Submitted:
14 April 2023
Posted:
17 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
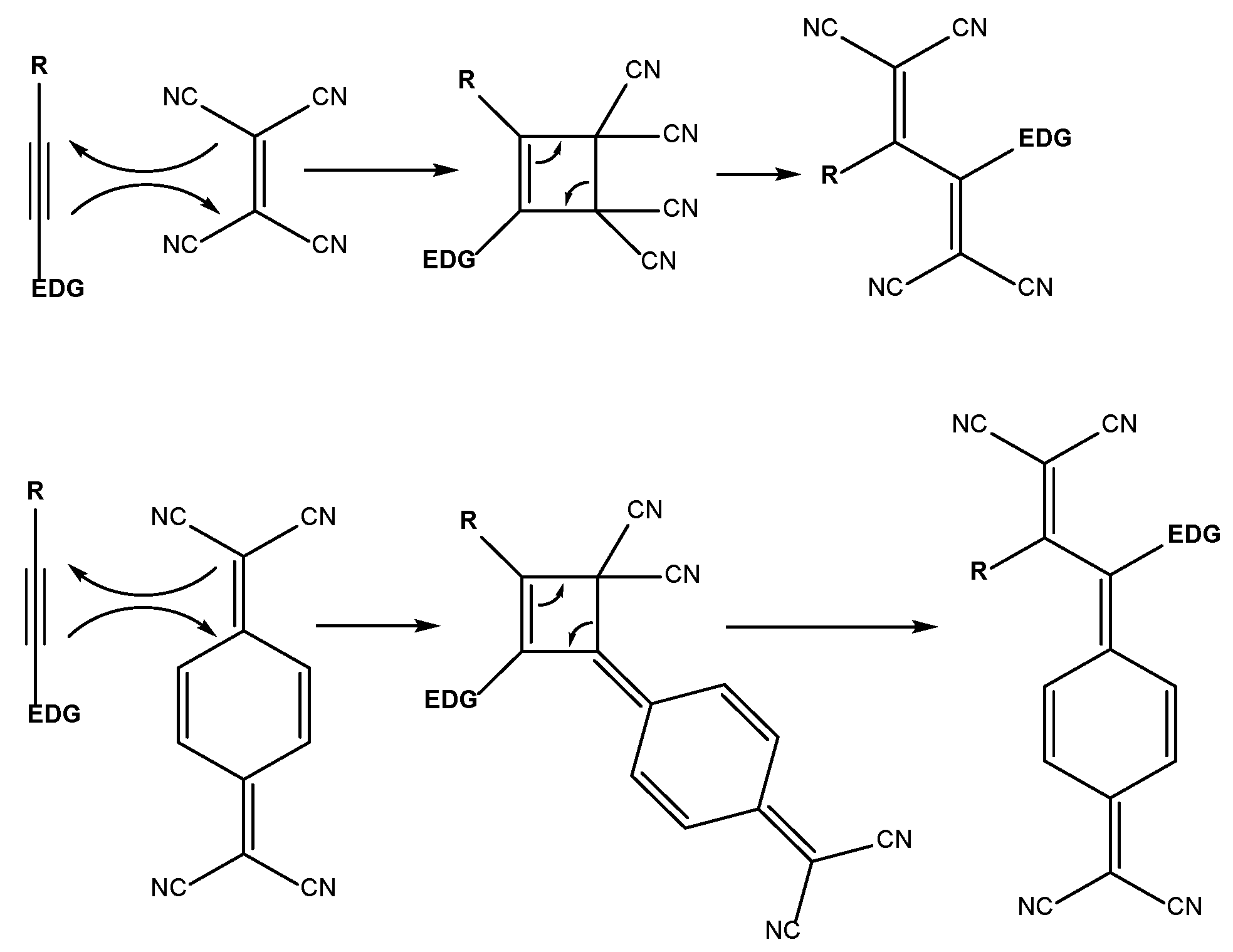
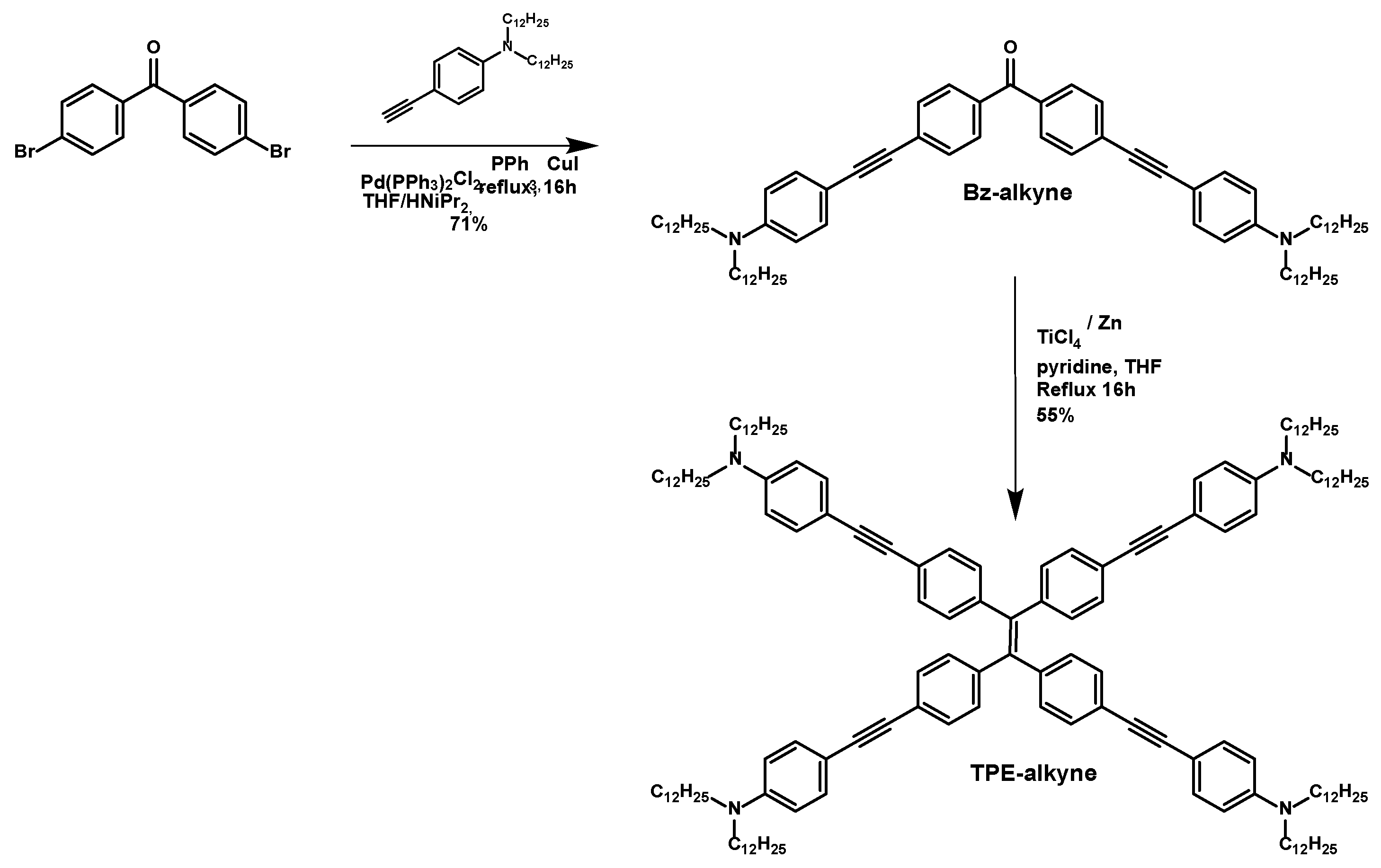
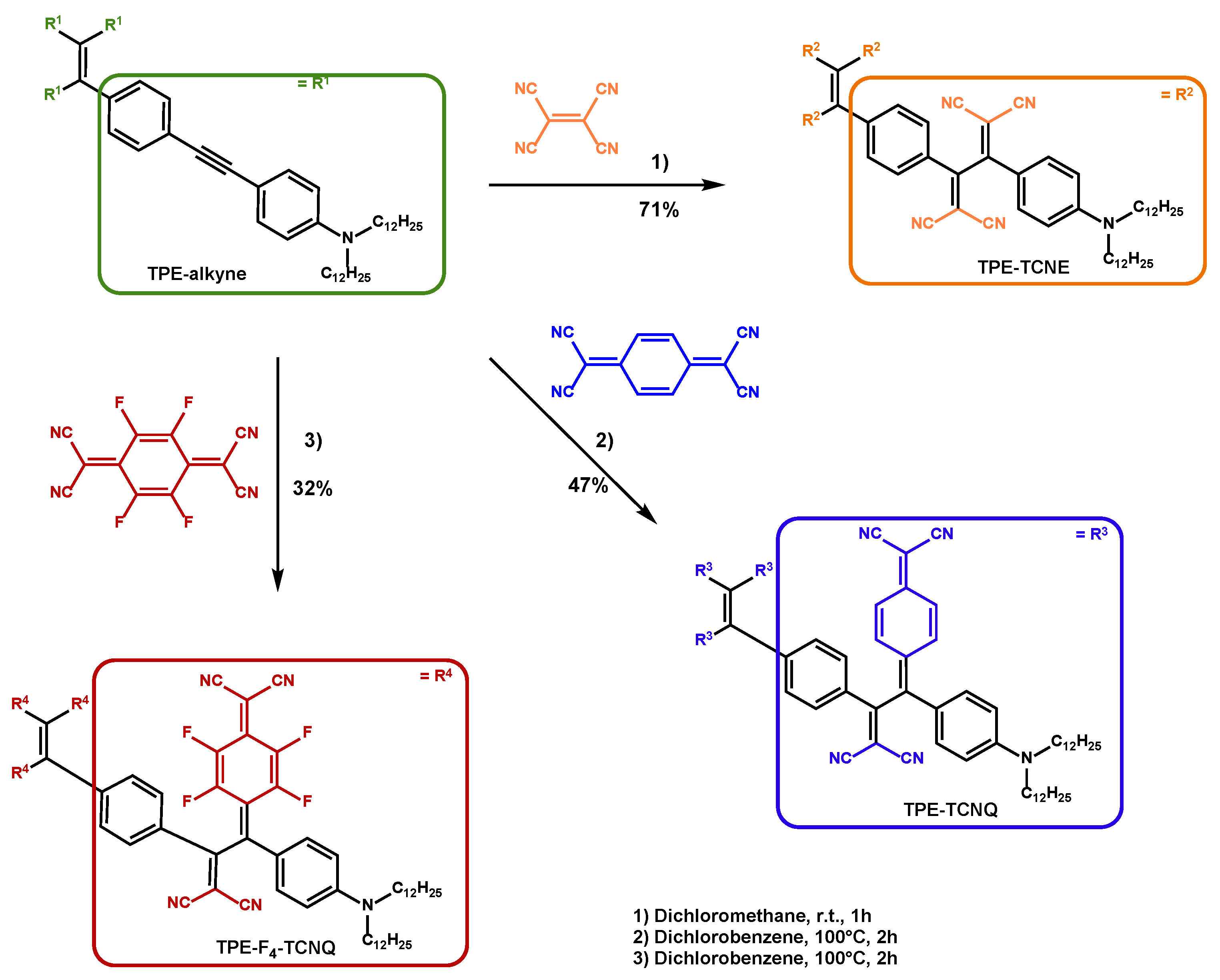
2.1. Synthesis
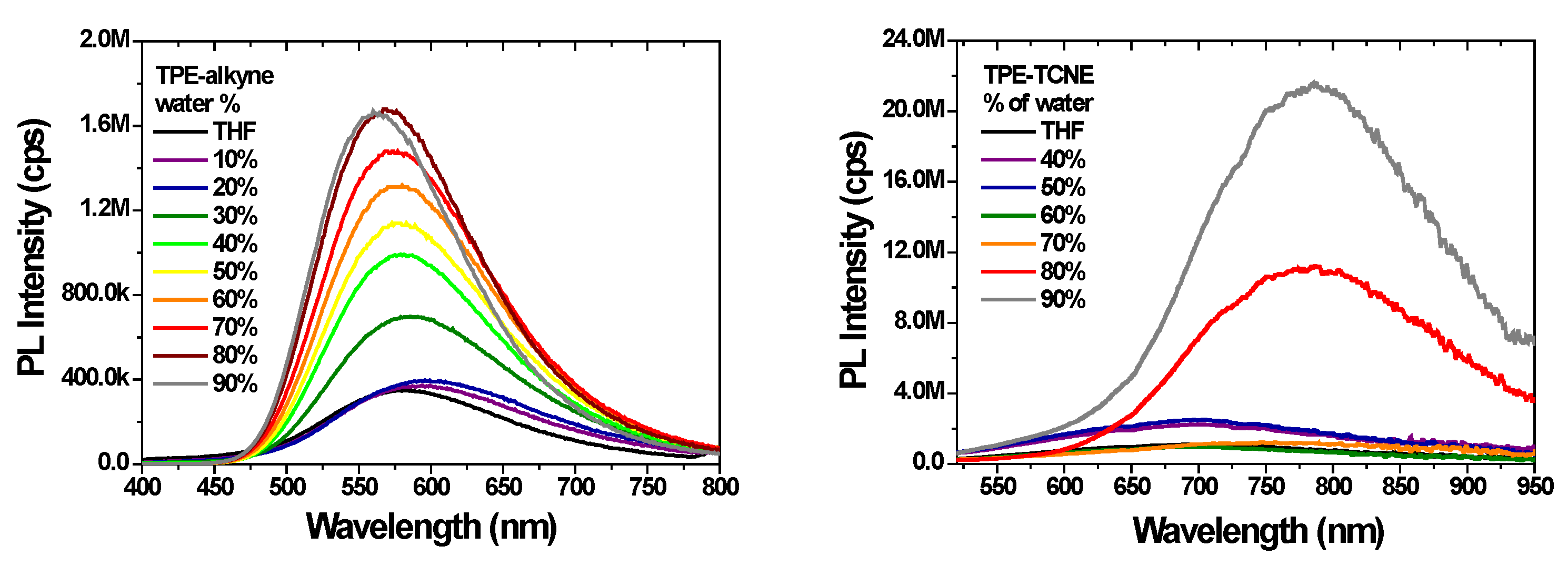
2.2. Photophysical properties
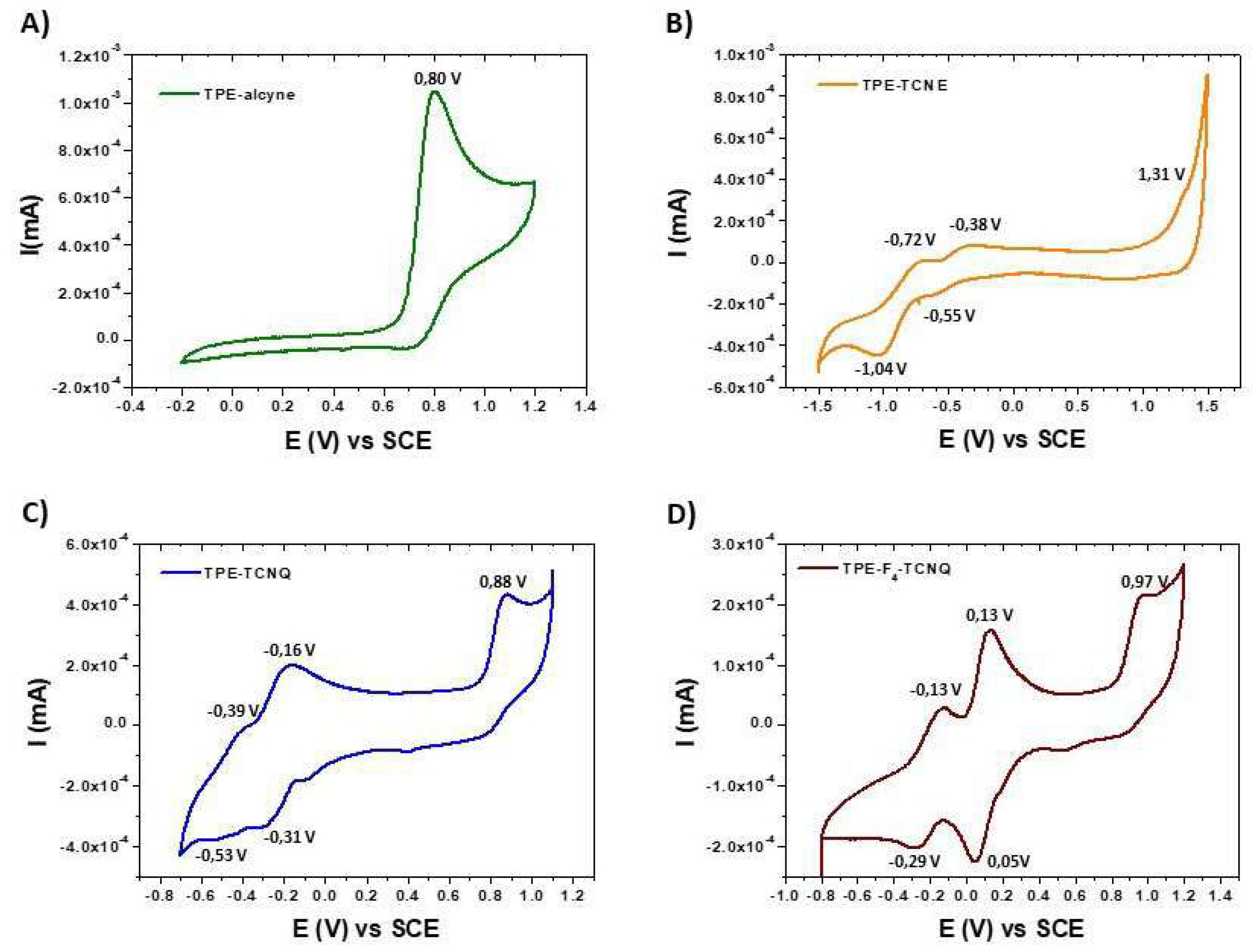
2.3. Electrochemistry
| TPE-alcyne | TPE-TCNE | TPE-TCNQ | TPE-F4-TCNQ | |
|---|---|---|---|---|
| E1/2 (V) a | - | -0.45 -0.88 |
-0.23 -0.46 |
+0.09 -0.21 |
| Ep b | +0.80 | +1.31 | +0.88 | +0.97 |
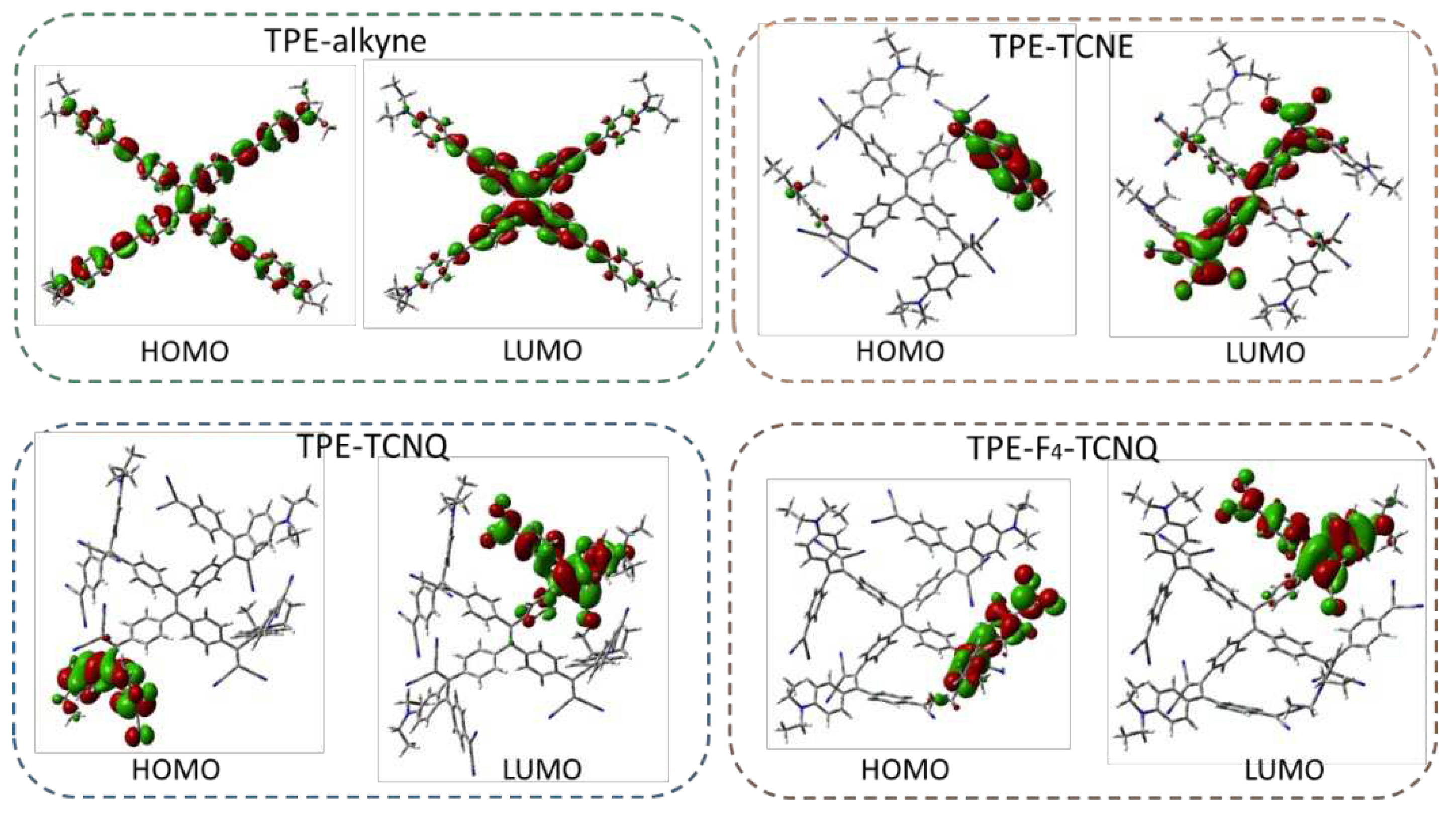
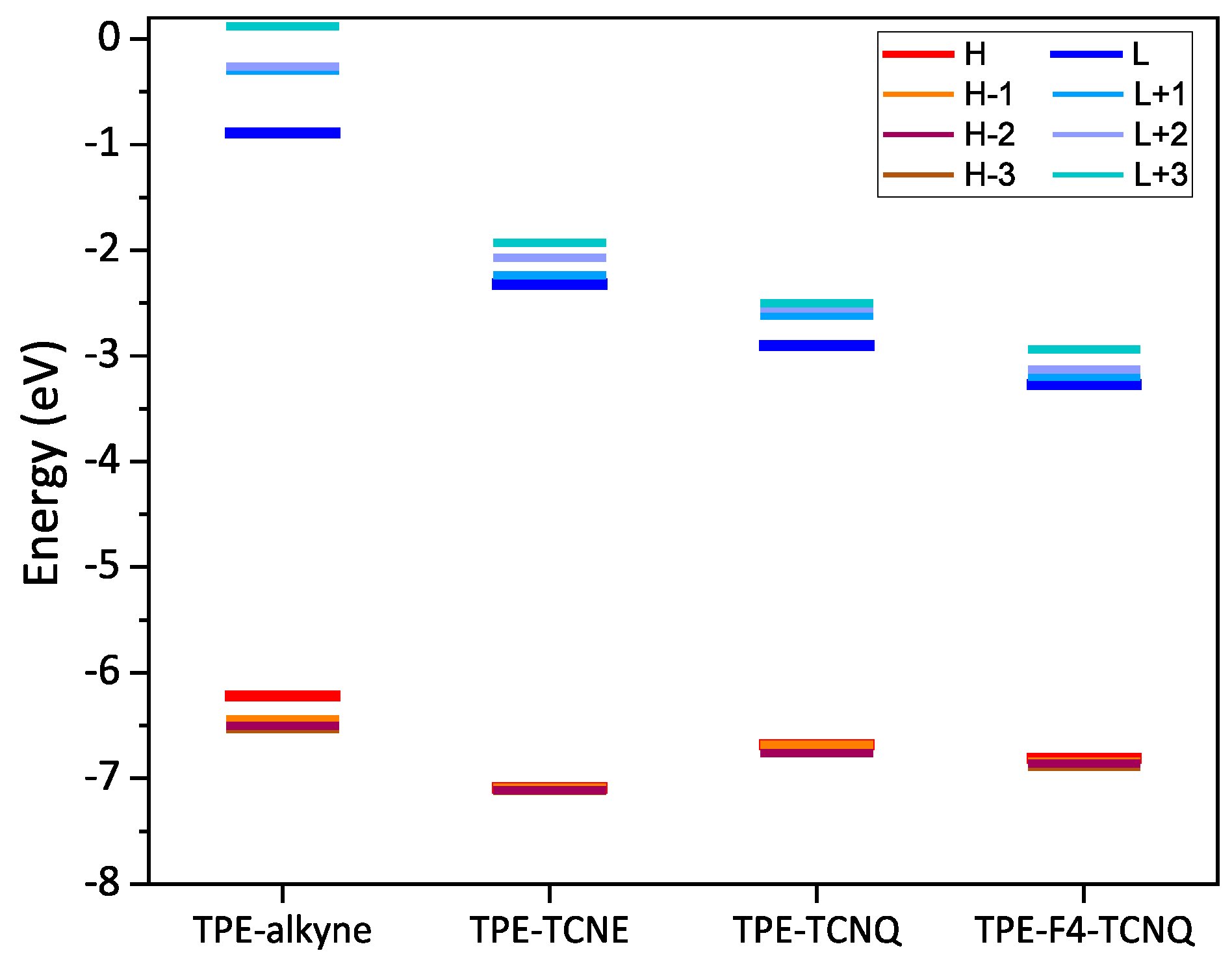
2.4. Electronic structure calculations
2.5. Excited-state calculations
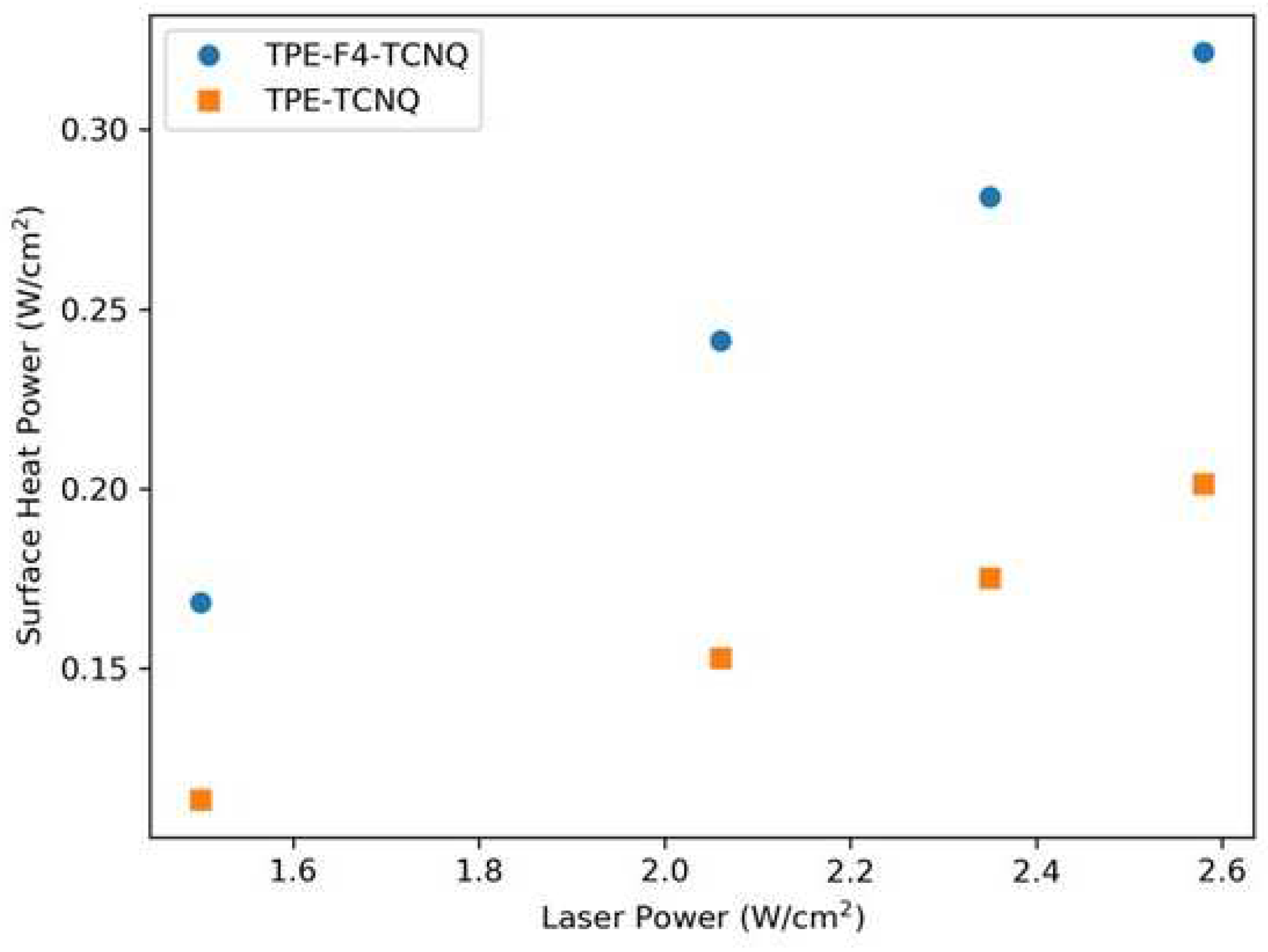
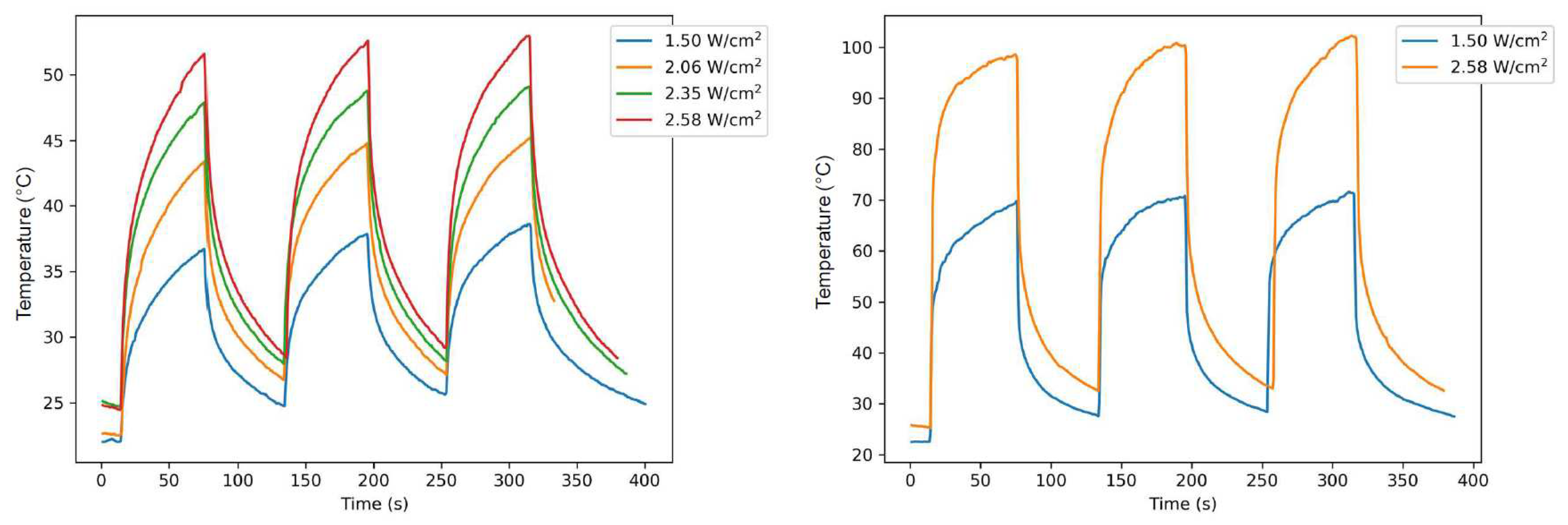
2.6. Photothermal properties and thermal stability
2.7. Concluding remarks
3. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braye, E.H.; Hübel, W.; Caplier, I. New Unsaturated Heterocyclic Systems. I. J. Am. Chem. Soc. 1961, 83, 4406–4413. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Y.; Li, Y.; Li, Y.; Feng, Z.; Fan, X.; Qian, J.; Lin, H. A historical review of aggregation-induced emission from 2001 to 2020: A bibliometric analysis. Aggregate 2022, 3, e152. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. Engl. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Du, L.; Ma, C.; Leung, N.L.C.; Niu, Y.; Qin, A.; Sun, J.; Peng, Q.; Sung, H.H.Y.; et al. Drawing a clear mechanistic picture for the aggregation-induced emission process. Mater. Chem. Front., 2019, 3, 1143–1150. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Turley, A.T.; Wang, L.; McGonigal, P.R.; Tu, Y.; Li, Y.; Wang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Aggregate Science: From Structures to Properties. Adv. Mater. (Weinheim, Ger.) 2020, 32, 2001457. [Google Scholar] [CrossRef]
- Xu, S.; Duan, Y.; Liu, B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2020, 32, 1903530. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Y.; Ding, K.; Xie, Y.; Fan, J.; Tong, J.; Zeng, Z.; Li, Y.; Zhao, C.; Wang, Z.; et al. Aggregation Effect on Multiperformance Improvement in Aryl-Armed Phenazine-Based Emitters. J. Am. Chem. Soc. 2023, 145, 1607–1616. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, G.; Zhao, G.; Wang, X.; Tian, W.; Sun, Y. Novel benzonitrile-based AIE host with high triplet energy for highly efficient solution-processed blue TADF OLEDs. Dyes Pigm. 2023, 210, 111037. [Google Scholar] [CrossRef]
- Hwang, J.; Nagaraju, P.; Cho, M.J.; Choi, D.H. Aggregation-induced emission luminogens for organic light-emitting diodes with a single-component emitting layer. Aggregate 2023, 4, e199. [Google Scholar] [CrossRef]
- Amro, K.; Thakur, A.K.; Rolland, M.; Van Der Lee, A.; Lemaur, V.; Lazzaroni, R.; Rault-Berthelot, J.; Poriel, C.; Hirsch, L.; Clément, S.; et al. Linking triptycene to silole: a fruitful association. Mater. Chem. Front. 2020, 4, 2006–2017. [Google Scholar] [CrossRef]
- Anitha, O.; Mathivanan, M.; Tharmalingam, B.; Thiruppathiraja, T.; Ghorai, S.; Natarajan, R.; Thiagarajan, V.; Lakshmipathi, S.; Murugesapandian, B. Multi-stimuli responsiveness of pyrimidine bishydrazone: AIE, tuneable luminescence, white light emission, mechanochromism, acidochromism and its anticounterfeiting applications. Dyes Pigm. 2023, 212, 111091. [Google Scholar] [CrossRef]
- Guo, X.; Song, T.; Chen, D.; Zhu, J.; Li, Z.; Xia, Q.; Wang, L.; Yang, W. Multi Stimuli-Responsive Aggregation-Induced Emission Active Polymer Platform Based on Tetraphenylethylene-Appended Maleic Anhydride Terpolymers. ACS Appl. Mater. Interfaces 2023, 15, 3543–3557. [Google Scholar] [CrossRef]
- Deng, D.-d.; Zou, Y.; Chen, Z.; Liu, S.; Yang, Y.; Pu, S. Finely regulated benzothiadiazole derivatives: Aggregation-induced emission (AIE), hypso- or bathochromic mechanofluorochromic behaviors, and multilevel information encryption applications. Dyes Pigm. 2023, 211, 111051. [Google Scholar] [CrossRef]
- Ahangar, A.A.; Ahmad, I.; Dar, A.A. AIE in the halogenated anils and their utilization as fluorescent probes for explosive nitro-aromatics. New J. Chem. 2023, 47, 4775–4783. [Google Scholar] [CrossRef]
- Amro, K.; Clément, S.; Déjardin, P.; Douglas, W.E.; Gerbier, P.; Janot, J.-M.; Thami, T. Supported thin flexible polymethylhydrosiloxane permeable films functionalised with silole groups: new approach for detection of nitroaromatics. J. Mater. Chem. 2010, 20, 7100–7103. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, B.; Hao, J.; Ma, L.; Huang, Y.; Shao, X.; Li, C.; Chu, Z.; Yi, C.; Wong, S.H.D.; et al. An AIEgen/graphene oxide nanocomposite (AIEgen@GO)-based two-stage "turn-on" nucleic acid biosensor for rapid detection of SARS-CoV-2 viral sequence. Aggregate 2023, 4, e195. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, P.; Shen, Q.; Zhou, Y.; Wang, Z.; Xu, Y.; Meng, L.; Dang, D.; Ben, Z.T. AIE nanocrystals: Emerging nanolights with ultra-high brightness for biological application. Coord. Chem. Rev. 2023, 477, 214944. [Google Scholar] [CrossRef]
- Luo, W.; Tan, Y.; Gui, Y.; Yan, D.; Wang, D.; Tang, B.Z. Near-Infrared-Emissive AIE Bioconjugates: Recent Advances and Perspectives. Molecules 2022, 27, 3914. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Xu, R.; Xu, Y.; Dang, D.; Shen, Q.; Meng, L.; Tang, B.Z. Seeing the unseen: AIE luminogens for super-resolution imaging. Coord. Chem. Rev. 2022, 451, 214279. [Google Scholar] [CrossRef]
- Yan, D.; Qin, Y.; Yan, S.; Sun, P.; Wang, Y.; Wang, D.; Tang, B.Z. Near-infrared emissive AIE nanoparticles for biomedical applications: From the perspective of different nanocarriers. Particuology 2023, 74, 103–118. [Google Scholar] [CrossRef]
- Chua, M.H.; Chin, K.L.O.; Loh, X.J.; Zhu, Q.; Xu, J. Aggregation-Induced Emission-Active Nanostructures: Beyond Biomedical Applications. ACS Nano 2023, 17, 1845–1878. [Google Scholar] [CrossRef]
- Ingle, J.; Basu, S. Mitochondria Targeted AIE Probes for Cancer Phototherapy. ACS Omega 2023, 8, 8925–8935. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Ge, J.; Deng, Y.; Ding, F.; Hu, L.; Wang, H. A deep-red emission AIE fluorescent probes based on coumarin for imaging lipid droplets in living cells. J. Mol. Struct. 2023, 1277, 134847. [Google Scholar] [CrossRef]
- Ingle, J.; Sengupta, P.; Basu, S. Illuminating Sub-Cellular Organelles by Small Molecule AIEgens. ChemBioChem 2023, 24, e202200370. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, S.; Liu, Z.; Cao, W.; Li, G.; Gao, W.; Tang, B. Novel AIE Probe for In Situ Imaging of Protein Sulfonation to Assess Cigarette Smoke-Induced Inflammatory Damage. Anal. Chem. 2023, 95, 1967–1974. [Google Scholar] [CrossRef]
- Kotras, C.; Fossepre, M.; Roger, M.; Gervais, V.; Richeter, S.; Gerbier, P.; Ulrich, S.; Surin, M.; Clement, S. A cationic tetraphenylethene as a light-up supramolecular probe for DNA G-quadruplexes. Front. Chem. 2019, 7, 493. [Google Scholar] [CrossRef]
- Arribat, M.; Remond, E.; Richeter, S.; Gerbier, P.; Clement, S.; Cavelier, F. Silole amino acids with aggregation-induced emission features synthesized by hydrosilylation. Eur. J. Org. Chem. 2019, 2019, 2275–2281. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, Y.; Chen, N.; Cao, G.; Zeng, Y.; Dong, J.; Liu, M.; Ye, Z.; Li, Y.; Huang, S.; et al. Aggregation-Induced emission photosensitizer with lysosomal response for photodynamic therapy against cancer. Bioorg. Chem. 2023, 132, 106349. [Google Scholar] [CrossRef]
- Wang, X.; Xue, K.; Wang, X.; Zhao, Y.; Deng, J.; Yang, L.; Liang, J.; Li, Y.; Qi, Z. An aggregation-induced emission photosensitizer with efficient singlet oxygen generation capacity for mitochondria targeted photodynamic therapy. Dyes Pigm. 2023, 213, 111181. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, L.; Chen, S.; Pu, Z.; Gu, M.; Shen, Y. Highly Efficient Photodynamic Therapy with Mitochondria-Targeting Aggregation-Induced Emission Photosensitizer for Retinoblastoma. Adv. Healthcare Mater. 2023, 12, 2202219. [Google Scholar] [CrossRef]
- Qu, R.; Zhen, X.; Jiang, X. Emerging designs of aggregation-induced emission agents for enhanced phototherapy applications. CCS Chem. 2022, 4, 401–419. [Google Scholar] [CrossRef]
- Lin, Y.; Yi, M.; Guan, X.; Chen, E.; Yang, L.; Li, S.; Li, Y.; Zhang, L. "Two birds with one stone" strategy for the lung cancer therapy with bioinspired AIE aggregates. J. Nanobiotechnol. 2023, 21, 49. [Google Scholar] [CrossRef]
- Jiang, W.; Cheng, C.; Qiu, X.; Chen, L.; Guo, X.; Luo, Y.; Wang, J.; Wang, J.; Xie, Z.; Li, P.; et al. Peptide Supramolecular Assembly-Instructed In Situ Self-Aggregation for Stratified Targeting Sonodynamic Therapy Enhancement of AIE Luminogens. Adv. Sci. 2023, 10, 2204989. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Chen, L.; Chen, Q.; Hu, R.; Xu, X.; Wang, Y.; Li, J.; Feng, S.; Dong, C.; Zhang, X.-L.; et al. Engineered Phage with Aggregation-Induced Emission Photosensitizer in Cocktail Therapy against Sepsis. Adv. Mater. 2023, 35, 2208578. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Yuan, C.; Pang, X.; Shangguan, P.; Liu, Y.; Han, L.; Sun, J.; Lam, J.W.Y.; Liu, Y.; et al. Near-Infrared Aggregation-Induced Emission Luminogens for In Vivo Theranostics of Alzheimer's Disease. Angew. Chem., Int. Ed. 2023, 62, e202211550. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Structural and process controls of AIEgens for NIR-II theranostics. Chem. Sci. 2020, 12, 3427–3436. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile Types of Inorganic/Organic NIR-IIa/IIb Fluorophores: From Strategic Design toward Molecular Imaging and Theranostics. Chem. Rev. 2022, 122, 209–268. [Google Scholar] [CrossRef]
- Xu, C.; Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 2021, 50, 1111–1137. [Google Scholar] [CrossRef]
- Shao, A.; Xie, Y.; Zhu, S.; Guo, Z.; Zhu, S.; Guo, J.; Shi, P.; James, T.D.; Tian, H.; Zhu, W.H. Far-Red and Near-IR AIE-Active Fluorescent Organic Nanoprobes with Enhanced Tumor-Targeting Efficacy: Shape-Specific Effects. Angew. Chem. Int. Ed. Engl. 2015, 54, 7275–7280. [Google Scholar] [CrossRef]
- Gu, X.; Yao, J.; Zhang, G.; Zhang, C.; Yan, Y.; Zhao, Y.; Zhang, D. New electron-donor/acceptor-substituted tetraphenylethylenes: aggregation-induced emission with tunable emission color and optical-waveguide behavior. Chem. Asian. J. 2013, 8, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lee, M.M.S.; Zhang, Z.; Sung, H.H.Y.; Williams, I.D.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, D.; Tang, B.Z. Facile synthesis of AIEgens with wide color tunability for cellular imaging and therapy. Chem. Sci. 2019, 10, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A. Donor–acceptor type low band gap polymers: polysquaraines and related systems. Chem. Soc. Rev. 2003, 32, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Jia, T.; Kang, B.; Li, F.; Fahlman, M.; Wang, Y. Nitrile-substituted QA derivatives: new acceptor materials for solution-processable organic bulk heterojunction solar cells. Adv. Energy Mater. 2011, 1, 431–439. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Michinobu, T.; Diederich, F. The [2+2] Cycloaddition-Retroelectrocyclization (CA-RE) Click Reaction: Facile Access to Molecular and Polymeric Push-Pull Chromophores. Angew. Chem. Int. Ed. 2018, 57, 3552–3577. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Ito, S. Azulene-based donor-acceptor systems: synthesis, optical, and electrochemical properties. Chem. - Eur. J. 2017, 23, 16696–16709. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.I.; Rodgers, J.R.; Snow, M.R.; Swincer, A.G. Cyclopentadienyl-ruthenium and -osmium chemistry. Cleavage of tetracyanoethylene under mild conditions: X-ray crystal structures of [Ru{η3-C(CN)2CPhCC(CN)2}(PPh3)(η-C5H5)] and [Ru{C[C(CN)2]CPhC(CN)2}-(CNBut)(PPh3)(η-C5H5)]. J. C. S, Chem Comm. 1981, 6, 271–272. [Google Scholar]
- Cai, C.; Liakatas, I.; Wong, M.-S.; Bösch, M.; Bosshard, C.; Günter, P.; Concilio, S.; Tirelli, N.; Suter, U.W. Donor−Acceptor-Substituted Phenylethenyl Bithiophenes: Highly Efficient and Stable Nonlinear Optical Chromophores. Org. Lett. 1999, 1, 1847–1849. [Google Scholar] [CrossRef]
- Wu, X.; Wu, J.; Liu, Y.; Jen, A.K.Y. Highly Efficient, Thermally and Chemically Stable Second Order Nonlinear Optical Chromophores Containing a 2-Phenyl-tetracyanobutadienyl Acceptor. J. Am. Chem. Soc. 1999, 121, 472–473. [Google Scholar] [CrossRef]
- Michinobu, T.; Boudon, C.; Gisselbrecht, J.P.; Seiler, P.; Frank, B.; Moonen, N.N.; Gross, M.; Diederich, F. Donor-substituted 1,1,4,4-tetracyanobutadienes (TCBDS): new chromophores with efficient intramolecular charge-transfer interactions by atom-economic synthesis. Chem. Eur. J 2006, 12, 1889–1905. [Google Scholar] [CrossRef]
- Michinobu, T.; May, J.C.; Lim, J.H.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Biaggio, I.; Diederich, F. A new class of organic donor–acceptor molecules with large third-order optical nonlinearities. Chem. Commun. 2005, 737–739. [Google Scholar] [CrossRef]
- Kivala, M.; Boudon, C.; Gisselbrecht, J.P.; Enko, B.; Seiler, P.; Muller, I.B.; Langer, N.; Jarowski, P.D.; Gescheidt, G.; Diederich, F. Organic super-acceptors with efficient intramolecular charge-transfer interactions by [2+2] cycloadditions of TCNE, TCNQ, and F4-TCNQ to donor-substituted cyanoalkynes. Chem. Eur. J. 2009, 15, 4111–4123. [Google Scholar] [CrossRef]
- Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Diederich, F. A novel reaction of 7,7,8,8-tetracyanoquinodimethane (TCNQ): charge-transfer chromophores by [2 + 2] cycloaddition with alkynes. Chem. Commun. 2007, 4731–4733. [Google Scholar] [CrossRef]
- Reutenauer, P.; Kivala, M.; Jarowski, P.D.; Boudon, C.; Gisselbrecht, J.P.; Gross, M.; Diederich, F. New strong organic acceptors by cycloaddition of TCNE and TCNQ to donor-substituted cyanoalkynes. Chem. Commun. 2007, 4898–4900. [Google Scholar] [CrossRef]
- Patil, Y.; Misra, R. Diketopyrrolopyrrole-Based and Tetracyano-Bridged Small Molecules for Bulk Heterojunction Organic Solar Cells. Chem. - Asian J. 2018, 13, 220–229. [Google Scholar] [CrossRef]
- Rout, Y.; Chauhan, V.; Misra, R. Synthesis and Characterization of Isoindigo-Based Push-Pull Chromophores. J. Org. Chem. 2020, 85, 4611–4618. [Google Scholar] [CrossRef]
- Rao, P.S.; More, V.G.; Jangale, A.D.; Bhosale, S.V.; Bhosale, R.S.; Puyad, A.L.; Chen, J.-Y.; Li, J.-L.; Bhosale, S.V.; Gupta, A.; et al. A series of V-shaped small molecule non-fullerene electron acceptors for efficient bulk-heterojunction devices. Dyes Pigm. 2019, 171, 107677. [Google Scholar] [CrossRef]
- Rao, P.S.; Puyad, A.L.; Bhosale, S.V.; Bhosale, S.V. Triphenylamine-merocyanine-based D1-A1-π-A2/A3-D2 chromophore system: synthesis, optoelectronic, and theoretical studies. Int. J. Mol. Sci. 2019, 20, 1621. [Google Scholar] [CrossRef]
- Rout, Y.; Misra, R.; Singhal, R.; Biswas, S.; Sharma, G.D. Phenothiazine-based small-molecule organic solar cells with power conversion efficiency over 7% and open circuit voltage of about 1. 0 V using solvent vapor annealing. Phys. Chem. Chem. Phys. 2018, 20, 6321–6329. [Google Scholar] [CrossRef]
- Srinivasa Rao, P.; Gupta, A.; Bhosale, S.V.; Bilic, A.; Xiang, W.; Evans, R.A.; Bhosale, S.V. Donor-acceptor-acceptor-based non-fullerene acceptors comprising terminal chromen-2-one functionality for efficient bulk-heterojunction devices. Dyes Pigm. 2017, 146, 502–511. [Google Scholar] [CrossRef]
- Gautam, P.; Misra, R.; Sharma, G.D. Dicyanoquinodimethane-substituted benzothiadiazole for efficient small-molecule solar cells. Phys. Chem. Chem. Phys. 2016, 18, 7235–7241. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Washino, Y.; Murata, K.; Nozaki, N.; Matsumoto, H.; Michinobu, T. [2+2] Cycloaddition-retroelectrocyclization reactivity and thin film transistor performances of carbazole-based platinum polyyne polymers. Mater. Chem. Phys. 2022, 281, 125861. [Google Scholar] [CrossRef]
- Leliège, A.; Blanchard, P.; Rousseau, T.; Roncali, J. Triphenylamine/Tetracyanobutadiene-Based D-A-D π-Conjugated Systems as Molecular Donors for Organic Solar Cells. Org. Lett. 2011, 13, 3098–3101. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.A.; Murugan, P.; Shanmugam, R.; Praveen, C. Azulene Bridged π-Distorted Chromophores: The Influence of Structural Symmetry on Optoelectrochemical and Photovoltaic Parameters. ChemPlusChem 2021, 86, 1451–1460. [Google Scholar] [CrossRef]
- Philippe, C.; Melan, J.; Barsella, A.; Vives, T.; Leroux, Y.R.; Robin-Le Guen, F.; Lemiegre, L.; Jacquemin, D.; Gauthier, S.; Trolez, Y. A comprehensive study of tetracyanobutadiene push-pull chromophores derived from γ-pyranylidene. Tetrahedron Chem 2023, 5. [Google Scholar] [CrossRef]
- Pokladek, Z.; Ripoche, N.; Betou, M.; Trolez, Y.; Mongin, O.; Olesiak-Banska, J.; Matczyszyn, K.; Samoc, M.; Humphrey, M.G.; Blanchard-Desce, M.; et al. Linear Optical and Third-Order Nonlinear Optical Properties of Some Fluorenyl- and Triarylamine-Containing Tetracyanobutadiene Derivatives. Chem. Eur. J. 2016, 22, 10155–10167. [Google Scholar] [CrossRef] [PubMed]
- Ripoche, N.; Betou, M.; Philippe, C.; Trolez, Y.; Mongin, O.; Dudek, M.; Pokladek, Z.; Matczyszyn, K.; Samoc, M.; Sahnoune, H.; et al. Two-photon absorption properties of multipolar triarylamino/tosylamido 1,1,4,4-tetracyanobutadienes. Phys. Chem. Chem. Phys. 2021, 23, 22283–22297. [Google Scholar] [CrossRef]
- Mammadova, F.; Inyurt, F.C.; Barsella, A.; Dengiz, C. Cyano-rich donor-acceptor-donor-type NLOphores containing dialkylated triazene and aniline groups. Dyes Pigm. 2023, 209, 110894. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, D.; Gao, H.; Zhang, J.; Xing, Y.; Yang, Z.; Cao, H.; He, W. Third-order nonlinear optical properties of the "clicked" closed-ring spiropyrans. Dyes Pigm. 2019, 162, 451–458. [Google Scholar] [CrossRef]
- Miao, Z.; Han, H.; Wang, D.; Gao, H.; Gu, J.; Hu, H. Nonlinear optical and energy-level modulation of organic alkynes by click chemistry. Tetrahedron 2016, 72, 4039–4046. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Gao, H.; Wang, D.; Yang, Z.; Cao, H.; He, W. Photoacoustic effect of azo derivatives modified by click reagents and parceled by liposomes. Dyes Pigm. 2020, 172, 107822. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, Z.; Liu, W.; Wang, D.; He, W.; Cao, H.; Yang, Z. Novel application of NIR photoacoustic absorbing dyes in thermosensitive micelles. Dyes Pigm. 2019, 164, 319–326. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, D.; Gao, H.; Yang, Z.; Cao, H.; He, W. Photoacoustic effect of near-infrared absorbing fullerene derivatives with click moieties. Dyes Pigm. 2019, 164, 182–187. [Google Scholar] [CrossRef]
- Li, L.; Wang, D.; Wang, L.; Ramella, D.; Wang, H.; Gao, H.; Zhang, J.; Xing, Y.; Li, B.; Yang, Z.; et al. The photoacoustic effect of near-infrared absorbing porphyrin derivatives prepared via click chemistry. Dyes Pigm. 2018, 148, 501–507. [Google Scholar] [CrossRef]
- Xu, A.-P.; Han, H.-H.; Lu, J.; Yang, P.-P.; Gao, Y.-J.; An, H.-W.; Zhanng, D.; Li, L.-Z.; Zhang, J.-P.; Wang, D.; et al. Charge transfer NIR dyes for improved photoacoustic effect. Dyes Pigm. 2016, 125, 392–398. [Google Scholar] [CrossRef]
- Shi, H.; Gu, R.; Xu, W.; Huang, H.; Xue, L.; Wang, W.; Zhang, Y.; Si, W.; Dong, X. Near-Infrared Light-Harvesting Fullerene-Based Nanoparticles for Promoted Synergetic Tumor Phototheranostics. ACS Appl. Mater. Interfaces 2019, 11, 44970–44977. [Google Scholar] [CrossRef]
- Tang, B.; Qin, A.; Han, P.; Zhang, G.; Xu, H. Preparation of organic near-infrared photothermal materials and its application. CN11504 3756, 2022. [Google Scholar]
- Zhao, Z.; Lu, P.; Lam, J.W.Y.; Wang, Z.; Chan, C.Y.K.; Sung, H.H.Y.; Williams, I.D.; Ma, Y.; Tang, B.Z. Molecular anchors in the solid state: Restriction of intramolecular rotation boosts emission efficiency of luminogen aggregates to unity. Chem. Sci. 2011, 2, 672–675. [Google Scholar] [CrossRef]
- Philippe, C.; Coste, M.; Bretonniere, Y.; Lemiegre, L.; Ulrich, S.; Trolez, Y. Quadruple Functionalization of a Tetraphenylethylene Aromatic Scaffold with Ynamides or Tetracyanobutadienes: Synthesis and Optical Properties. Eur. J. Org. Chem. 2022, 2022, e202200049. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, C.; Wu, W.; Wang, F.; Du, L.; Zhang, X.; Xiong, Y.; He, X.; Cai, Y.; Kwok, R.T.K.; et al. Highly efficient photothermal nanoagent achieved by harvesting energy via excited-state intramolecular motion within nanoparticles. Nat. Commun. 2019, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Fesser, P.; Iacovita, C.; Wäckerlin, C.; Vijayaraghavan, S.; Ballav, N.; Howes, K.; Gisselbrecht, J.-P.; Crobu, M.; Boudon, C.; Stöhr, M.; et al. Visualizing the Product of a Formal Cycloaddition of 7,7,8,8-Tetracyano-p-quinodimethane (TCNQ) to an Acetylene-Appended Porphyrin by Scanning Tunneling Microscopy on Au(111). Chem. Eur. J. 2011, 17, 5246–5250. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, D.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.; Wang, H.; Gu, J.; Hu, H. Nonlinear optical properties of symmetrical and asymmetrical porphyrin derivatives with click chemistry modification. Dyes Pigm. 2016, 134, 155–163. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Xing, Y.; Gao, H.; Wang, X.; Zhao, Y.; Yang, H. Energy-level modulation of organic alkynes by click chemistry. Tetrahedron 2013, 69, 895–901. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, Q.; Guo, Z.; He, Z.; Zhang, H.; Ma, C.; Gao, J.; Zhao, Y.; Wang, D. Preparation of Polyphenylene Ring Derivative Dyes with Wide Wave Absorption Properties and Their Performance Study. Molecules 2022, 27, 5551. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Zhao, X.; Li, Q.; Zhao, Y.; Guo, Z.; He, Z.; Zhang, H.; Gao, J.; Miao, Z. Preparation of symmetrical and asymmetrical multi-phenylene ring nonlinear optical materials with click chemical modifications and their properties. Tetrahedron 2022, 127, 132992. [Google Scholar] [CrossRef]
- Dar, A.H.; Gowri, V.; Gopal, A.; Muthukrishnan, A.; Bajaj, A.; Sartaliya, S.; Selim, A.; Ali, E.M.; Jayamurugan, G. Designing of Push-Pull Chromophores with Tunable Electronic and Luminescent Properties Using Urea as the Electron Donor. J. Org. Chem. 2019, 84, 8941–8947. [Google Scholar] [CrossRef]
- Simon Marques, P.; Castan, J.M.A.; Raul, B.A.L.; Londi, G.; Ramirez, I.; Pshenichnikov, M.S.; Beljonne, D.; Walzer, K.; Blais, M.; Allain, M.; et al. Triphenylamine/Tetracyanobutadiene-Based π-Conjugated Push-Pull Molecules End-Capped with Arene Platforms: Synthesis, Photophysics, and Photovoltaic Response. Chem. - Eur. J. 2020, 26, 16422–16433. [Google Scholar] [CrossRef]
- Philippe, C.; Bui, A.T.; Beau, M.; Bloux, H.; Riobe, F.; Mongin, O.; Roisnel, T.; Cordier, M.; Paul, F.; Lemiegre, L.; et al. Synthesis and Photophysical Properties of 1,1,4,4-Tetracyanobutadienes Derived from Ynamides Bearing Fluorophores. Chem. - Eur. J. 2022, 28, e202200025. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-functional Theory of Atoms and Molecules; Clarendon Press: 1989.
- Runge, E.; Gross, E.K.U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Trickey, S.B. Recent Advances in Density Functional Methods -Part I by Delano P. Chong. Int. J. Quantum Chem. 1999, 72, 155–156. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Etienne, T.; Assfeld, X.; Monari, A. Toward a Quantitative Assessment of Electronic Transitions' Charge-Transfer Character. J. Chem. Theory Comput. 2014, 10, 3896–3905. [Google Scholar] [CrossRef]
- Etienne, T.; Assfeld, X.; Monari, A. New Insight into the Topology of Excited States through Detachment/Attachment Density Matrices-Based Centroids of Charge. J. Chem. Theory Comput. 2014, 10, 3906–3914. [Google Scholar] [CrossRef]
- Han, P.; Zhang, G.; Xu, H.; Hu, R.; Qin, A.; Tang, B.Z. Organic near infrared photothermal materials with temperatures up to 450°C constructed by cycloaddition-retroelectrocyclization click reaction. ChemRxiv 2022, 1–32. [Google Scholar]
- Banziger, S.D.; Clendening, R.A.; Oxley, B.M.; Ren, T. Spectroelectrochemical and Computational Analysis of a Series of Cycloaddition-Retroelectrocyclization-Derived Donor-Acceptor Chromophores. J. Phys. Chem. B 2020, 124, 11901–11909. [Google Scholar] [CrossRef]
- Gautam, P.; Maragani, R.; Misra, R. Tuning the HOMO-LUMO gap of donor-substituted benzothiazoles. Tetrahedron Lett. 2014, 55, 6827–6830. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, D.; Wang, X.; Liang, P.; Mi, Y.; Yang, H. Efficient modification of pyrene-derivative featuring third-order nonlinear optics via the click post-functionalization. Tetrahedron Lett. 2013, 54, 4859–4864. [Google Scholar] [CrossRef]
- Liang, P.; Du, Z.; Wang, D.; Yang, Z.; Sheng, H.; Liang, S.; Cao, H.; He, W.; Yang, H. Optoelectronic and Self-assembly Properties of Porphyrin Derivatives with Click Chemistry Modification. ChemPhysChem 2014, 15, 3523–3529. [Google Scholar] [CrossRef]
- Mi, Y.; Liang, P.; Jin, Z.; Wang, D.; Yang, Z. Synthesis and Third-Order Nonlinear Optical Properties of Triphenylene Derivatives Modified by Click Chemistry. ChemPhysChem 2013, 14, 4102–4108. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Gautam, P. Tuning of the HOMO-LUMO gap of donor-substituted symmetrical and unsymmetrical benzothiadiazoles. Org. Biomol. Chem. 2014, 12, 5448–5457. [Google Scholar] [CrossRef]
- Rout, Y.; Gautam, P.; Misra, R. Unsymmetrical and Symmetrical Push-Pull Phenothiazines. J. Org. Chem. 2017, 82, 6840–6845. [Google Scholar] [CrossRef]
- Rout, Y.; Jang, Y.; Gobeze, H.B.; Misra, R.; D'Souza, F. Conversion of Large-Bandgap Triphenylamine-Benzothiadiazole to Low-Bandgap, Wide-Band Capturing Donor-Acceptor Systems by Tetracyanobutadiene and/or Dicyanoquinodimethane Insertion for Ultrafast Charge Separation. J. Phys. Chem. C 2019, 123, 23382–23389. [Google Scholar] [CrossRef]
- Sharma, R.; Maragani, R.; Misra, R. C3-Symmetric star shaped donor-acceptor truxenes: synthesis and photophysical, electrochemical and computational studies. New J. Chem. 2018, 42, 882–890. [Google Scholar] [CrossRef]
- Sharma, R.; Thomas, M.B.; Misra, R.; D'Souza, F. Strong Ground- and Excited-State Charge Transfer in C3-Symmetric Truxene-Derived Phenothiazine-Tetracyanobutadine and Expanded Conjugates. Angew. Chem., Int. Ed. 2019, 58, 4350–4355. [Google Scholar] [CrossRef]
- Zhang, W.-S.; Wang, D.; Cao, H.; Yang, H. Energy level tunable pre-click functionalization of [60]fullerene for nonlinear optics. Tetrahedron 2014, 70, 573–577. [Google Scholar] [CrossRef]
- Zhang, Z.; Gou, G.; Wan, J.; Li, H.; Wang, M.; Li, L. Synthesis, Structure, and Significant Energy Gap Modulation of Symmetrical Silafluorene-Cored Tetracyanobutadiene and Tetracyanoquinodimethane Derivatives. J. Org. Chem. [CrossRef]
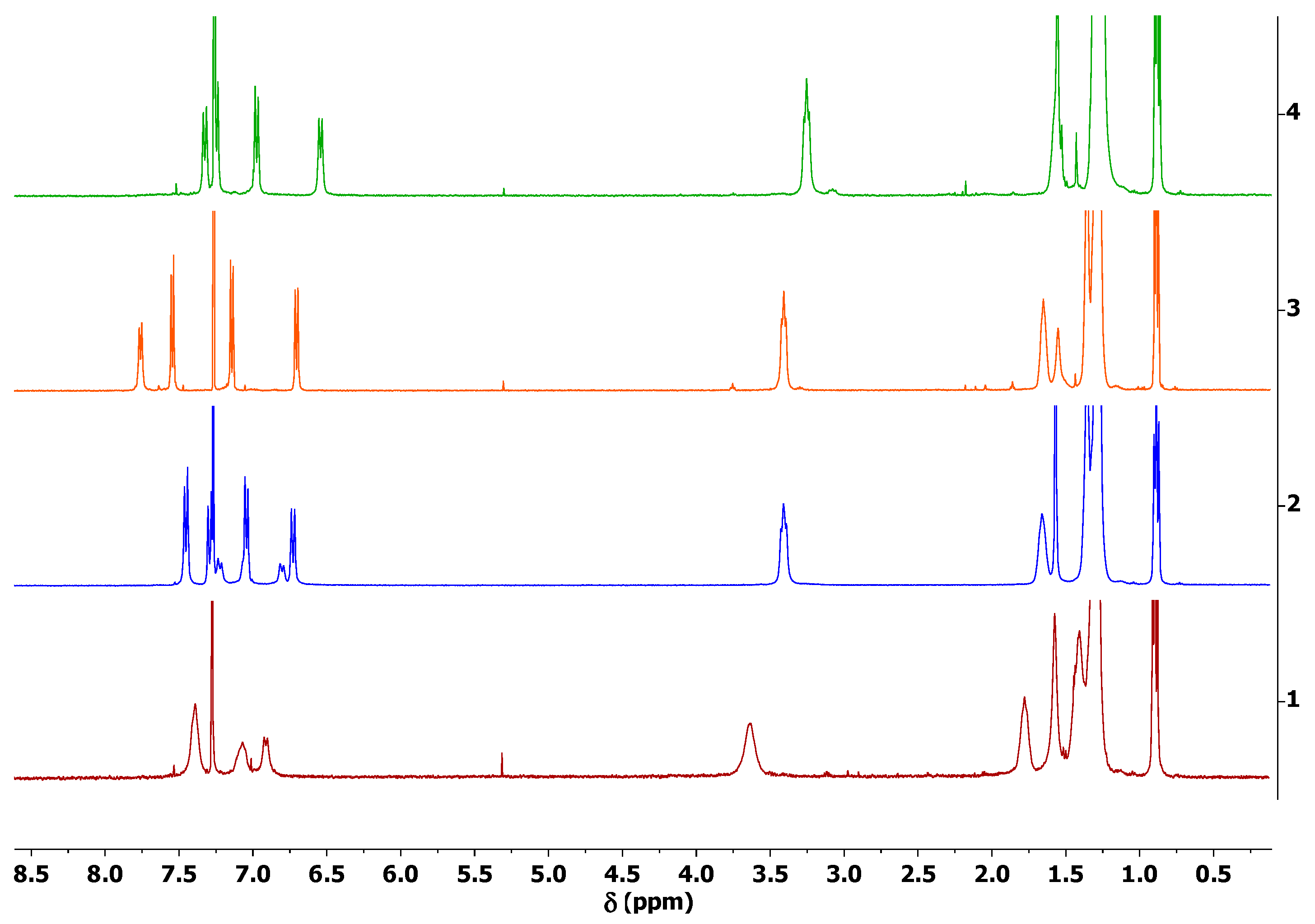
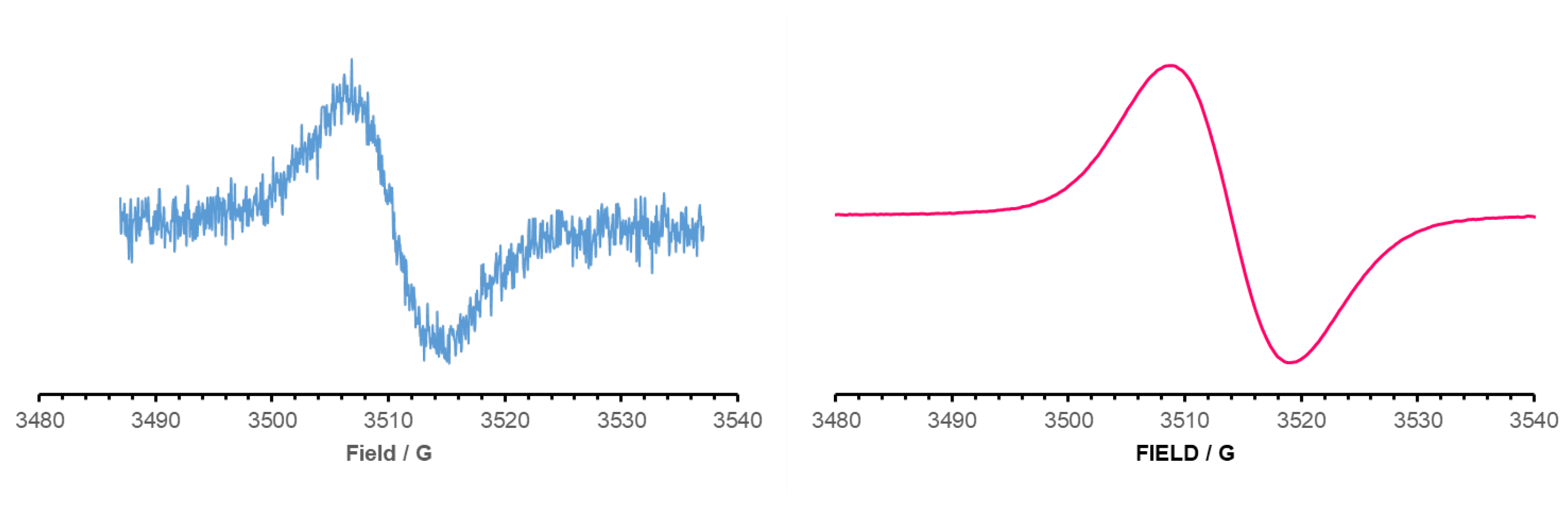
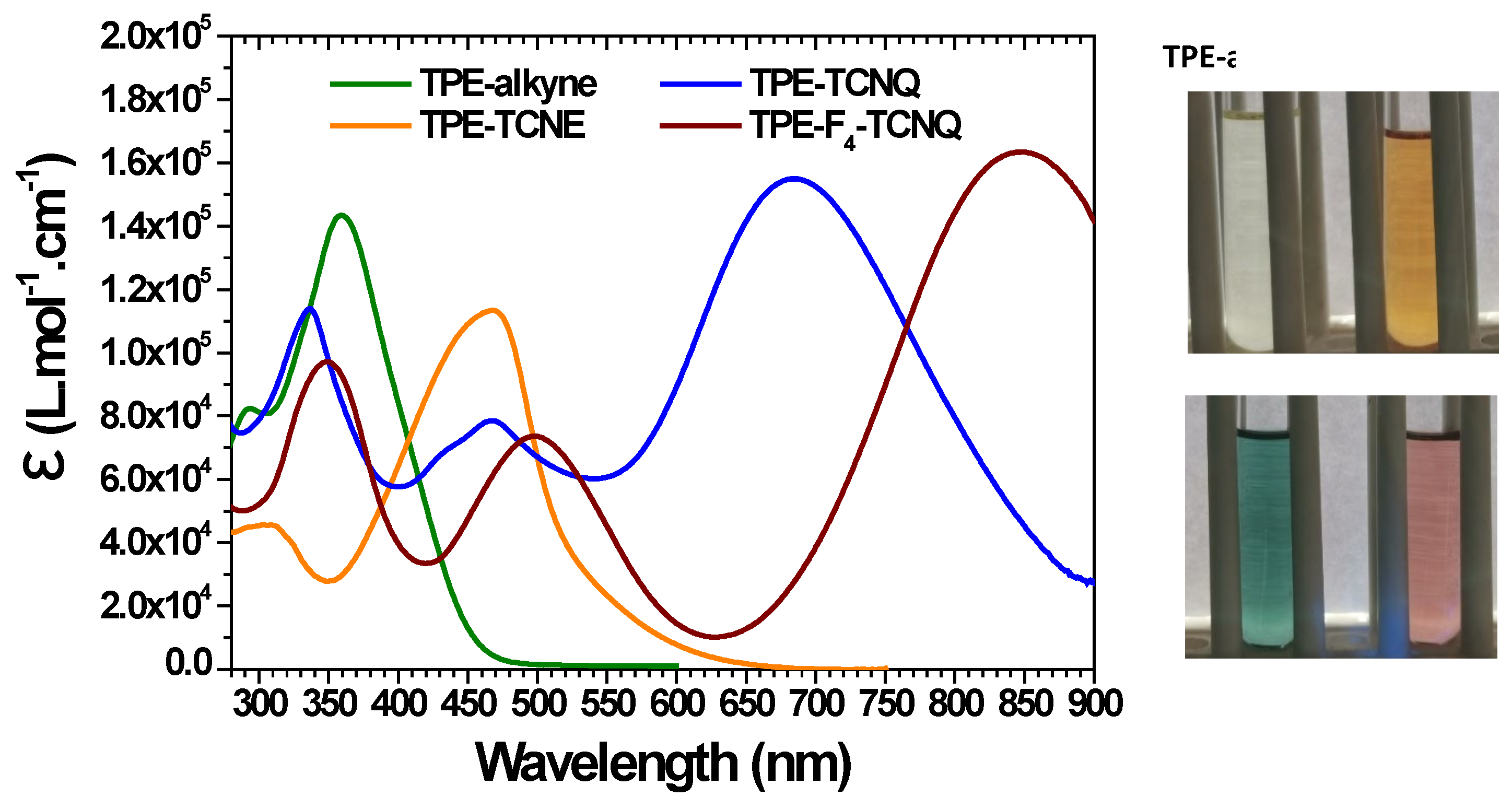
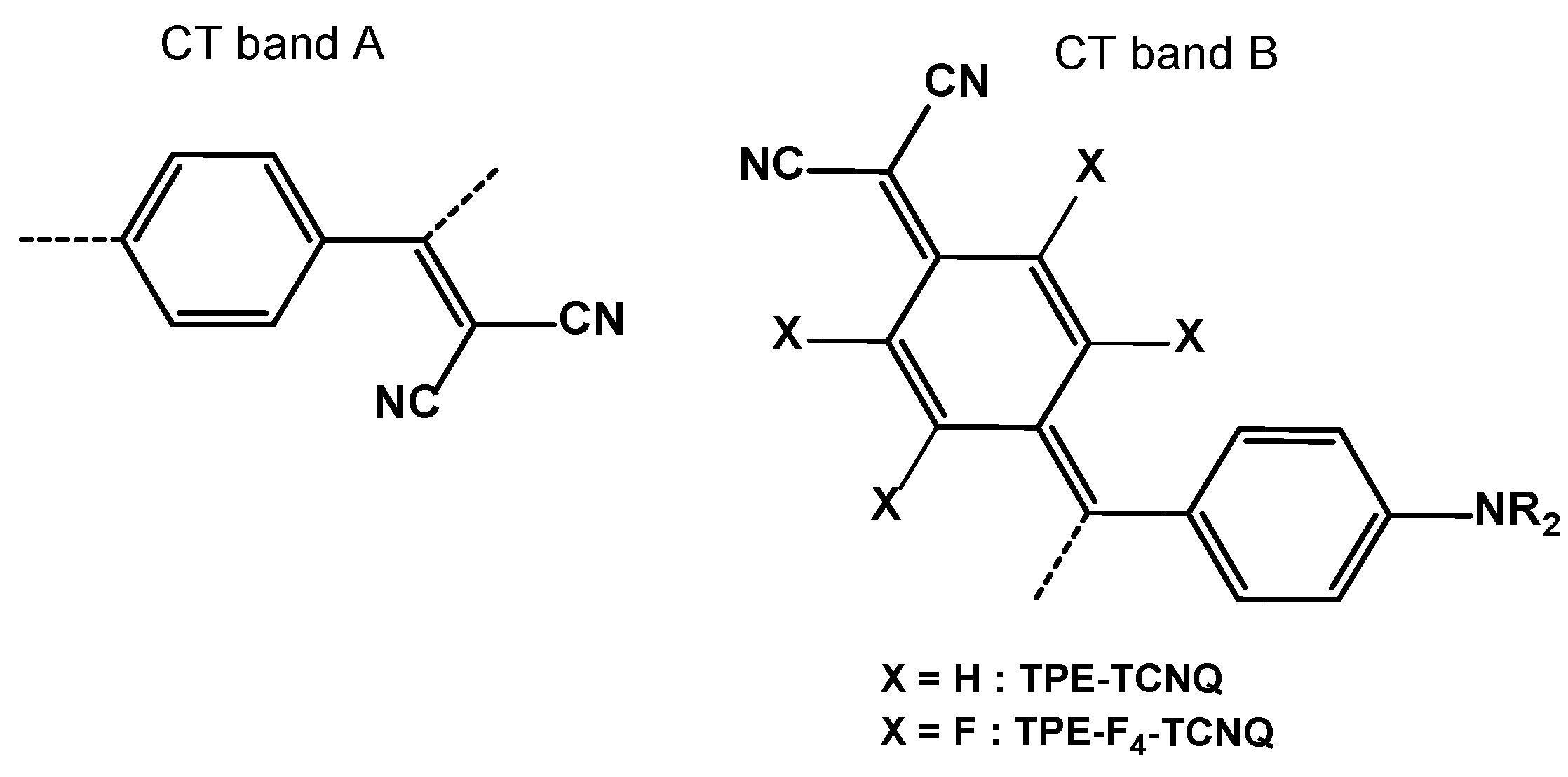
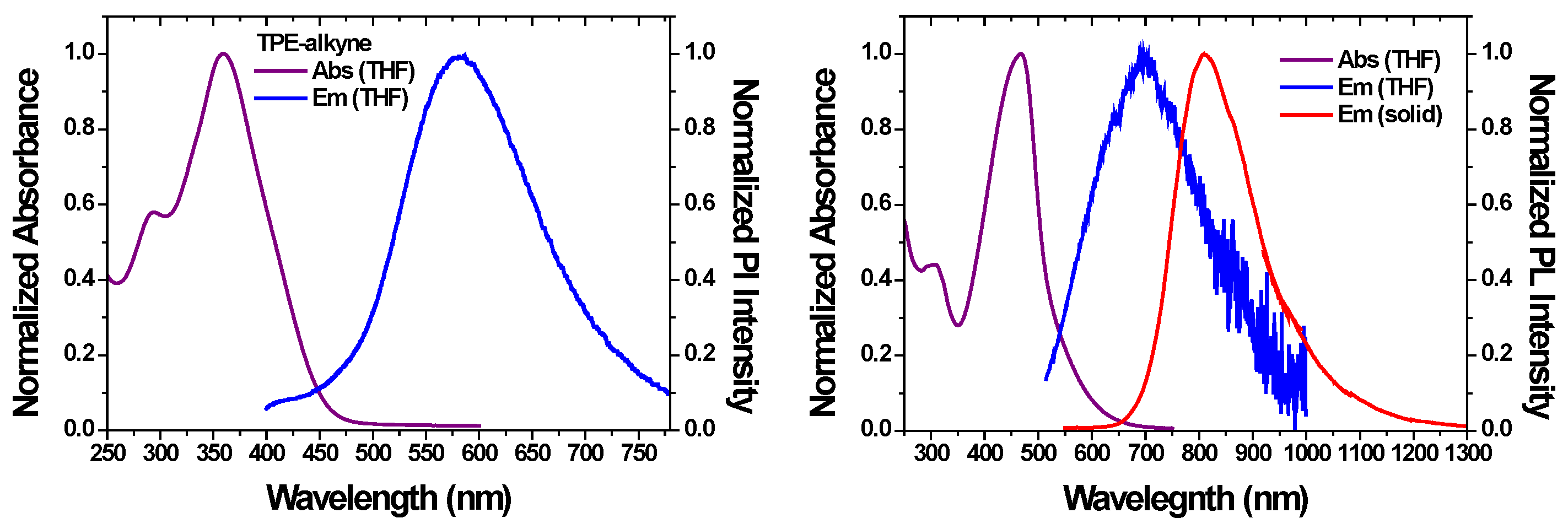
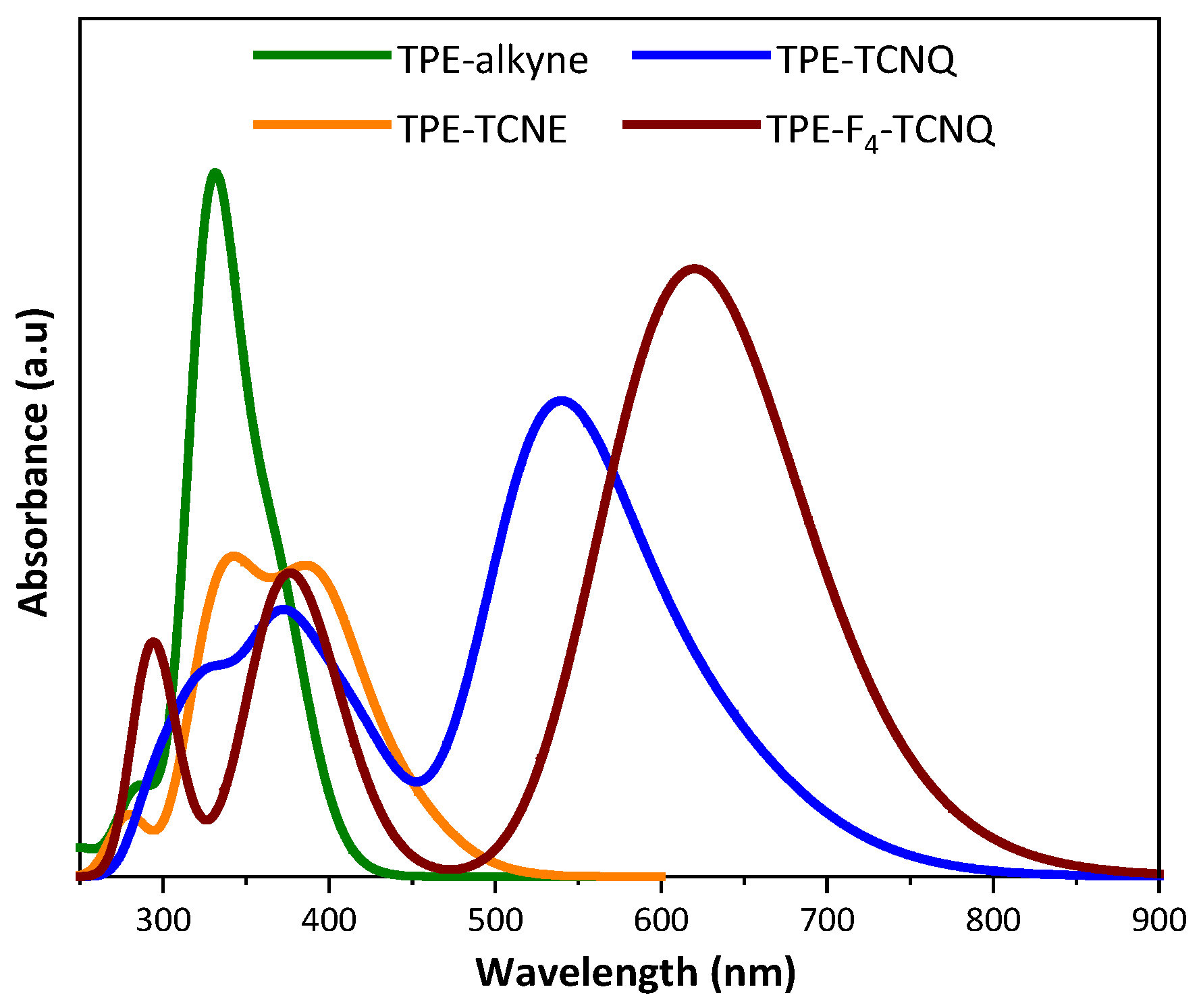
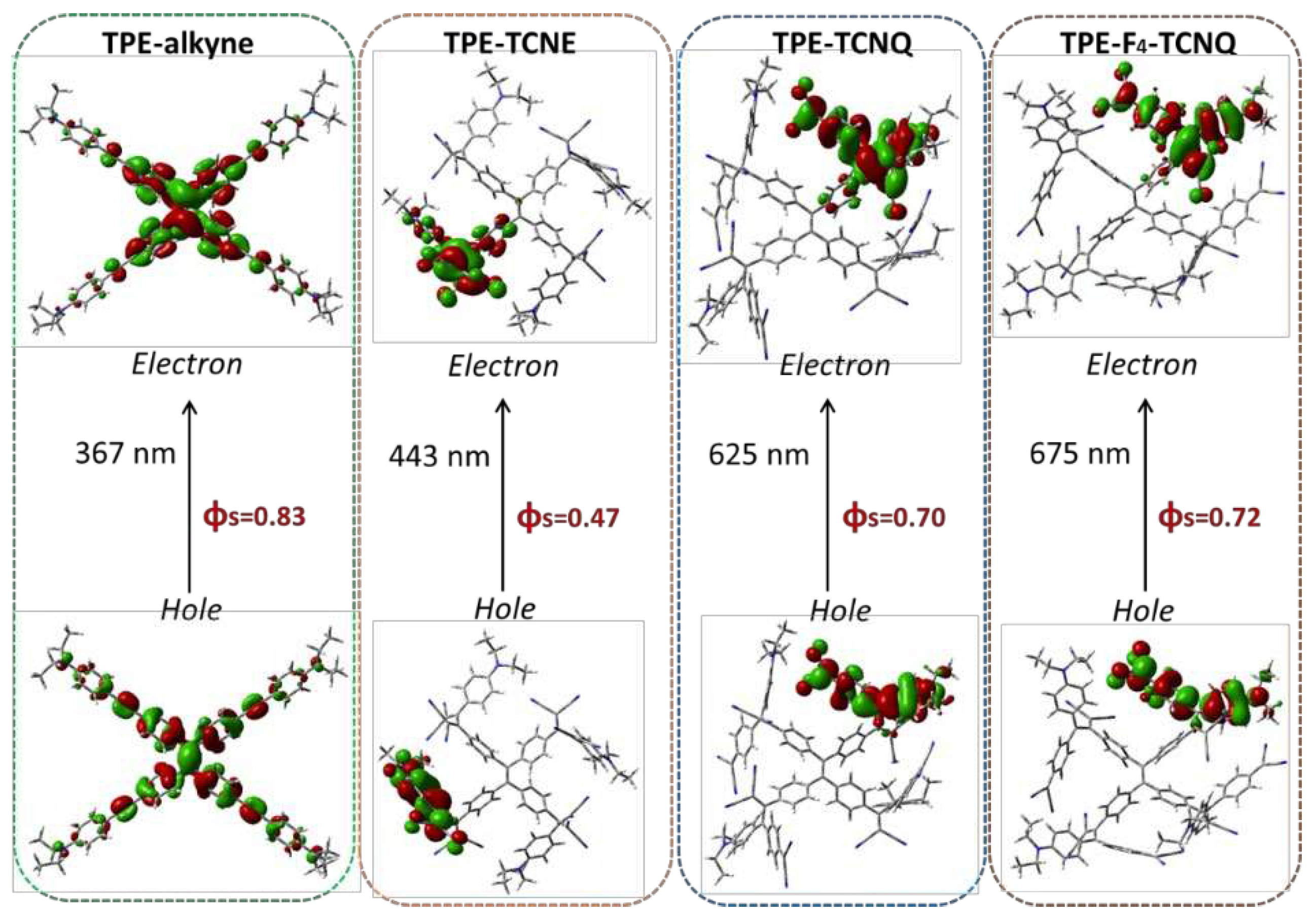
| TPE-alkyne | TPE-TCNE | TPE-TCNQ | TPE-F4-TCNQ | |
|---|---|---|---|---|
| Absorbance in solutiona (nm) /(ε(L.mol-1.cm-1)) | 294 (82 400) 359 (143 400) |
307 / (45 600) 469 (113 400) |
337 (113 900) 468 (78 500) 688 (155 000) |
348 (97 500) 497 (73 600) 849 (163 400) |
| Absorbance as thin films (nm) | - | 476 | 471, 738 | 498, 864 |
| λem max(solutiona / aggregatedb) | 582 / 560 / - | ≈700 / 786 | - | - |
| Φsol(%)a | 13% | - | - | - |
| Φaggr(%)b | 21% | NM | - | - |
| λonset(nm)c | 460 | 540 | 900 | - |
| Eg opt (eV)d | 2.70 | 2.30 | 1.38 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).