Submitted:
13 April 2023
Posted:
13 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Plant secondary metabolites
3. Alkaloids: the major plant secondary metabolites
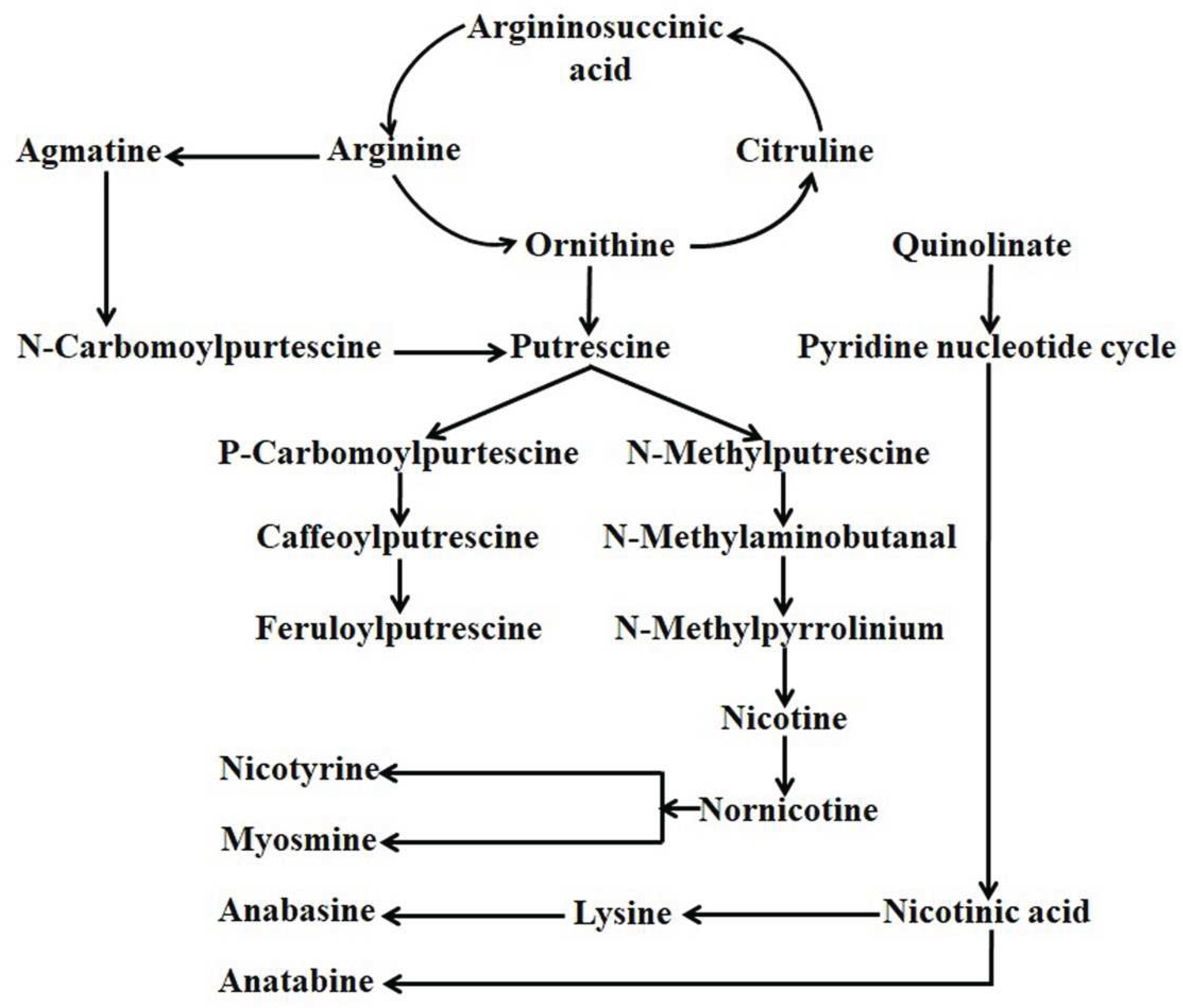
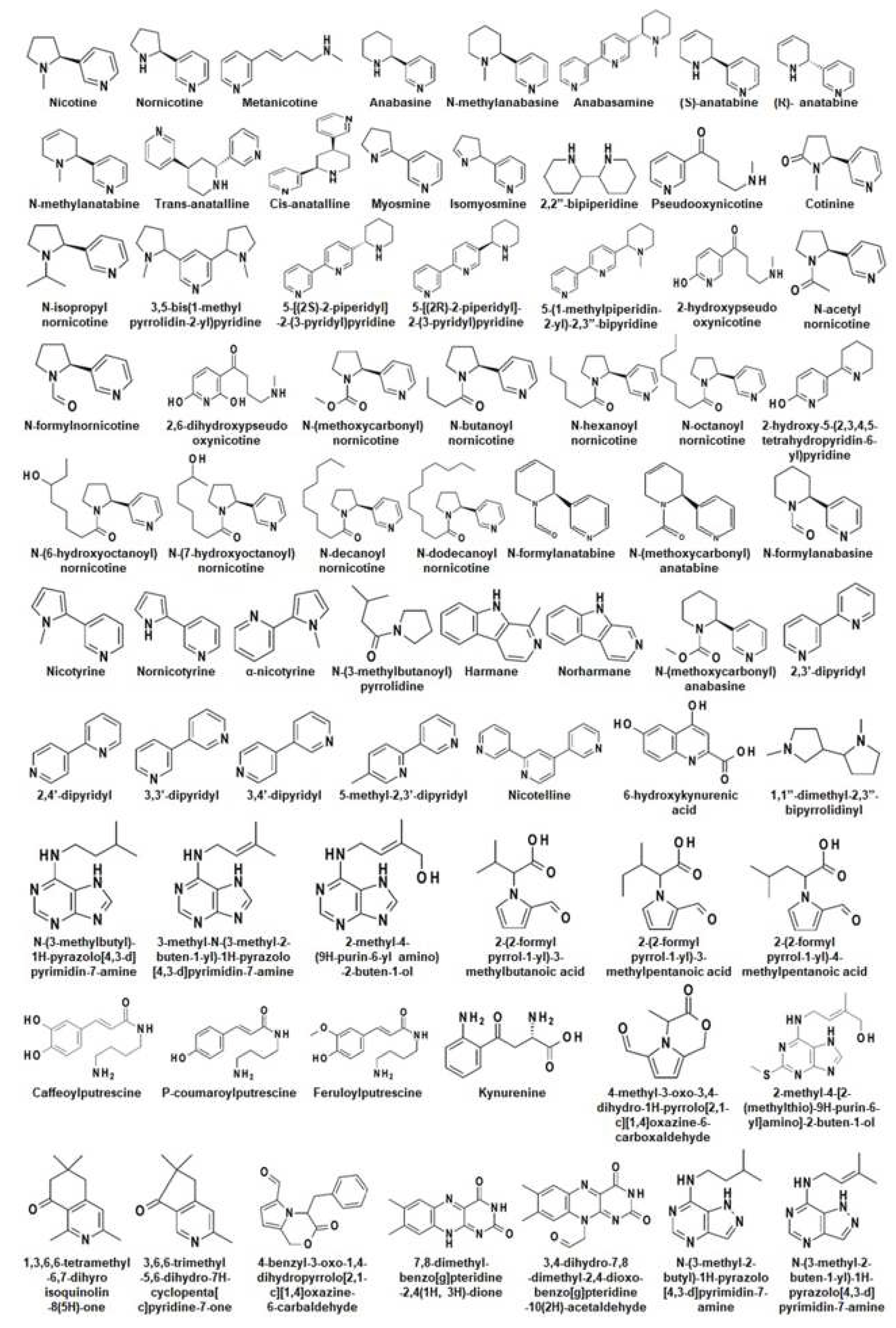
4. Alkaloid present in Nicotiana
5. Anticancer alkaloids present in Nicotiana
5.1. β-carboline
5.2. Kynurenines
5.3. Nicotine and nornicotine
6. Metabolic engineering of Nicotiana for anticancer compound
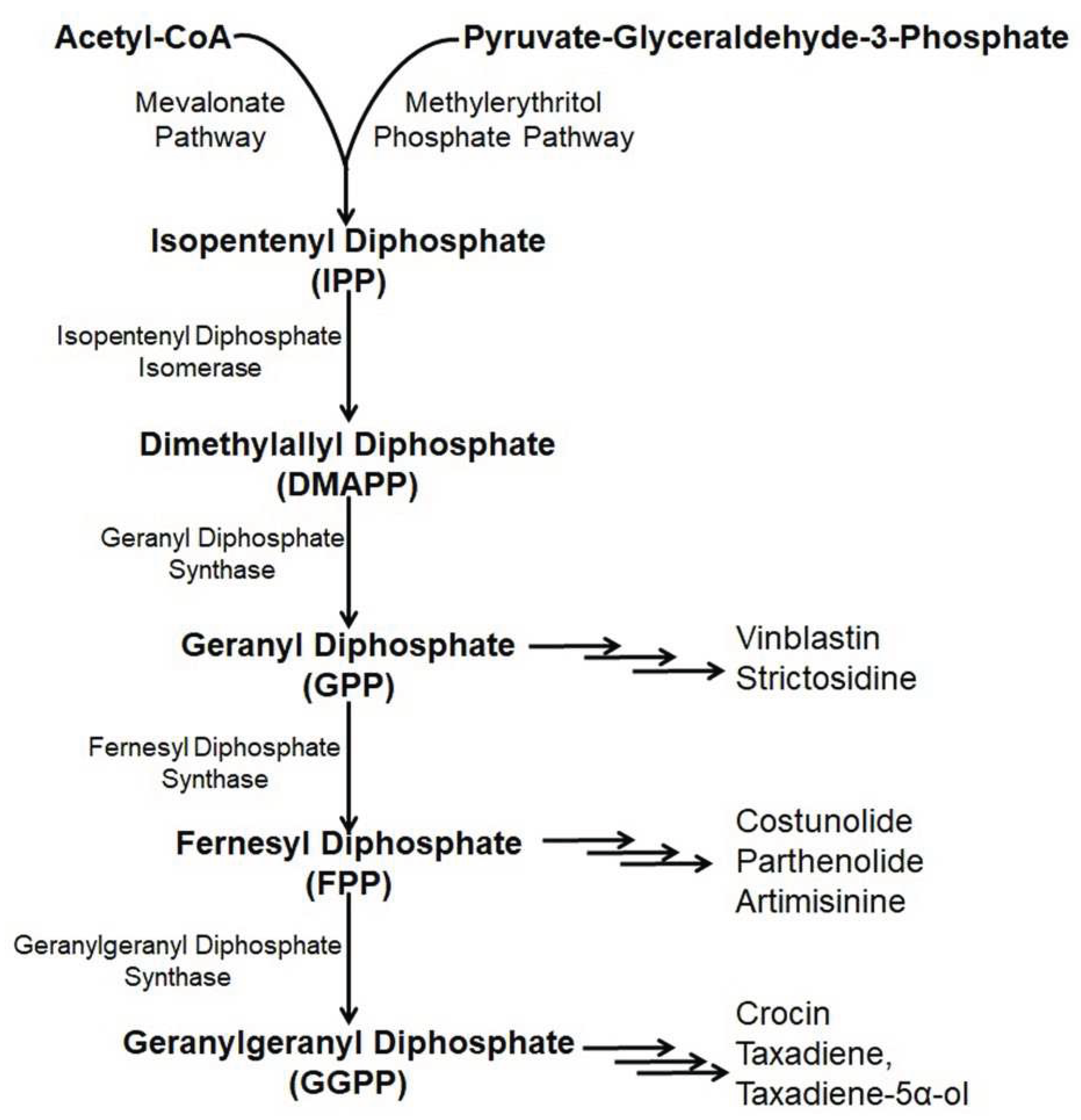
6.1. Taxol
6.2. Artemisinin
6.3. Parthenolide
6.4. Costunolide
6.5. Etoposide and related anticancer molecules
6.6. Crocin
6.7. Vinblastine
6.8. Strictosidine
7. Challenges and Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemos, R.D.C.; Pádua, J.M.V.; Bruzi, A.T.; Oliveira, V.B.D.; Ramalho, M.A.P. Comparison between doubled haploid lines and lines obtained via the bulk method in tobacco. Crop. Breed. Appl. Biotechnol. 2022, 22, e42992249. [Google Scholar] [CrossRef]
- Camlica, M.; Yaldiz, G. Genetic diversity of one cultivar and 29 genotypes of tobacco based on morphological and yield properties. J. Anim. Plant Sci. 2020, 30, 442–453. [Google Scholar]
- Leal, M.; Moreno, M.A.; Albornoz, P.L.; Mercado, M.I.; Zampini, I.C.; Isla, M.I. Nicotiana tabacum leaf waste: Morphological characterization and chemical-functional analysis of extracts obtained from powder leaves by using green solvents. Molecules 2023, 28, 1396. [Google Scholar] [CrossRef] [PubMed]
- Jaber, A.; Soukariyeh, R.; Khalil, A.; Abdel-Sater, F.; Cheble, E. Biological activities of total oligomeric flavonoids enriched extracts of Nicotiana tabacum from eight lebanese regions. Int. J. Pharm. Sci. Rev. Res. 2020, 6, 70–77. [Google Scholar]
- Coseri, S. Natural products and their analogues as efficient anticancer drugs. Mini-Rev. Med. Chem. 2009, 9, 560–571. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Chen, M.W.; Cheng, K.J.; Yu, P.Z.; Wei, X.; Shi, Z. Therapeutic potential of steroidal alkaloids in cancer and other diseases. Med. Res. Rev. 2016, 36, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, F.; Noma, M.; Kawashima, N. The alkaloid contents of sixty Nicotiana species. Phytochemistry 1985, 24, 477–480. [Google Scholar] [CrossRef]
- Dewey, R.E.; Xie, J. Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 2013, 94, 10–27. [Google Scholar] [CrossRef]
- Mishra, A.; Chaturvedi, P.; Datta, S.; Sinukumar, S.; Joshi, P.; Garg, A. Harmful effects of nicotine. Indian J. Med. Paediatr. Oncol. 2015, 36, 24–31. [Google Scholar] [CrossRef]
- Khan, H.; Patel, S.; A Kamal, M. Pharmacological and toxicological profile of harmane-β-Carboline alkaloid: Friend or foe. Curr. Drug Metab. 2017, 18, 853–857. [Google Scholar] [CrossRef]
- Echeverria, V.; Grizzell, J.A.; Barreto, G.E. Neuroinflmmation: a therapeutic target of cotinine for the treatment of psychiatric disorders? Curr. Pharmaceut. Des. 2016, 22, 1324–1333. [Google Scholar] [CrossRef]
- Tantawy, M.A.; Nafie, M.S.; Elmegeed, G.A.; Ali, I.A.I. Auspicious Role of the Steroidal Heterocyclic Derivatives as a Platform for Anti-Cancer Drugs. Bioorg. Chem. 2017, 73, 128–146. [Google Scholar] [CrossRef]
- Yuan, X.L.; Mao, X.X.; Du, Y.M.; Yan, P.Z.; Hou, X.D.; Zhang, Z.F. Anti-tumor activity of cembranoid-type diterpenes isolated from Nicotiana tabacum L. Biomoleculars 2019, 9, 45. [Google Scholar] [CrossRef]
- Pickens, L.B.; Tang, Y.; Chooi, Y.H. Metabolic engineering for the production of natural products. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 211–236. [Google Scholar] [CrossRef]
- Hasan, M.M.; Kim, H.S.; Jeon, J.H.; Kim, S.H.; Moon, B.K.; Song, J.Y.; Shim, S.H.; Baek, K.H. Metabolic engineering of Nicotiana benthamiana for the increased production of taxadiene. Plant Cell Rep. 2014, 33, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Campos, A.L.; Gutiérrez-Ortega, A. Agrobacterium-mediated transformation of Nicotiana tabacum cv. Xanthi leaf explants. Bio. 2019, 101, e3150. [Google Scholar]
- Dudley, Q.M.; Jo, S.; Guerrero, D.A.S.; Chhetry, M.; Smedley, M.A.; Harwood, W.A.; Sherden, N.H.; O’Connor, S.E.; Caputi, L.; Patron, N.J. Reconstitution of monoterpene indole alkaloid biosynthesis in genome engineered Nicotiana benthamiana. Commun. Biol. 2022, 5, 1–12. [Google Scholar] [CrossRef]
- Chadwick, D.J.; Whelan, J. Secondary metabolites: Their function and evolution. John Wiley & Sons. 1992, pp.3-4.
- Teoh, E.S. Secondary metabolites of plants. In Medicinal Orchids of Asia; Springer International Publishing: Cham, Switzerland, 2016; pp. 59–73. ISBN 9783319242743. [Google Scholar]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; Siddiqui, K.S.; Hermandez-Sotomayor, S.M.T.; Faisal, M. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; Calina, D.; Cho, W.C. Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P. Plant alkaloids: Classification, isolation and drug development. In Medicinal Plants: Chemistry, Pharmacology and Therapeutic Applications; Swamy, M.K., Patra, J.K., Rudramurthy, G.R., Eds.; Taylor & Francis Ltd.: Boca Raton, FL, USA, 2019; pp. 131–137. [Google Scholar]
- Salehi, B.; Sharifi-Rad, J.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K. Cucurbita plants: From farm to industry. Appl. Sci. 2019, 9, 3387. [Google Scholar] [CrossRef]
- Kurek, J. Introductory chapter: Alkaloids-Their importance in nature and for human life. In Alkaloids—Their Importance in Nature and for Human Life; Kurek, J., Ed.; Intech Open: London, UK, 2019. [Google Scholar]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Zenkner, F.F.; Margis-Pinheiro, M.; Cagliari, A. Nicotine biosynthesis in Nicotiana: A Metabolic Overview. Tobacco Sci. 2019, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alijevic, O.; McHugh, D.; Rufener, L.; Mazurov, A.; Hoeng, J.; Peitsch, M. An electrophysiological characterization of naturally occurring tobacco alkaloids and their action on human α4β2 and α7 nicotinic acetylcholine receptors. Phytochemistry 2020, 170, 1121187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Kumar, K.; Kumar, V. Recent insights into synthetic β-carbolines with anti-cancer activities. Eur. J. Med. Chem. 2017, 142, 48–73. [Google Scholar] [CrossRef]
- Herraiz, T. β-Carbolines in foods. In bioactive compounds in foods; Gilbert, J.; Senyuva, H.Z., Eds.; Blackwell Publishing, UK, 2008; pp 199- 223.
- Cao, R.H.; Peng, W.L.; Wang, Z.H.; Xu, A.L. β-Carboline alkaloids: biochemical and pharmacological functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive beta-Carbolines in food: A Review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef]
- Poindexter, E.H.; Carpenter, R.D. The isolation of harmane and norharmane from tobacco and cigarette smoke. Phytochemistry 1962, 1, 215–221. [Google Scholar] [CrossRef]
- Manasa, K.L.; Yadav, S.S.; Srikanth, D.; Nagesh, N.; Alvalaa, M. Recent insights into β-Carboline alkaloids with anticancer potential. IOSR J. Pharm. Biol. Sci. 2020, 15, 01–27. [Google Scholar]
- Chen, Y.F.; Lin, Y.C.; Chen, J.P.; Chan, H.C.; Hsu, M.H.; Lin, H.Y.; Kuo, S.C.; Huang, L.J. Synthesis and biological evaluation of novel 3,9-substituted β-carboline derivatives as anticancer. Bioorg. Med. Chem. Lett. 2015, 25, 3873–3877. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Paidesetty, S.K.; Padhy, R.N. Norharmane as a potential chemical entity for development of anticancer drugs. Eur. J. Med. Chem. 2019, 162, 752–764. [Google Scholar] [CrossRef]
- Cox, E.D.; Cook, J.M. The pictet-spengler condensation: A new direction for an old reaction. Chem. Rev. 1995, 95, 1797–1842. [Google Scholar] [CrossRef]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; Moll, N.; Schutze, G.; Schwarz, M.J.; Waetzig, G.H.; Rosenstiel, P.; Krawczak, M.; Szymczak, S.; Schreiber, S. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 2017, 153, 1504–1516.e2. [Google Scholar] [CrossRef] [PubMed]
- Steiner, N.; Müller, U.; Hajek, R.; Sevcikova, S.; Borjan, B.; Jöhrer, K.; Göbel, G.; Pircher, A.; Gunsilius, E. The metabolomic plasma profile of myeloma patients is considerably different from healthy subjects and reveals potential new therapeutic targets. PLoS ONE 2018, 13, e0202045. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Cheng, M.L.; Tang, H.Y.; Huang, C.Y.; Wu, Y.R.; Chen, C.M. Alternations of metabolic profile and kynurenine metabolism in the plasma of parkinson’s disease. Mol. Neurobiol. 2018, 55, 6319–6328. [Google Scholar] [CrossRef] [PubMed]
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows- current knowledge on its fate and function. Pharmacol. Ther. 2021, 225, 107845. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; Jugold, M.; Guillemin, G.J.; Miller, C.L.; Lutz, C.; Radlwimmer, B.; Lehmann, I.; von Deimling, A.; Wick, W.; Platten, M. An endogenous tumour-promoting ligands of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Zgrajka, W.; Bartnik, M.; Kocki, T.; Turski, W.A. Distribution, synthesis, and absorption of kynurenic acid in plants. Planta Med. 2011, 77, 858–864. [Google Scholar] [CrossRef]
- Mo, Z.; Duan, L.; Pu, Y.; Tian, Z.; Ke, Y.; Luo, W.; Pi, K.; Huang, Y.; Nie, Q.; Liu, R. Proteomics and co-expression network analysis reveal the importance of hub proteins and metabolic pathways in nicotine synthesis and accumulation in tobacco (Nicotiana tabacum L.). Front. Plant Sci. 2022, 13, 860455. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Secondary metabolites. Compr. Biotechnol. 2019, 10, 131–143. [Google Scholar]
- Sanner, T.; Grimsrud, T.K. Nicotine: Carcinogenicity and effects on response to cancer treatment–A review. Front. Oncol. 2015, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Gavilano, L.B.; Coleman, N.P.; Burnley, L.E.; Bowman, M.L.; Kalengamaliro, N.E.; Hayes, A.; Bush, L.; Siminszky, B. Genetic engineering of Nicotiana tabacum for reduced nornicotine content. J. Agric. Food Chem. 2006, 54, 9071–9078. [Google Scholar] [CrossRef] [PubMed]
- Pakdeechanuan, P.; Teoh, S.; Shoji, T.; Hashimoto, T. Non-functionalization of two CYP82E Nicotine N-demethylase genes abolishes nornicotine formation in Nicotiana langsdorffii. Plant Cell Physiol. 2012, 53, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Knezevich, A.; Muzic, J.; Hatsukami, D.K.; Hecht, S.S.; Stepanov, I. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N′-nitrosonornicotine in humans. Nicotine Tob. Res. 2013, 15, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Kadohama, N.; Shintani, K.; Osawa, Y. Tobacco alkaloid derivatives as inhibitors of breast cancer aromatase. Cancer Lett. 1993, 75, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Talo, M.; Yildirim, O.; Karabatak, M.; Acharya, U.R. Automated invasive ductal carcinoma detection based using deep transfer learning with whole-slide images. Pattern Recognit. Lett. 2020, 133, 232–239. [Google Scholar] [CrossRef]
- Reed, J.; Osbourn, A. Engineering terpenoid production through transient expression in Nicotiana benthamiana. Plant Cell. Rep. 2018, 37, 1431–1441. [Google Scholar] [CrossRef]
- Bally, J.; Jung, H.; Mortimer, C.; Naim, F.; Philips, J.G.; Hellens, R.; Bombarely, A.; Goodin, M.M.; Waterhouse, P.M. The rise and rise of Nicotiana benthamiana: a plant for all reasons. Annu. Rev. Phytopathol. 2018, 56, 405–426. [Google Scholar] [CrossRef]
- Heldman, A.W.; Cheng, L.; Jenkins, G.M.; Heller, P.F.; Kim, D.W.; Ware, M.J.; Nater, C.; Hruban, R.H.; Rezai, B.; Abella, B.S.; Bunge, K.E.; Kinsella, J.L.; Sollott, S.J.; Lakatta, E.G.; Brinker, J.A.; Hunter, W.L.; Froehlich, J.P. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 2001, 103, 2289–2295. [Google Scholar] [CrossRef]
- Ahn, J.H.; Eum, K.H.; Lee, M. Spry2 does not directly modulate Raf-1 kinase activity in v-Ha-ras-transformed NIH 3T3 fibroblasts. BMB Rep. 2010, 43, 205–211. [Google Scholar] [CrossRef]
- Hata, K.; Osaki, M.; Dhar, D.K.; Nakayama, K.; Fujiwaki, R.; Ito, H.; Nagasue, N.; Miyazaki, K. Evaluation of the antiangiogenic effect of taxol in a human epithelial ovarian carcinoma cell line. Cancer Chemother. Pharmacol. 2004, 53, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.; Ketchum, R.E.B.; Long, R.M.; Kaspera, R.; Wildung, M.R. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 2006, 5, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Kaspera, R.; Croteau, R. Cytochrome P450 oxygenases of taxol biosynthesis. Phytochem. Rev. 2006, 5, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Karuppusamy, S.; Pullaiah, T. 6-Botany of paclitaxel producing plants. In Paclitaxel; Swamy, M.K., Pullaiah, T., Chen, Z.-S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 155–170. [Google Scholar]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.; Walsh, V. The story of taxol. Cambridge University Press, Cambridge. 2001, pp 282.
- Hasan, M.M.; Dhakal, R.; Baek, K.H. Accumulation of taxadiene by root culture of Nicotiana benthamiana domin transformed with taxadiene synthase. Bangladesh J. Bot. 2017, 46, 899–905. [Google Scholar]
- Li, S. Review and expectation of the study on quantitative analysis of steroidal alkaloid. Drug Stanoaros China 2001, 2, 8–11. [Google Scholar]
- Kovacs, K.; Zhang, L.; Linforth, R.T.; Whittaker, B.; Hayes, C.; Fray, R. Redirection of carotenoid metabolism for the efficient production of taxadiene [taxa-4(5),11(12)-diene] in transgenic tomato fruit. Transgen. Res. 2007, 16, 121–126. [Google Scholar] [CrossRef]
- Numonov, S.; Sharopov, F.; Salimov, A.; Sukhrobov, P.; Atolikshoeva, S.; Safarzoda, R.; Habasi, M.; Aisa, H. Assessment of artemisinin contents in selected Artemisia species from Tajikistan (Central Asia). Medicines 2019, 6, 23. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Mu, L.; Yang, X. Artemisinins as anticancer drugs: Novel therapeutic approaches, molecular mechanisms, and clinical trials. Front. Pharmacol. 2020, 11, 1608. [Google Scholar] [CrossRef]
- Brown, G.D.; Sy, L.K. In vivo transformations of dihydroartemisinic acid in Artemisia annua plants. Tetrahedron 2004, 60, 1139–1159. [Google Scholar] [CrossRef]
- Chen, R.; Bu, Y.; Ren, J.; Pelot, K.A.; Hu, X.; Diao, Y.; Zhang, L. Discovery and modulation of diterpenoid metabolism improves glandular trichome formation, artemisinin production and stress resilience in Artemisia annua. New Phytol. 2021, 230, 2387–2403. [Google Scholar] [CrossRef] [PubMed]
- Picaud, S.; Olofsson, L.; Brodelius, M.; Brodelius, P.E. Expression, purification and characterization of recombinant amorpha-4,11-diene synthase from Artemisia annua L. Arch. Biochem. Biophys. 2005, 436, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Teoh, K.H.; Polichuk, D.R.; Reed, D.W.; Nowak, G.; Covello, P.S. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006, 580, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; Kapoor, V.; Celermajer, D.S.; Mellor, A.L.; Jr, J.F.K.; Hunt, N.H.; Stocker, R. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Rydén, A.-M.; Ruyter-Spira, C.; Quax, W.J.; Osada, H.; Muranaka, T.; Kayser, O.; Bouwmeester, H. The molecular cloning of dihydroartemisinic aldehyde reductase and its implication in artemisinin biosynthesis in Artemisia annua. Planta Med. 2010, 76, 1778. [Google Scholar] [CrossRef]
- Liu, M.; Shi, P.; Fu, X.; Brodelius, P.E.; Shen, Q.; Jiang, W.; He, Q.; Tang, K. Characterization of a trichome-specific promoter of the aldehyde dehydrogenase 1 (ALDH1) gene in Artemisia annua. Plant Cell Tissue Organ Cult. 2016, 126, 469–480. [Google Scholar] [CrossRef]
- Czechowski, T.; Larson, T.R.; Catania, T.M.; Harvey, D.; Brown, G.D.; Graham, I.A. Artemisia annua mutant impaired in artemisinin synthesis demonstrates importance of nonenzymatic conversion in terpenoid metabolism. Proc. Natl. Acad. Sci. USA 2016, 113, 15150–15155. [Google Scholar] [CrossRef]
- Farhi, M.; Marhevka, E.; Ben-Ari, J.; Algamas-Dimantov, A.; Liang, Z.; Zeevi, V.; Edelbaum, O.; Spitzer-Rimon, B.; Abeliovich, H.; Schwartz, B.; Tzfira, T.; Vainstein, A. Generation of the potent anti-malarial drug artemisinin in tobacco. Nat. Biotechnol. 2011, 29, 1072–107. [Google Scholar] [CrossRef]
- Zhang, Y.; Nowak, G.; Reed, D.W.; Covello, P.S. The production of artemisinin precursors in tobacco. Plant Biotechnol. J. 2011, 9, 445–454. [Google Scholar] [CrossRef]
- Ting, H.M.; Wang, B.; Rydén, A.M.; Woittiez, L.; van Herpen, T.; Verstappen, F.W.; Ruyter-Spira, C.; Beekwilder, J.; Bouwmeester, H.J.; van der Krol, A. The metabolite chemotype of Nicotiana benthamiana transiently expressing artemisinin biosynthetic pathway genes is a function of CYP71AV1 type and relative gene dosage. New Phytol. 2013, 199, 352–366. [Google Scholar] [CrossRef]
- Malhotra, K.; Subramaniyan, M.; Rawat, K.; Kalamuddin, M.; Qureshi, M.I.; Malhotra, P.; Mohmmed, A.; Cornish, K.; Daniell, H.; Kumar, S. Compartmentalized metabolic engineering for artemisinin biosynthesis and effective malaria treatment by oral delivery of plant cells. Mol. Plant 2016, 9, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Wallaart, T.E.; Bouwmeester, H.J.; Hille, J.; Poppinga, L.; Maijers, N.C.A. Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 2001, 212, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Van Herpen, T.W.J.M.; Cankar, K.; Nogueira, M.; Bosch, D.; Bouwmeester, H.J.; Beekwilder, J. Nicotiana benthamiana as a production platform for artemisinin precursors. PLoS ONE 2010, 5, e14222. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, P.; Zhou, F.; Erban, A.; Karcher, D.; Kopka, J.; Bock, R. A new synthetic biology approach allows transfer of an entire metabolic pathway from a medicinal plant to a biomass crop. eLife 2016, 5, e13664. [Google Scholar] [CrossRef] [PubMed]
- Ikram, N.K.B.K.; Simonsen, H.T. A review of biotechnological artemisinin production in plants. Front. Plant Sci. 2017, 8, 1966. [Google Scholar] [CrossRef] [PubMed]
- Tiuman, T.S.; Ueda-Nakamura, T.; Cortez, D.A.G.; Dias, B.P.; Morgado-Diaz, J.A.; de Souza, W.; Nakamura, C.V. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum Parthenium. Antimicrob. Agents Chemother. 2005, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Majdi, M.; Liu, Q.; Karimzadeh, G.; Malboobi, M.A.; Beekwilder, J.; Cankar, K.; De Vos, R.; Todorović, S.; Simonović, A.; Bouwmeester, H. Biosynthesis and localization of parthenolide in glandular trichomes of feverfew (Tanacetum parthenium L. Schulz Bip.). Phytochemistry 2011, 72, 1739–1750. [Google Scholar] [CrossRef]
- Liu, Q.; Manzano, D.; Tanic, N.; Pesic, M.; Bankovic, J.; Pateraki, I.; Ricard, L.; Ferrer, A.; de Vos, R.; van de Krol, S.; Bouwmeester, H. Elucidation and in planta reconstitution of the parthenolide biosynthetic pathway. Metab. Eng. 2014, 23, 145–153. [Google Scholar] [CrossRef]
- Rasul, A.; Parveen, S.; Ma, T. Costunolide: A novel anti-cancer sesquiterpene lactone. Bangladesh J. Pharmacol. 2012, 7, 6–13. [Google Scholar] [CrossRef]
- Butturini, E.; Cavalieri, E.; de Prati, A.C.; Darra, E.; Rigo, A.; Shoji, K.; Murayama, N.; Yamazaki, H.; Watanabe, Y.; Suzuki, H.; Mariotto, S. Two naturally occurring terpenes, dehydrocostuslactone and costunolide, decrease intracellular GSH content and inhibit STAT3 activation. PLoS ONE 2011, 6, e20174. [Google Scholar] [CrossRef]
- Pitchai, D.; Roy, A.; Banu, S. In vitro and in silico evaluation of NF-κB targeted costunolide action on estrogen receptor-negative breast cancer cells-a comparison with normal breast cells. Phytother. Res. 2014, 28, 1499–1505. [Google Scholar] [CrossRef]
- Liu, Q.; Majdi, M.; Cankar, K.; Goedbloed, M.; Charnikhova, T.; Verstappen, F.W.; de Vos, R.C.; Beekwilder, J.; van der Krol, S.; Bouwmeester, H.J. Reconstitution of the costunolide biosynthetic pathway in yeast and Nicotiana benthamiana. PLoS ONE 2011, 6, e23255. [Google Scholar] [CrossRef]
- Thirumaran, R.; Prendergast, G.C.; Gilman, P.B. Cytotoxic chemotherapy in clinical treatment of cancer. In Cancer Immunotherapy: Immune Suppression and Tumor Growth; Prendergast, G.C., Jaffee, E.M., Eds.; Elsevier Inc.: Burlington, VT, USA.; San Diego, CA USA.; London UK, 2007; pp. 101–116. [Google Scholar]
- Davey, S.G. Engineering etoposide. Nat. Rev. Chem. 2020, 4, 63. [Google Scholar] [CrossRef]
- Kim, SS.; Wengier, D.L.; Ragland, C.J.; Sattely, E.S. Transcriptional reactivation of lignin biosynthesis for the heterologous production of etoposide aglycone in Nicotiana benthamian. ACS Synth. Biol. 2022, 11, 3379–3387. [Google Scholar] [CrossRef]
- Schultz, B.J.; Kim, S.Y.; Lau, W.; Sattely, E.S. Total biosynthesis for milligram-scale production of etoposide intermediates in a plant chassis. J. Am. Chem. Soc. 2019, 141, 19231–19235. [Google Scholar] [CrossRef]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef]
- Ranjbar, R.; Shayanfar, P.; Maniati, M. In vitro antileishmanial effects of saffron compounds, crocin and stigmasterol, on iranian strain of Leishmania major (MHOM/IR/75/ER). Iran. J. Parasitol. 2021, 16, 151. [Google Scholar] [CrossRef]
- Bakshi, H.A.; Zoubi, M.S.A.; Faruck, H.L.; Aljabali, A.A.A.; Rabi, F.A.; Hafiz, A.A.; Al-Batanyeh, K.M.; Al-Trad, B.; Ansari, P.; Nasef, M.M.; Charbe, N.B.; Satija, S.; Mehta, M.; Mishra, V.; Gupta, G.; Abobaker, S.; Negi, P.; Azzouz, I.M.; Dardouri, A.A.K.; Dureja, H.; Prasher, P.; Chellappan, D.K.; Dua, K.; Silva, M.W.D.; Tanani, M.E.; McCarron, P.A.; Tambuwala, M.M. Dietary crocin is protective in pancreatic cancer while reducing radiation-induced hepatic oxidative damage. Nutrients 2020, 12, 1901. [Google Scholar] [CrossRef]
- Ahrazem, O.; Zhu, C.; Huang, X.; Rubio-Moraga, A.; Capell, T.; Christou, P.; Gómez-Gómez, L. Metabolic engineering of crocin biosynthesis in Nicotiana species. Front. Plant Sci. 2022, 13, 861140. [Google Scholar] [CrossRef]
- Grzech, D.; Hong, B.; Caputi, L.; Sonawane, P.D.; O’Connor, S.E. Engineering the biosynthesis of late-stage vinblastine precursors precondylocarpine acetate, catharanthine, tabersonine in Nicotiana benthamiana. ACS Synth. Biol. 2023, 12, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sarrion-Perdigones, A.; Vazquez-Vilar, M.; Palací, J.; Castelijns, B.; Forment, J.; Ziarsolo, P.; Blanca, J.; Granell, A.; Orzaez, D. GoldenBraid 2.0: A comprehensive DNA assembly framework for plant synthetic biology. Plant Physiol. 2013, 162, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Stoeckigt, J.; Ruppert, L. Strictosidine, the biosynthetic key to monoterpenoid indole alkaloids. In Comprehensive Natural Products Chemisty; Kelly, J.W., Ed.; Elsevier, B. V.: Amsterdam, The Netherlands, 1999; Volume 4, pp. 109–138. [Google Scholar]
- De Luca, V.; Salim, V.; Levac, D.; Atsumi, S.M.; Yu, F. Discovery and functional analysis of monoterpenoid indole alkaloid pathways in plants. Methods Enzymol. 2012, 515, 207–229. [Google Scholar] [PubMed]
- O’Connor, S.E.; Maresh, J.J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep. 2006, 23, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.F. Rigorous biogenetic network for a group of indole alkaloids derived from strictosidine. Molecules 2008, 13, 1875–1896. [Google Scholar] [CrossRef]
- Miettinen, K.; Dong, L.; Navrot, N.; Schneider, T.; Burlat, V.; Pollier, J.; Woittiez, L.; van der Krol, S.; Lugan, R.; Ilc, T.; Verpoorte, R.; Oksman-Caldentey, K.M.; Martinoia, E.; Bouwmeester, H.; Goossens, A.; Memelink, J.; Werck-Reichhart, D. The seco-iridoid pathway from Catharanthus roseus. Nat. Commun. 2014, 5, 3606. [Google Scholar] [CrossRef]
- Kumar, S.; Hahn, F.M.; Baidoo, E.; Kahlon, T.S.; Wood, D.F.; McMahan, C.M.; Cornish, K.; Keasling, J.D.; Daniell, H.; Whalen, M.C. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mevalonate pathway in chloroplasts. Metab. Eng. 2012, 14, 19–28. [Google Scholar] [CrossRef]
- Wellen, K.E.; Snyder, N.W. Should we consider subcellular compartmentalization of metabolites, and if so, how do we measure them? Curr Opin Clin Nutr Metab Care. 2019, 22, 347–354. [Google Scholar] [CrossRef]
- Lynch, J.H.; Orlova, I.; Zhao, C.; Guo, L.; Jaini, R.; Maeda, H.; Akhtar, T.; Cruz-Lebron, J.; Rhodes, D.; Morgan, J. Multifaceted plant responses to circumvent Phehyperaccumulation by downregulation of flux through the shikimate pathway and by vacuolar Phe sequestration. Plant J. 2017, 92, 939–950. [Google Scholar] [CrossRef]
- Garg, A.; Sharma, S.; Srivastava, P.; Ghosh, S. Application of virus-induced gene silencing in Andrographis paniculata, an economically important medicinal plant. Protoplasma 2021, 258, 1155–1162. [Google Scholar] [CrossRef]
- Shih, M.-L.; Morgan, J.A. Metabolic flux analysis of secondary metabolism in plants. Metab. Eng. Commun. 2020, 10, e00123. [Google Scholar] [CrossRef]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Stevenson, P.C. Cost: Benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Prot. 2014, 57, 71–76. [Google Scholar] [CrossRef]
- Fester, K. Plant alkaloids. Encycl. Life Sci. 2010, 11, 74–81. [Google Scholar]
- Sisson, V.; Severson, R. Alkaloid composition of the Nicotiana species. Beitr. Tabakforsch. Int. 1990, 14, 327–339. [Google Scholar] [CrossRef]
- Dawson, R.F. Nicotine synthesis in excised tobacco roots. Amer. Jour. Bot. 1942, 29, 813–815. [Google Scholar] [CrossRef]
- Saunders, J.A. Investigations of vacuoles isolated from tobacco: I. Quantitation of nicotine. Plant Physiol. 1979, 64, 74–78. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Lu, Y.; Stegemann, S.; Agrawal, S.; Karcher, D.; Ruf, S.; Bock, R. Horizontal transfer of a synthetic metabolic pathway between plant species. Curr. Biol. 2017, 27, 3034–3041. [Google Scholar] [CrossRef]
- Demurtas, O.C.; De Brito Francisco, R.; Diretto, G.; Ferrante, P.; Frusciante, S.; Pietrella, M.; Aprea, G.; Borghi, L.; Feeney, M.; Frigerio, L.; Coricello, A.; Costa, G.; Alcaro, S.; Martinoia, E.; Giuliano, G. ABCC transporters mediate the vacuolar accumulation of crocins in saffron stigmas. Plant cell 2019, 31, 2789–2804. [Google Scholar] [CrossRef]
- Sonawane, P.D.; Pollier, J.; Panda, S.; Szymanski, J.; Massalha, H.; Yona, M.; Unger, T.; Malitsky, S.; Arendt, P.; Pauwels, L.; Almekias-Siegl, E.; Rogachev, I.; Meir, S.; Cardenas, P.D.; Masri, A.; Petrikov, M.; Schaller, H.; Schaffer, A.A.; Kamble, A.; Giri, A.P.; Goossens, A.; Aharoni, A. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat. Plants 2016, 3, 16205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




