Submitted:
17 April 2023
Posted:
17 April 2023
Read the latest preprint version here
Abstract
Keywords:
INTRODUCTION
HYPOTHESIS
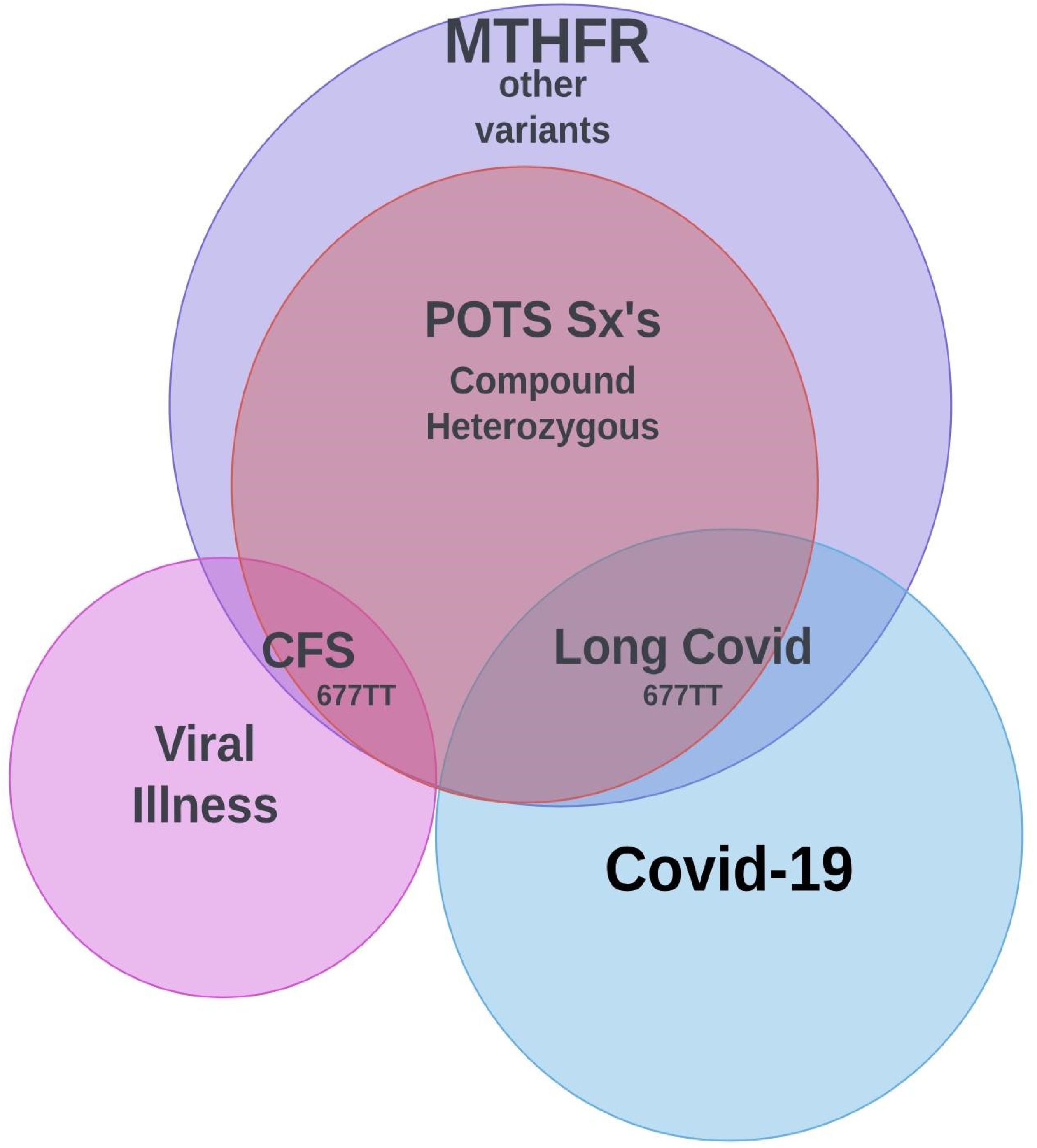
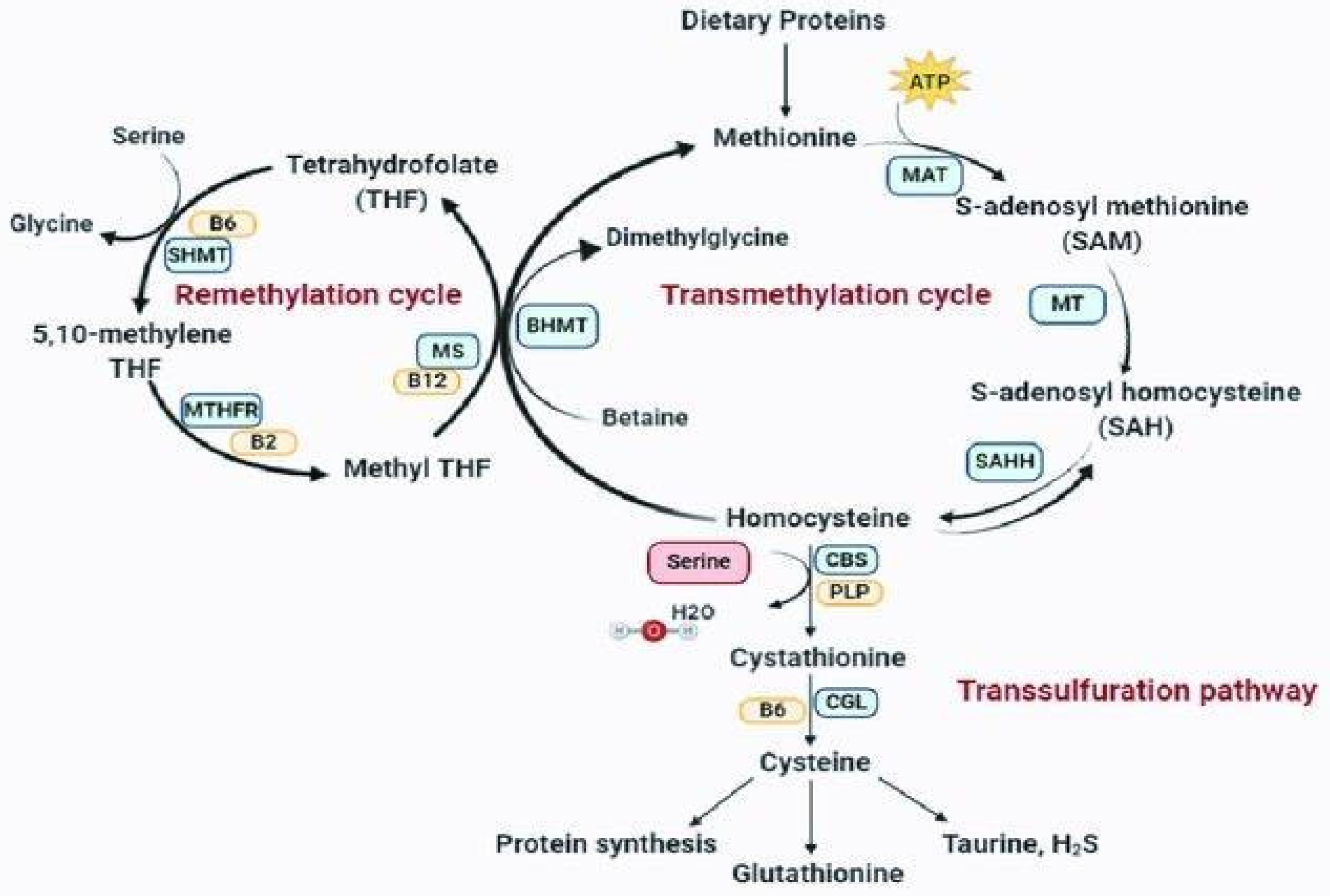
1. MTHFR and Homocysteine
2. POSTURAL ORTHOSTATIC TACHYCARDIA SYNDOME
3. Gut Microbiome
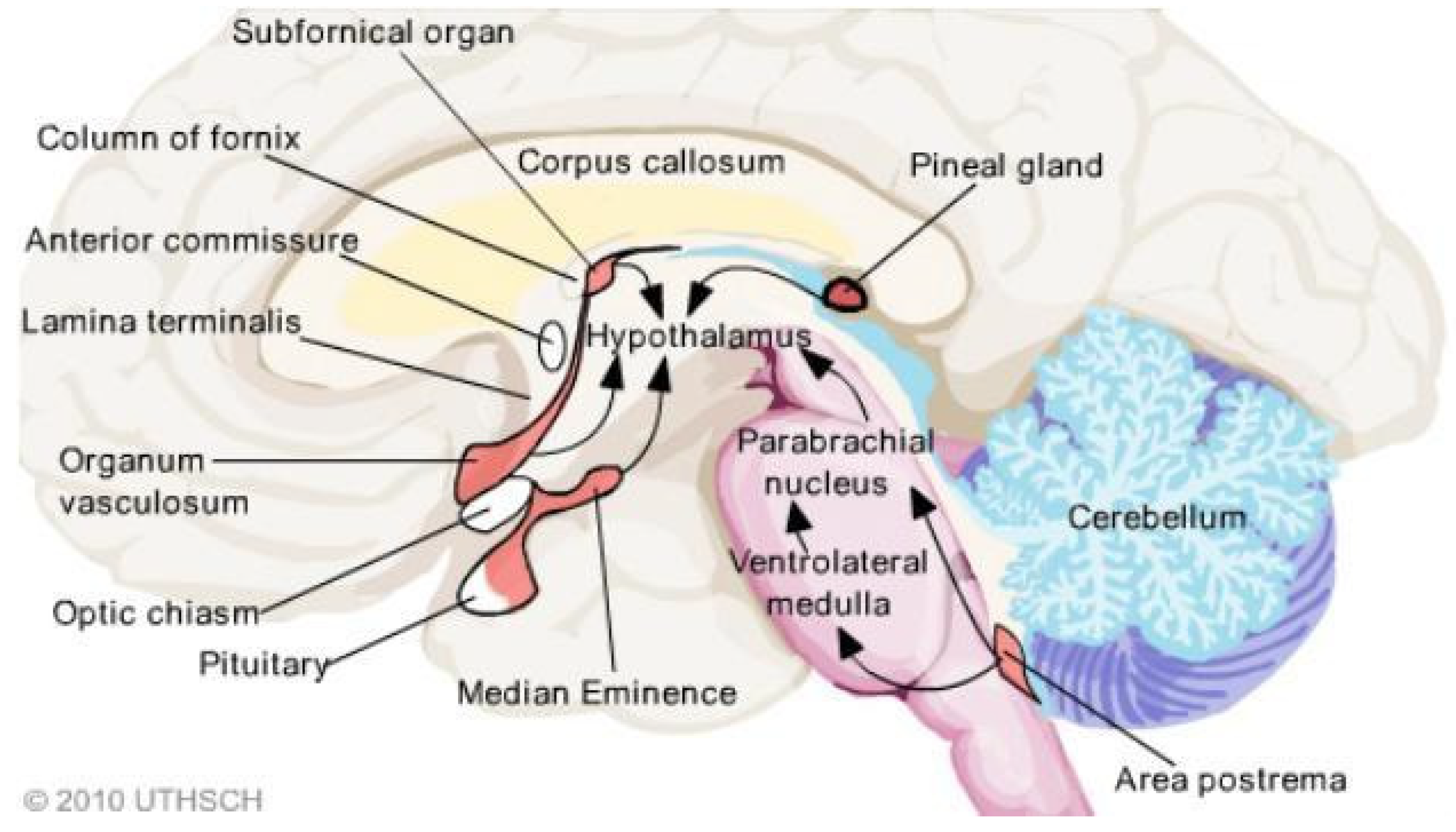
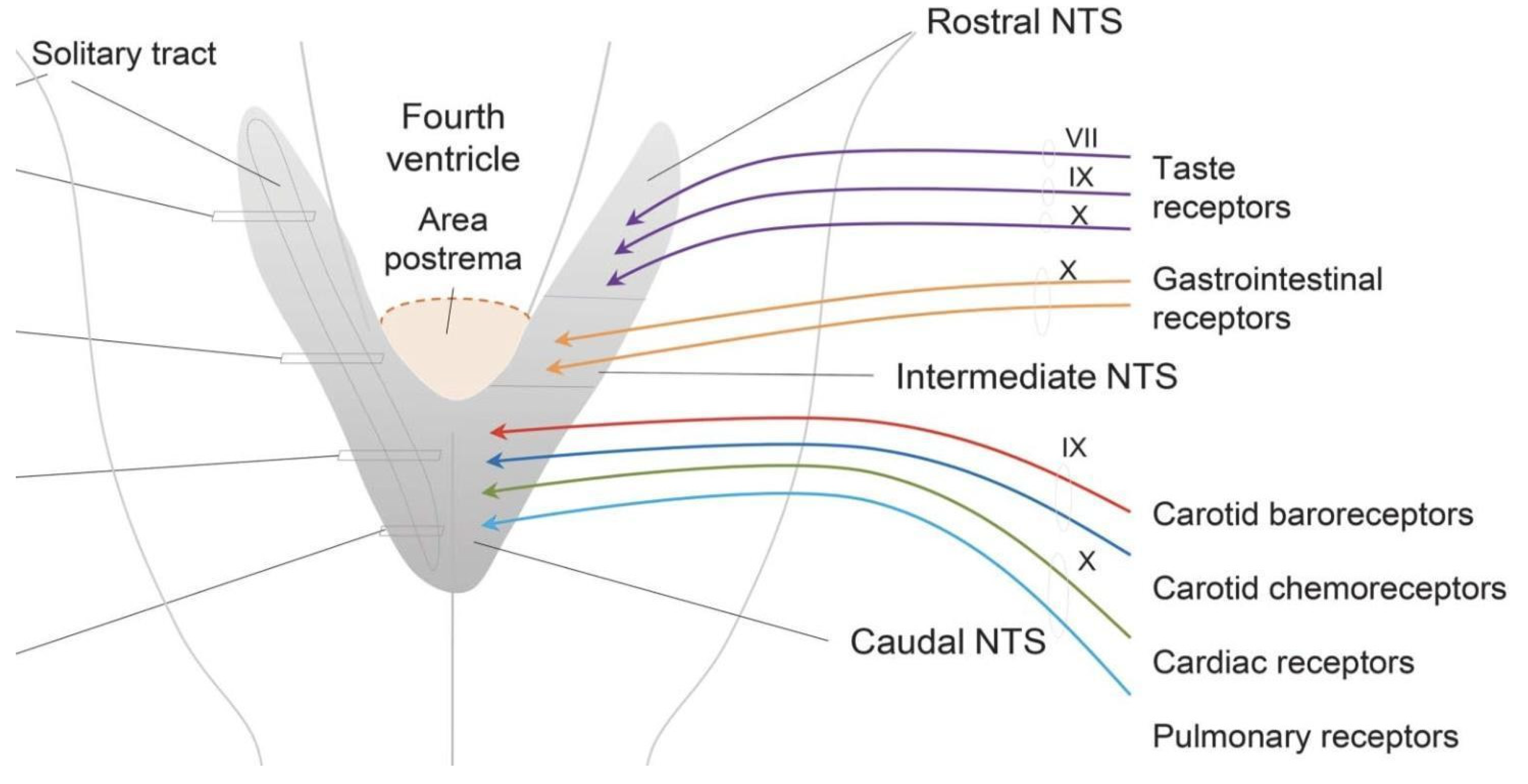
4. CVOs
5. Methylation and Shingles
6. Autoimmunity in Females
7. A Mitochondria and Oxidative Stress
7. B Magnesium and Vitamins
8. Thoughts on Therapy
CONCLUSION
References
- Karst, M.; Hollenhorst, J.; Achenbach, J. Life-threatening course in coronavirus disease 2019 (COVID-19): Is there a link to methylenetetrahydrofolic acid reductase (MTHFR) polymorphism and hyperhomocysteinemia? Med Hypotheses 2020, 144, 110234–110234. [Google Scholar] [CrossRef] [PubMed]
- Ponti, G.; Pastorino, L.; Manfredini, M.; Ozben, T.; Oliva, G.; Kaleci, S.; Iannella, R.; Tomasi, A. COVID-19 spreading across world correlates with C677T allele of the methylenetetrahydrofolate reductase (MTHFR) gene prevalence. J. Clin. Lab. Anal. 2021, 35, e23798. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadeq, D.W.; Nasrallah, G.K. The Spectrum of Mutations of Homocystinuria in the MENA Region. Genes 2020, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, Y.; Li, Y.; Fan, S.; Zhi, X.; Lu, X.; Wang, D.; Zheng, Q.; Wang, Y.; Wang, Y.; et al. Geographical Distribution of MTHFR C677T, A1298C and MTRR A66G Gene Polymorphisms in China: Findings from 15357 Adults of Han Nationality. PLoS ONE 2013, 8, e57917. [Google Scholar] [CrossRef] [PubMed]
- Carpenè, G.; Negrini, D.; Henry, B.M.; Montagnana, M.; Lippi, G. Homocysteine in coronavirus disease (COVID-19): A systematic literature review. Diagnosis 2022, 9, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Regland, B.; Andersson, M.; Abrahamsson, L.; Bagby, J.; Dyrehag, L.E.; Gottfries, C.G. Increased Concentrations of Homocysteine in the Cerebrospinal Fluid in Patients with Fibromyalgia and Chronic Fatigue Syndrome. Scand. J. Rheumatol. 1997, 26, 301–307. [Google Scholar] [CrossRef]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.P.; Reynolds, E.H. Homocysteine, folate, methylation, and monoamine metabolism in depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef]
- Oner, P.; Yilmaz, S.; Doğan, S. High Homocysteine Levels Are Associated with Cognitive Impairment in Patients Who Recovered from COVID-19 in the Long Term. J. Pers. Med. 2023, 13, 503. [Google Scholar] [CrossRef]
- Ren, J.-C.; Wu, Y.-X.; Wu, Z.B.; Zhang, G.-H.; Wang, H.B.; Liu, H.B.; Cui, J.-P.B.; Chen, Q.; Liu, J.; Frank, A.; et al. MTHFR Gene Polymorphism Is Associated With DNA Hypomethylation and Genetic Damage Among Benzene-Exposed Workers in Southeast China. J. Occup. Environ. Med. 2018, 60, e188–e192. [Google Scholar] [CrossRef]
- Fryar-Williams, S. Fundamental Role of Methylenetetrahydrofolate Reductase 677 C → T Genotype and Flavin Compounds in Biochemical Phenotypes for Schizophrenia and Schizoaffective Psychosis. Front. Psychiatry 2016, 7, 172. [Google Scholar] [CrossRef]
- Ashfield-Watt, P.A.; et al. “Methylenetetrahydrofolate reductase 677C-->T genotype modulates homocysteine responses to a folate-rich diet or a low-dose folic acid supplement: A randomized controlled trial”. Am. J. Clin. Nutr. 2002, 76, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.J. Nutrient Power: Heal Your Biochemistry and Heal Your Brain. Skyhorse Publishing 2012 USA.
- Fedorowski, A. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. J. Intern. Med. 2018, 285, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Chadda, K.R.; Blakey, E.E.; Huang, C.L.H.; Jeevaratnam, K. Long COVID-19 and Postural Orthostatic Tachycardia Syndrome- Is Dysautonomia to Be Blamed? Front. Cardiovasc. Med. 2022, 9, 860198. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J. Betrayal by the Brain: The Neurologic Basis of Chronic Fatigue Syndrome, Fibromyalgia Syndrome, and Related Neural Network. 1st edition Routledge 1996 USA.
- Okamoto, L.E.; Raj, S.R.; Peltier, A.; Gamboa, A.; Shibao, C.; Diedrich, A.; Black, B.K.; Robertson, D.; Biaggioni, I. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin. Sci. 2011, 122, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.M.; Glover, J.L.; Medow, M.S. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin. Sci. 2006, 110, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, H.; Murphy, T.A.; Nuss, Z.; Liles, J.; Liles, C.; Aston, C.E.; Raj, S.R.; Fedorowski, A.; Kem, D.C. Angiotensin II Type 1 Receptor Autoantibodies in Postural Tachycardia Syndrome. J. Am. Hear. Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Fedorowski, A.; Li, H.; Yu, X.; Koelsch, K.A.; Harris, V.M.; Liles, C.; Murphy, T.A.; Quadri, S.M.S.; Scofield, R.H.; Sutton, R.; et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace 2016, 19, 1211–1219. [Google Scholar] [CrossRef]
- Badiudeen, T.; Forsythe, E.A.; Bennett, G.; Li, H.; Yu, X.; Beel, M.; Nuss, Z.; Blick, K.E.; Okamoto, L.E.; Arnold, A.C.; et al. A functional cell-based bioassay for assessing adrenergic autoantibody activity in postural tachycardia syndrome. J. Transl. Autoimmun. 2019, 2, 100006. [Google Scholar] [CrossRef]
- Blecher, M. “Receptors, antibodies, and disease”. Clin Chem. 1984, 30, 1137–1356. [Google Scholar] [CrossRef]
- Wirth, K.; Scheibenbogen, C. A Unifying Hypothesis of the Pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Recognitions from the finding of autoantibodies against ß2-adrenergic receptors. Autoimmun. Rev. 2020, 19, 102527. [Google Scholar] [CrossRef]
- Briquez, P.S.; Rouhani, S.J.; Yu, J.; Pyzer, A.R.; Trujillo, J.; Dugan, H.L.; Stamper, C.T.; Changrob, S.; Sperling, A.I.; Wilson, P.C.; et al. Severe COVID-19 induces autoantibodies against angiotensin II that correlate with blood pressure dysregulation and disease severity. Sci. Adv. 2022, 8, eabn3777. [Google Scholar] [CrossRef]
- Barki-Harrington, L.; Luttrell, L.M.; Rockman, H.A. Dual Inhibition of β-Adrenergic and Angiotensin II Receptors by a Single Antagonist. Circulation 2003, 108, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Medow, M.S.; et al. Decreased Microvascular Nitric Oxide–Dependent Vasodilation in Postural Tachycardia Syndrome. Circulation 2005, 112, 2611–2618. [Google Scholar] [CrossRef]
- Stewart, J.M.; Taneja, I.; Glover, J.; Medow, M.S.; Lang, J.A.; Krajek, A.C.; McNeely, B.D.; Meade, R.D.; Fujii, N.; Seely, A.J.E.; et al. Angiotensin II type 1 receptor blockade corrects cutaneous nitric oxide deficit in postural tachycardia syndrome. Am. J. Physiol. Circ. Physiol. 2008, 294, H466–H473. [Google Scholar] [CrossRef] [PubMed]
- Laith, AIK; et al. “Association between the level of Bradykinin and viral infection in patient suffering from respiratory infection, renal transplant, and renal failure”. Ann. Trop. Med. Public Health 22020, 3, SP231203. [Google Scholar]
- Kiowski, W.; Linder, L.; Kleinbloesem, C.; van Brummelen, P.; Bühler, F.R. Blood pressure control by the renin-angiotensin system in normotensive subjects. Assessment by angiotensin converting enzyme and renin inhibition. Circulation 1992, 85, 1–8. [Google Scholar] [CrossRef]
- Persson, P.B. Renin: Origin, secretion and synthesis. J. Physiol. 2003, 552, 667–671. [Google Scholar] [CrossRef]
- Ichihara, A.; Suzuki, H.; Saruta, T. Effects of magnesium on the renin-angiotensin-aldosterone system in human subjects. J. Lab. Clin. Med. 1993, 122, 432–440. [Google Scholar]
- Yeoh, YK, et al “Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19”. Gut 2021, 70, 698–706. [CrossRef]
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front. Immunol. 2022, 12, 628741. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol. Motil. 2016, 29. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Lin, H.-T.V.; Tsai, G.J. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process. Biochem. 2013, 48, 559–568. [Google Scholar] [CrossRef]
- Mayengbam, S.; Chleilat, F.; Reimer, R.A. Dietary Vitamin B6 Deficiency Impairs Gut Microbiota and Host and Microbial Metabolites in Rats. Biomedicines 2020, 8, 469. [Google Scholar] [CrossRef]
- Dev, S.; Mizuguchi, H.; Das, A.K.; Matsushita, C.; Maeyama, K.; Umehara, H.; Ohtoshi, T.; Kojima, J.; Nishida, K.; Takahashi, K.; et al. Suppression of Histamine Signaling by Probiotic Lac-B: A Possible Mechanism of Its Anti-allergic Effect. J. Pharmacol. Sci. 2008, 107, 159–166. [Google Scholar] [CrossRef]
- Yu, X.; Ye, Z.; Houston, C.M.; Zecharia, A.Y.; Ma, Y.; Zhang, Z.; Uygun, D.S.; Parker, S.; Vyssotski, A.L.; Yustos, R.; et al. Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron 2015, 87, 164–178. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Esguevillas, G.; Alonso-Navarro, H.; Zurdo, M.; Turpín-Fenoll, L.; Millán-Pascual, J.; Adeva-Bartolomé, T.; Cubo, E.; Navacerrada, F.; Amo, G.; et al. Gamma-aminobutyric acid (GABA) receptors genes polymorphisms and risk for restless legs syndrome. Pharmacogenomics J. 2018, 18, 565–577. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Restless legs syndrome is associated with long-COVID in women. Sleep Med. 2022, 18, 1413–1418. [Google Scholar] [CrossRef]
- Civelek, G.M.; Ciftkaya, P.O.; Karatas, M. Evaluation of restless legs syndrome in fibromyalgia syndrome: An analysis of quality of sleep and life. J. Back Musculoskelet. Rehabilitation 2014, 27, 537–544. [Google Scholar] [CrossRef]
- Dodson, C.; Bagai, K.; Weinstock, L.B.; Thompson, E.; Okamoto, L.E.; Peltier, A.; Raj, S.R.; Walters, A.S. Restless legs syndrome is increased in postural orthostatic tachycardia syndrome. Sleep Med. 2021, 17, 791–795. [Google Scholar] [CrossRef]
- Jammoul, M.; Naddour, J.; Madi, A.; Reslan, M.A.; Hatoum, F.; Zeineddine, J.; Abou-Kheir, W.; Lawand, N. Investigating the possible mechanisms of autonomic dysfunction post-COVID-19. Auton. Neurosci. 2022, 245, 103071–103071. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Ling, E.-A. The circumventricular organs. Clin Exp Pharmacol Physiol. 2017, 32, 879–892. [Google Scholar] [CrossRef]
- Cytokines Driving Sympathetic Nervous System Activation In The Subfornical Organ: Implications For Heart Failure And Hypertension (2015) Gabriel Bassi https://brainimmune.com/subfornical-organ-heart-failure-hypertension/).
- Cutsforth-Gregory, J.K.; Benarroch, E.E. Nucleus of the solitary tract, medullary reflexes, and clinical implications. Neurology 2017, 88, 1187–1196. [Google Scholar] [CrossRef]
- Page, M.C.; Cassaglia, P.A.; Brooks, V.L.; Erdos, B.; Clifton, R.R.; Liu, M.; Li, H.; McCowan, M.L.; Sumners, C.; Scheuer, D.A.; et al. GABA in the paraventricular nucleus tonically suppresses baroreflex function: Alterations during pregnancy. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R1452–R1458. [Google Scholar] [CrossRef] [PubMed]
- Haam, J.; Popescu, I.R.; Morton, L.A.; Halmos, K.C.; Teruyama, R.; Ueta, Y.; Tasker, J.G. GABA Is Excitatory in Adult Vasopressinergic Neuroendocrine Cells. J. Neurosci. 2012, 32, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, Y.-B.; Kim, J.S.; Bin Kim, W.; Kim, Y.S.; Han, H.C.; Colwell, C.S.; Cho, Y.-W.; Kim, Y.I. GABAergic inhibition is weakened or converted into excitation in the oxytocin and vasopressin neurons of the lactating rat. Mol. Brain 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Unnikrishnan P, Diabetes Insipidus, Dept of Neuroanesthesia SCTIMST, Trivandrum, Kerala, India https://www.uzhnu.edu.ua/uk/infocentre/get/23985.
- Kakizawa, K.; Watanabe, M.; Mutoh, H.; Okawa, Y.; Yamashita, M.; Yanagawa, Y.; Itoi, K.; Suda, T.; Oki, Y.; Fukuda, A. A novel GABA-mediated corticotropin-releasing hormone secretory mechanism in the median eminence. Sci. Adv. 2016, 2, e1501723–1501723. [Google Scholar] [CrossRef]
- Moore, A.M.; Abbott, G.; Mair, J.; Prescott, M.; Campbell, R.E. Mapping GABA and glutamate inputs to gonadotrophin-releasing hormone neurones in male and female mice. J. Neuroendocr. 2018, 30, e12657. [Google Scholar] [CrossRef]
- Wiens, S.C.; Trudeau, V.L. Thyroid hormone and γ-aminobutyric acid (GABA) interactions in neuroendocrine systems. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 332–344. [Google Scholar] [CrossRef]
- McKinley, M.J.; Gerstberger, R.; Mathai, M.L.; Oldfield, B.J.; Schmid, H. The lamina terminalis and its role in fluid and electrolyte homeostasis. J. Clin. Neurosci. 1999, 6, 289–301. [Google Scholar] [CrossRef]
- Miwa, K. Down-regulation of renin–aldosterone and antidiuretic hormone systems in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J. Cardiol. 2017, 69, 684–688. [Google Scholar] [CrossRef]
- Pereira, G.; Gillies, H.; Chanda, S.; Corbett, M.; Vernon, S.D.; Milani, T.; Bateman, L. Acute Corticotropin-Releasing Factor Receptor Type 2 Agonism Results in Sustained Symptom Improvement in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Syst. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Coffin, S.T.; Black, B.K.; Biaggioni, I.; Paranjape, S.Y.; Orozco, C.; Black, P.W.; Dupont, W.D.; Robertson, D.; Raj, S.R. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Hear. Rhythm. 2012, 9, 1484–1490. [Google Scholar] [CrossRef]
- Diep, P.-T.; Chaudry, M.; Dixon, A.; Chaudry, F.; Kasabri, V. Oxytocin, the panacea for long-COVID? a review. Horm. Mol. Biol. Clin. Investig. 2022, 43, 363–371. [Google Scholar] [CrossRef]
- Amir, S. Stimulation of the paraventricular nucleus with glutamate activates interscapular brown adipose tissue thermogenesis in rats. Brain Res. 1990, 508, 152–155. [Google Scholar] [CrossRef]
- Nikesjö, F.; Sayyab, S.; Karlsson, L.; Apostolou, E.; Rosén, A.; Hedman, K.; Lerm, M. Defining post-acute COVID-19 syndrome (PACS) by an epigenetic biosignature in peripheral blood mononuclear cells. Clin. Epigenetics 2022, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Balnis, J.; Madrid, A.; Hogan, K.J.; Drake, L.A.; Adhikari, A.; Vancavage, R.; Singer, H.A.; Alisch, R.S.; Jaitovich, A. Persistent blood DNA methylation changes one year after SARS-CoV-2 infection. Clin. Epigenetics 2022, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sarı, I.K.; Keskin, O.; Keskin, A.S.; Ellidağ, H.Y.; Harmandar, O. Is Homocysteine Associated with the Prognosis of Covid-19 Pneumonia. Int. J. Clin. Pr. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- American Myalgic Encephalomyelitis and Chronic Fatigue Syndrome Society https://ammes.org/diet/.
- Waltz, E. Could long COVID be linked to herpes viruses? Early data offer a hint. Nature 2022. [Google Scholar] [CrossRef]
- Cruz, C.; Della Rosa, M.; Krueger, C.; Gao, Q.; Horkai, D.; King, M.; Field, L.; Houseley, J.; The Babraham Institute; Kingdom, U. Tri-methylation of histone H3 lysine 4 facilitates gene expression in ageing cells. eLife 2018, 7. [Google Scholar] [CrossRef]
- De Benedetti, F.; Prencipe, G.; Bracaglia, C.; Marasco, E.; Grom, A.A. Targeting interferon-γ in hyperinflammation: Opportunities and challenges. Nat. Rev. Rheumatol. 2021, 17, 678–691. [Google Scholar] [CrossRef]
- Varghese, J.; Sandmann, S.; Ochs, K.; Schrempf, I.-M.; Frömmel, C.; Dugas, M.; Schmidt, H.H.; Vollenberg, R.; Tepasse, P.-R. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuklina, E.M. T Lymphocytes as Targets for SARS-CoV-2. Biochem. (Moscow) 2022, 87, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Bossi, F.; Peerschke, E.I.; Ghebrehiwet, B.; Tedesco, F. Cross-talk between the complement and the kinin system in vascular permeability. Immunol. Lett. 2011, 140, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Hao, Z.-W.; Wang, Y.-F. The effect of estrogen in coronavirus disease 2019. Am. J. Physiol. Cell. Mol. Physiol. 2021, 321, L219–L227. [Google Scholar] [CrossRef]
- Herrera, A.Y.; Hodis, H.N.; Mack, W.J.; Mather, M. Estradiol Therapy After Menopause Mitigates Effects of Stress on Cortisol and Working Memory. J. Clin. Endocrinol. Metab. 2017, 102, 4457–4466. [Google Scholar] [CrossRef]
- Stelzig, K.E.; Canepa-Escaro, F.; Schiliro, M.; Berdnikovs, S.; Prakash, Y.S.; Chiarella, S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1280–L1281. [Google Scholar] [CrossRef]
- Mendes, G.M.d.M.; Nascimento, I.J.B.D.; Marazzi-Diniz, P.H.; Da Silveira, I.B.; Itaborahy, M.F.; Viana, L.E.; Silva, F.A.; Santana, M.F.; Pinto, R.A.; Dutra, B.G.; et al. The des-Arg9-bradykinin/B1R axis: Hepatic damage in COVID-19. Front. Physiol. 2022, 13, 1080837. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G.; van Deuren, M.; van der Meer, J.W.; de Mast, Q.; Brüggemann, R.J.; van der Hoeven, H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020, 9. [Google Scholar] [CrossRef]
- Thomas, R.L.; Jiang, L.; Adams, J.S.; Xu, Z.Z.; Shen, J.; Janssen, S.; Ackermann, G.; Vanderschueren, D.; Pauwels, S.; Knight, R.; et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Wood, E.; et al. “Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 'long-haulers'? ” Chronic Dis Transl Med. 2021, 7, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Riera, J.J.; Schousboe, A.; Waagepetersen, H.S.; Howarth, C.; Hyder, F. The micro-architecture of the cerebral cortex: Functional neuroimaging models and metabolism. NeuroImage 2008, 40, 1436–1459. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, N.; Koeva, Y. Hydrohysteroid Dehydrogenases—Biological Role and Clinical Importance—Review; Canuto, R.A., Ed.; Dehydrogenases IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Chambers, P. Antioxidants and Long Covid. OALib 2022, 9, 1–19. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef]
- Bikle, DD. “Vitamin D metabolism, mechanism of action, and clinical applications”. Chem Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef] [PubMed]
- Hadtstein, F.; Vrolijk, M. Vitamin B-6-Induced Neuropathy: Exploring the Mechanisms of Pyridoxine Toxicity. Adv. Nutr. Int. Rev. J. 2021, 12, 1911–1929. [Google Scholar] [CrossRef]
- Rose, DP. “The interactions between vitamin B6 and hormones”. Vitam Horm. 1978, 36, 53–99. [Google Scholar] [CrossRef]
- Sfera, A.; Thomas, K.G.; Sasannia, S.; Anton, J.J.; Andronescu, C.V.; Garcia, M.; Sfera, D.O.; Cummings, M.A.; Kozlakidis, Z. Neuronal and Non-Neuronal GABA in COVID-19: Relevance for Psychiatry. Reports 2022, 5, 22. [Google Scholar] [CrossRef]
- Campbell, D.J.; Kladis, A.; Valentijn, A.J. Effects of Losartan on Angiotensin and Bradykinin Peptides and Angiotensin-Converting Enzyme. J. Cardiovasc. Pharmacol. 1995, 26, 233–240. [Google Scholar] [CrossRef]
- Singh, PK; et al. “Increased plasma bradykinin level is associated with cognitive impairment in Alzheimer's patients”. Neurobiol Dis. 2020, 139, 104833. [Google Scholar] [CrossRef]
- Zaheer, J.; Kim, H.; Kim, J.S. Correlation of ACE2 with RAS components after Losartan treatment in light of COVID-19. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Kehoe, P.G.; Wong, S.; AL Mulhim, N.; Palmer, L.E.; Miners, J.S. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-β and tau pathology. Alzheimer's Res. Ther. 2016, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chambers, P. “Long Covid and Neurodegenerative Disease”. J Path Lab Med, /: https, 3673. [Google Scholar]
- Yang, T.; Yang, Y.; Wang, D.; Li, C.; Qu, Y.; Guo, J.; Shi, T.; Bo, W.; Sun, Z.; Asakawa, T. The clinical value of cytokines in chronic fatigue syndrome. J. Transl. Med. 2019, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X; et al. “TGF-β1 factor in the cerebrovascular diseases of Alzheimer's disease”. Eur Rev Med Pharmacol Sci. 2016, 20, 5178–5185.
- Zhang, Z.; Liu, C.; Gan, Z.; Wang, X.; Yi, Q.; Liu, Y.; Wang, Y.; Lu, B.; Du, H.; Shao, J.; et al. Improved Glucose-Stimulated Insulin Secretion by Selective Intraislet Inhibition of Angiotensin II Type 1 Receptor Expression in Isolated Islets of db/db Mice. Int. J. Endocrinol. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Chang, Y.-C.; Wu, L.-C.; Lin, J.-W.; Chuang, L.-M.; Lai, M.-S. Different angiotensin receptor blockers and incidence of diabetes: A nationwide population-based cohort study. Cardiovasc. Diabetol. 2014, 13, 91–91. [Google Scholar] [CrossRef]
- Villapol, S.; Saavedra, J.M. Neuroprotective Effects of Angiotensin Receptor Blockers. Am. J. Hypertens. 2014, 28, 289–299. [Google Scholar] [CrossRef]
- Hoffman, L.B.; Schmeidler, J.; Lesser, G.T.; Beeri, M.S.; Purohit, D.P.; Grossman, H.T.; Haroutunian, V. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology 2009, 72, 1720–1726. [Google Scholar] [CrossRef]
- van Dalen, J.W.; Marcum, Z.A.; Gray, S.L.; Barthold, D.; van Charante, E.P.M.; van Gool, W.A.; Crane, P.K.; Larson, E.B.; Richard, E. Association of Angiotensin II–Stimulating Antihypertensive Use and Dementia Risk. Neurology 2020, 96, e67–e80. [Google Scholar] [CrossRef]
- Arvin, AM; et al. “Varicella-zoster virus: Aspects of pathogenesis and host response to natural infection and varicella vaccine”. Adv Virus Res. 1996, 46, 263–309. [Google Scholar] [CrossRef]
- Hasan, T.; Arora, R.; Bansal, A.K.; Bhattacharya, R.; Sharma, G.S.; Singh, L.R. Disturbed homocysteine metabolism is associated with cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.A.; Jiang, P.; Chan, C.W.M.; Sun, K.; Wong, J.; Hui, E.P.; Chan, S.L.; Chan, W.C.; Hui, D.S.C.; Ng, S.S.M.; et al. Noninvasive detection of cancer-associated genome-wide hypomethylation and copy number aberrations by plasma DNA bisulfite sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 18761–18768. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rawat, A.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ashangari, C.; Suleman, A. Abstract 121: Vitamin D Deficiency Study in Postural Orthostatic Tachycardia Syndrome. Circ. Cardiovasc. Qual. Outcomes 2015, 8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




