natrevmats.2016.71.
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review.. 1989, 49, 6449–65.
- Schmaljohann, D., Thermo-and pH-responsive polymers in drug delivery. Advanced drug delivery reviews, 2006. 58(15): p. 1655-1670.
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884, . [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C 2016, 60, 560–568, . [CrossRef]
- Klouda, L. and A.G. Mikos, Thermoresponsive hydrogels in biomedical applications. European journal of pharmaceutics and biopharmaceutics, 2008. 68(1): p. 34-45.
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770, . [CrossRef]
- Bergueiro, J.; Calderón, M. Thermoresponsive Nanodevices in Biomedical Applications. Macromol. Biosci. 2015, 15, 183–199, . [CrossRef]
- Lu, X.; Sun, M.; Barron, A.E. Non-ionic, thermo-responsive DEA/DMA nanogels: Synthesis, characterization, and use for DNA separations by microchip electrophoresis. J. Colloid Interface Sci. 2011, 357, 345–353, . [CrossRef]
- Medeiros, S.; Santos, A.; Fessi, H.; Elaissari, A. Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm. 2011, 403, 139–161, . [CrossRef]
- Zha, L.; Banik, B.; Alexis, F. Stimulus responsive nanogels for drug delivery. Soft Matter 2011, 7, 5908–5916, . [CrossRef]
- Murakami, Y.; Maeda, M. DNA-Responsive Hydrogels That Can Shrink or Swell. Biomacromolecules 2005, 6, 2927–2929, . [CrossRef]
- Satarkar, N.S.; Biswal, D.; Hilt, J.Z. Hydrogel nanocomposites: a review of applications as remote controlled biomaterials. Soft Matter 2010, 6, 2364–2371, . [CrossRef]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive Intelligent Drug Delivery Systems. Photochem. Photobiol. 2009, 85, 848–860, . [CrossRef]
- Jiang, H.Y.; Kelch, S.; Lendlein, A. Polymers Move in Response to Light. Adv. Mater. 2006, 18, 1471–1475, . [CrossRef]
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely Triggerable Drug Delivery Systems. Adv. Mater. 2010, 22, 4925–4943, . [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderón, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186, . [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.-E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnology 2018, 16, 1–10, . [CrossRef]
- Zhang, F.; Gong, S.; Wu, J.; Li, H.; Oupicky, D.; Sun, M. CXCR4-Targeted and Redox Responsive Dextrin Nanogel for Metastatic Breast Cancer Therapy. Biomacromolecules 2017, 18, 1793–1802, . [CrossRef]
- Tian, Y.; Lei, M.; Yan, L.; An, F. Diselenide-crosslinked zwitterionic nanogels with dual redox-labile properties for controlled drug release. Polym. Chem. 2020, 11, 2360–2369, . [CrossRef]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198, . [CrossRef]
- Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Intracellular drug release nanosystems. Mater. Today 2012, 15, 436–442, . [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




