1. Introduction
Coagulation factor XIII (FXIII) present in the plasma (pFXIII) is a tetrameric pro-transglutaminase that consists of two potentially active A subunits (FXIII-A) and two inhibitory/protective B subunits (FXIII-B). The dimer of FXIII-A is also expressed in several cell types (cFXIII; FXIII-A
2), including platelets, megakaryocytes, monocytes, macrophages, dendritic cells, chondrocytes, osteoblasts, preadipocytes, corneal keratocytes. The transformation of pFXIII into an active transglutaminase (FXIIIa) needs proteolytic removal of an activation peptide from the N-terminus of FXIII-A and Ca
2+ induced dissociation of FXIII-B from the tetramer. For the cellular form the elevation of intracellular Ca
2+ concentration is sufficient to bring about the active configuration. The function of FXIII in the plasma has been elucidated a long time ago. In addition to mechanically stabilizing fibrin and protecting it from fibrinolytic degradation, it is essential for maintaining pregnancy, it is important for proper wound healing and it is involved in angiogenesis. Earlier and more recent data on the structure and functions of FXIII are reviewed in references [
1,
2,
3,
4,
5,
6].
In the eighties we reported for the first time that monocytes and peritoneal macrophages express FXIII-A but not FXIII-B [
7,
8]. This finding was soon confirmed in McDonagh’s laboratory [
9]. FXIIIa is a member of the transglutaminase family, other members (transglutaminase 1-7) are involved in a number of transglutaminases related functions, an excellent review on transglutaminases in monocytes and macrophages is provided in reference [
10]. The transglutaminase activity in monocytes from FXIII-A deficient patients was below the limit of detection and only traces of tissue transglutaminase (TGM2) could be detected in freshly prepared, non-stimulated monocytes [
11,
12]. However, the TGM2 content rapidly increased during culturing or stimulation of the cells [
12]. Monocyte/macrophage cFXIII might exert both intracellular, and if becomes externalized, extracellular function. Intracellular FXIIIa activity supports phagocytosis mediated by the Fc region of IgG and complement receptor
[13]. cFXIII, lacking signal peptide is not secreted by the usual secretory pathway, however, it could become externalized through alternative mechanisms. An unorthodox secretory mechanism is suggested by the finding that in monocytes and macrophages in association with Golgi vesicles FXIIIa is directed to the plasma membrane [
14]. Most recently it was shown that FXIII-A becomes externalized and accumulated on the membrane of human monocytes in response to stimulation by IL-4 or IL-10 and becomes capable of exerting extracellular functions [
15].
Macrophages by up-taking lipids, particularly oxidized LDL (oxLDL), are easily transformed into foam cells. Foam cells play a vital role in the initiation of atherosclerosis and in the development of atherosclerotic plaque. In the present study we investigated how the transformation of macrophages into foam cells influences the cFXIII content of such cells. A further question was if foam cells developed from other cells, particularly from vascular smooth muscle cells (VMSCs), also express cFXIII. We also explored the distribution of FXIII-A in the atherosclerotic plaque and attempted to verify the presence of complex protein structures cross-linked by FXIIIa.
2. Results
2.1. Investigation of Macrophage and HAoSMC Derived Foam Cells by Immunofluorescens Microscopy for FXIII-A Expression and LDL Ingestion
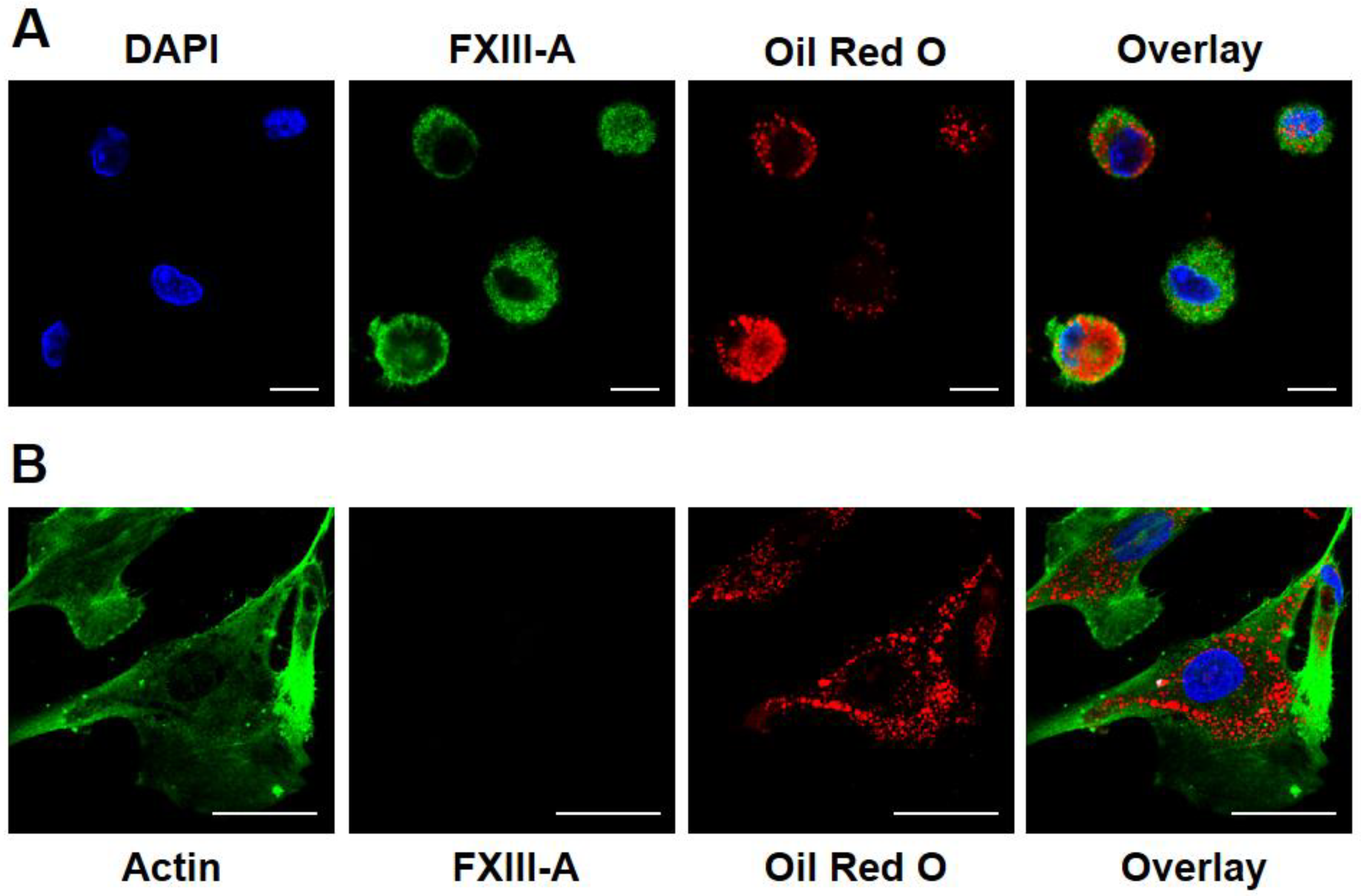
First, we investigated if FXIII-A is retained in macrophages undergoing transition into foam cells by immunohistochemistry. On Figure 1A four macrophages, all intensively stained for FXIII-A are shown. These macrophages accumulated oxLDL to different extent. In cells with considerable accumulation of lipid particles (two macrophages on the left side of the picture) FXIII-A became marginalized in the cytoplasm. Evidently the ingested lipid particles occupied a significant part of the central cytoplasm pushing other cytoplasmic constituents toward the sub-membranous region.
In addition to macrophages several other cell types present in the atherosclerotic plaque might potentially accumulate lipids and could get transformed into foam cells. Vascular smooth muscle cells, other major constituents of the atherosclerotic plaque, are also capable of transformation into foam cells. oxLDL is a relatively poor inducer of such transformation, but these cells can easily uptake enzyme-modified LDL (eLDL) that had been digested by trypsin plus cholesterol-esterase. The uptake of eLDL particle by human aortic smooth muscle cells (HAoSMCs), showing positive staining for actin, is demonstrated on
Figure 1B. Their transformation into foam cells, however, was not accompanied by the expression of FXIII-A.
2.2. Transformation of Macrophages into Foam Cells Results in Elevated Expression of Cellular FXIII
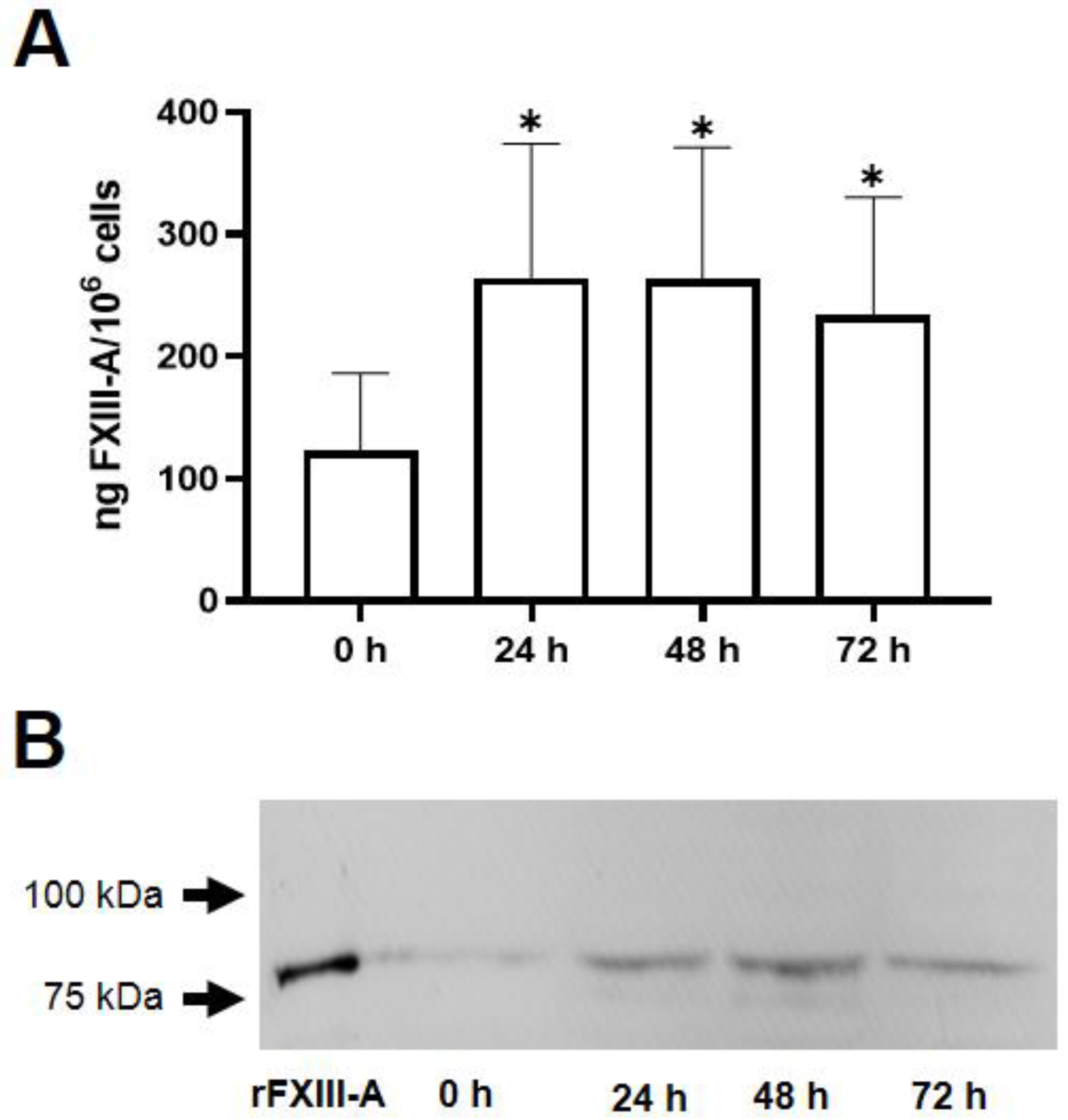
The next question we addressed was if the ingestion of oxLDL particle influence the FXIII-A content of the macrophages. Using FXIII-A ELISA it was shown that 24 hours after a single dose of oxLDL the formed foam cells exhibited more than double FXIII-A as compared to their non-transformed counterparts (
Figure 2A). The elevated FXIII-A content only slightly decreased during the following 48 hours. These results were confirmed by Wester blotting technique, as well. The Western blot shown on
Figure 2B also demonstrates that FXIII-A content of macrophages considerably increased 24 hours after the ingestion of oxLDL and only slightly decreased afterwards.
2.3. Macrophages and FXIII-A in the Atherosclerotic Plaque
After demonstrating that macrophage derived foam cells contain a considerable amount of cellular FXIII we explored if FXIII-A is present in the atherosclerotic plaque, and if yes, it is of intracytoplasmic and/or extracellular localization.
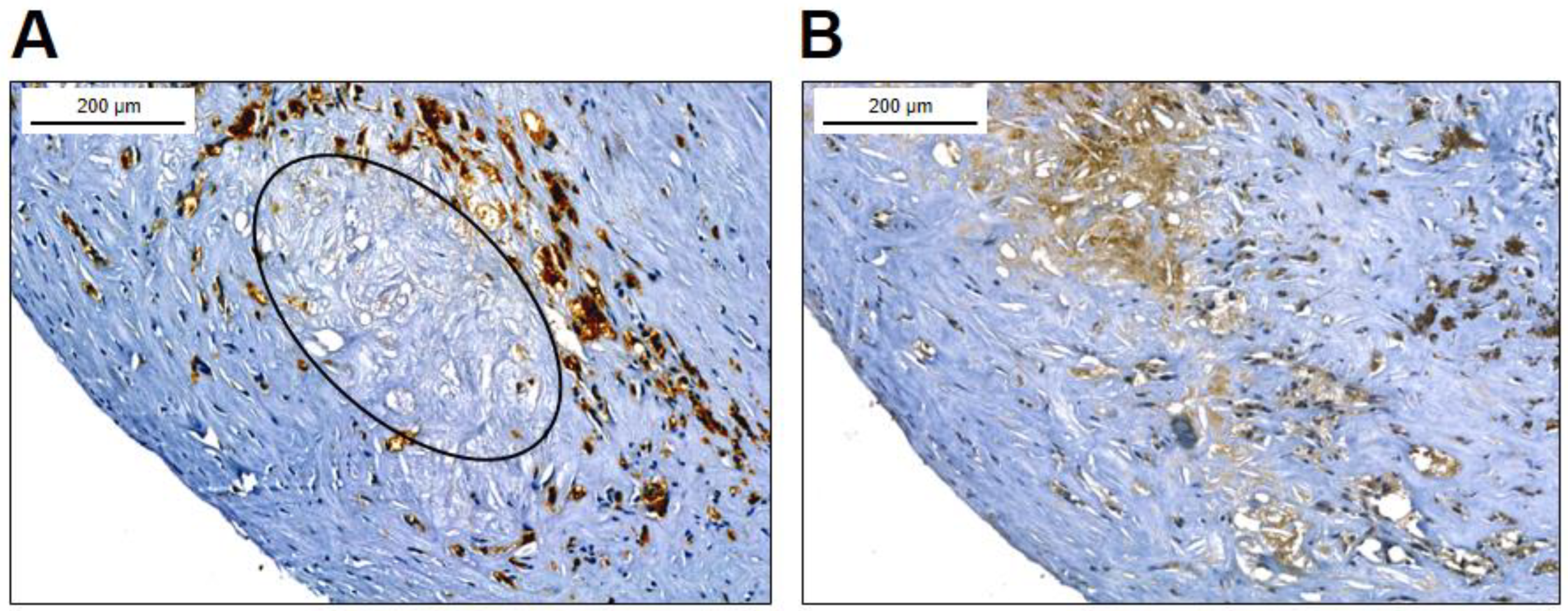
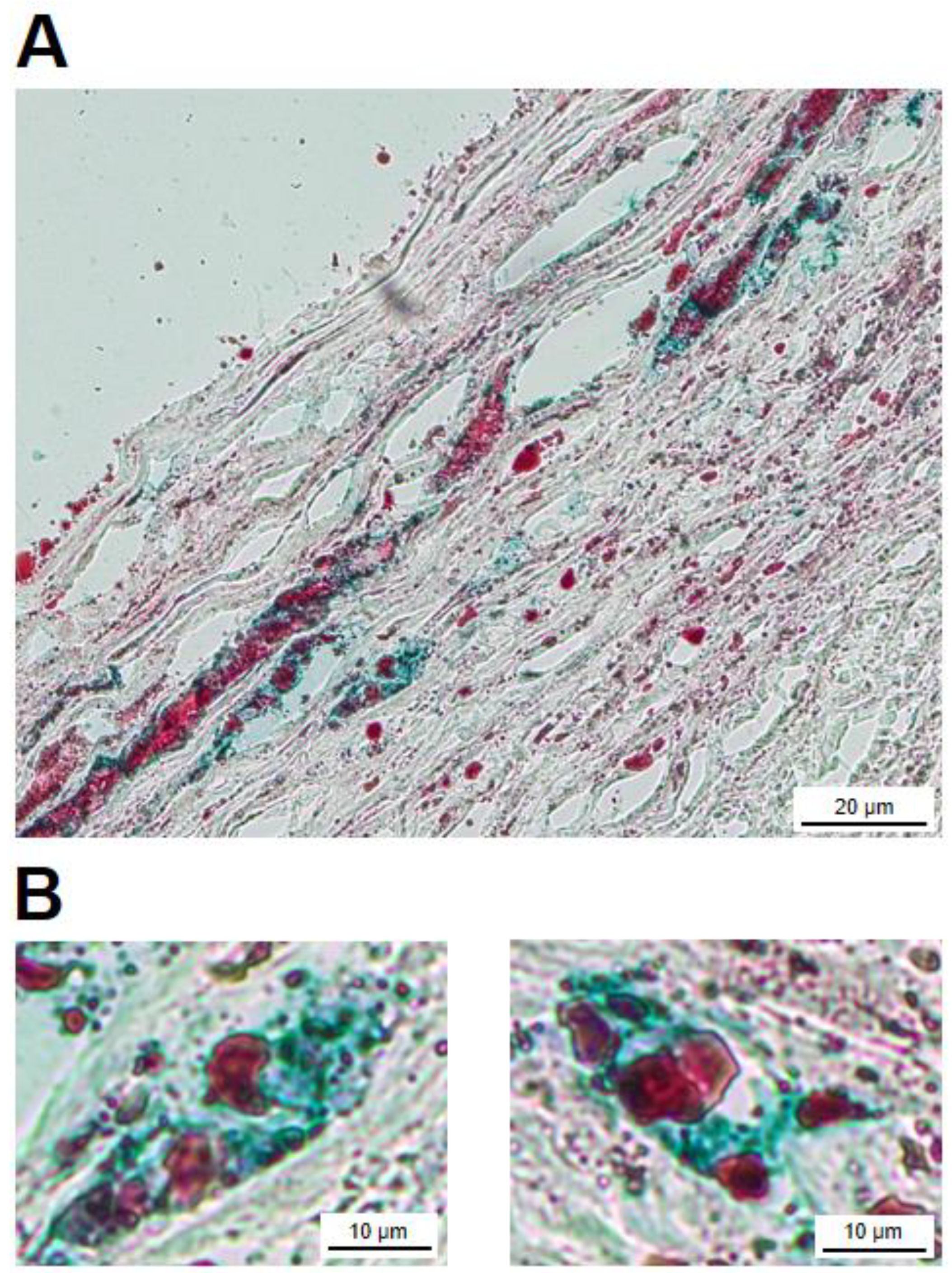
Figure 3A demonstrates a type IV carotid artery plaque with a lipid core and surrounding CD68 positive macrophages. Another plaque stained for FXIII-A is shown on
Figure 3B. Here, beside cells expressing FXIII-A staining of extracellular component can also be observed. More detailed localization of FXIII-A in the plaque is demonstrated on
Figure 4.
2.4. Visualization of FXIII-A and Isopeptide Cross-links within the Atherosclerotic Plaque by Immunohistochemistry
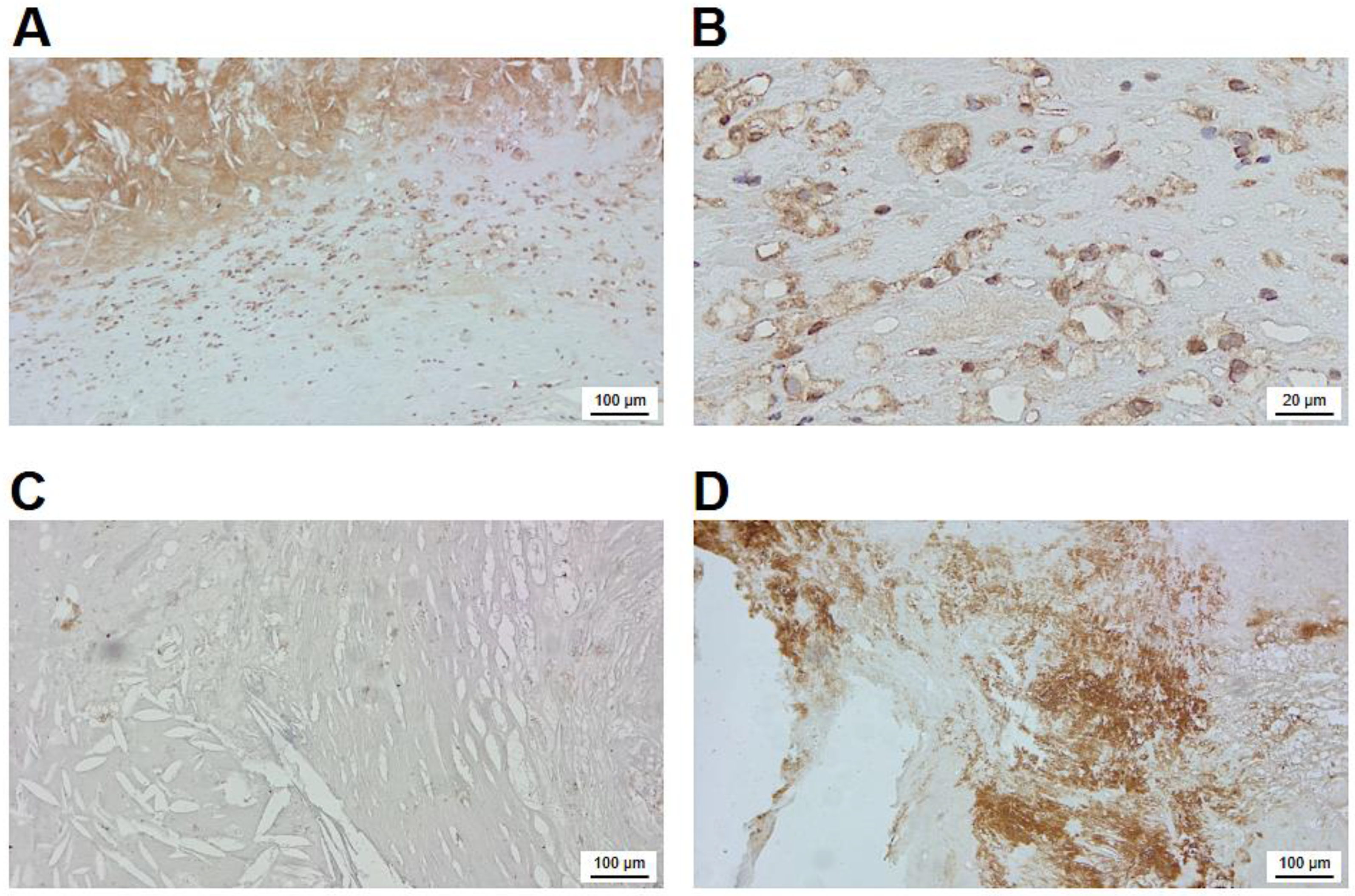
Figure 4 provides a detailed overview on the immunohistochemical localization of FXIII-A in the atherosclerotic plaque. The intense intra-cytoplasmatic brown immune-peroxidase staining demonstrates that FXIII-A is present in numerous macrophage-like cells underneath the lipid core (Figure 4A). In many of these cells empty non-stained part of the cytoplasm (Figure 4B) indicates that lipids that had been ingested by the cells were solubilized and removed by solvents used for the fixation/staining procedure. The intense extracellular staining of the lipid containing core for FXIII-A suggests that FXIII-A derived from the plasma and/or released from apoptotic/necrotic macrophages are bound to core constituents. The possibility of non-specific binding of primary or secondary antibodies used for the visualization of FXIII-A was excluded by the lack of staining in experiments with negative controls (Figure 4C).
A further question was if FXIII present in the atherosclerotic plaque was active and, as an active transglutaminase, was it involved in cross-linking proteins. Using a specific antibody that detects ε-(𝛾-glutamyl)lysyl bonds it was shown that the non-cellular part of the plaque is loaded by cross-linked protein structures (
Figure 4D). This result clearly indicates that FXIII is not just present in the atherosclerotic plaque, but it actively contributes to its structurization.
Figure 4.
Immunohistochemical visualization of FXIII-A and cross-linked protein structures in the atherosclerotic plaque. (A) FXIII-A of cellular and extracellular localization in the atherosclerotic plaque. (B) FXIII-A containing macrophages in the plaque at higher magnification. (C) Negative control for FXIII-A staining. (D) Protein mass cross-linked through Nε-(𝛾-L-glutamyl)-L-lysyl bonds in a non-cellular part of the sclerotic plaque.
Figure 4.
Immunohistochemical visualization of FXIII-A and cross-linked protein structures in the atherosclerotic plaque. (A) FXIII-A of cellular and extracellular localization in the atherosclerotic plaque. (B) FXIII-A containing macrophages in the plaque at higher magnification. (C) Negative control for FXIII-A staining. (D) Protein mass cross-linked through Nε-(𝛾-L-glutamyl)-L-lysyl bonds in a non-cellular part of the sclerotic plaque.
2.5. FXIII-A Containing Foam Cells within the Atherosclerotic Plaque
After establishing the presence of FXIII-A containing cells in the atherosclerotic plaque and its cross-linked protein products it was attempted to show that part of the FXIII-A containing macrophages housing the plaque were transformed into foam cells. In cryosections Oil Red O (ORO) stained droplets are observed both in the intracellular and extracellular compartments (
Figure 5A). Several cells show co-staining for ORO and anti-FXIII-A antibody. Cells depicted at higher magnification clearly demonstrate such a combination (
Figure 5B). This finding shows that FXIII-A containing foam cells are not only in vitro experimental products, but also exist in vivo in the atherosclerotic plaque.
3. Discussion
Macrophages are multipotent, multifunctional cells which may undergo considerable transformation in response to various inducers. In different environmental conditions they may remain non-polarized or by different polarizing agents they could be transformed into cells with pro-inflammatory M1 or anti-inflammatory M2 phenotype [
16]. Recent studies suggested a more detailed classification, and distinguished among CD14
++/CD16
-, CD14
+/CD16
++ and CD14
++/CD16
+ cells representing classical, non-classical and intermediate subtypes [
17]. Most recently single cell RNA sequencing technique made it possible to identify three main clusters of macrophages in the atherosclerotic plaque [
18]. The resident-like macrophages, with a phenotype resembling the M2 subtype can infiltrate the plaque. The inflammatory macrophages are non-foamy subtypes which are the major source of inflammatory cytokines. The triggering receptor expressed on myeloid cell-2, due their impaired cholesterol efflux capacity are lipid-laden cells, also resemble M2 phenotype. Macrophages are affected by a number of factors in the vessel wall which may influence their actual dynamic state of polarization[
19].
Monocyte/macrophages are capable of up-taking lipids and transform into foam cells. Foam cells play a major role in the initiation and progression of atherosclerosis. Going through apoptosis, autophagy, necroptosis and pyroptosis they provide the major source of the necrotic core in the atherosclerotic plaque. In addition to macrophages, in certain conditions vascular smooth muscle cells (VSMCs), stem/progenitor cells and endothelial cells might also ingest lipids and become transformed into foam cells [
20,
21]. VSMCs derived foam cells undergoing phenotypic transformation represent a considerable proportion, approximately 40%, of such cell type in the plaque [
22]. As shown on
Figure 1 both macrophage and VSMC derived cells are capable of ingesting lipid particles. As the capacity of VSMCs for ingesting oxLDL is low, in these experiments we used eLDL which was easily taken up by HAoSMCs. HAoMSCs do not express FXIII-A and its transformation into foam cells did not change the situation. This finding suggests that transformation into foam cells is not responsible for the additional FXIII-A acquired by macrophage derived foam cells, it is not the characteristic of foam cell formation, in general.
FXIII-A content of macrophages show a drastic increase when stimulated by interleukin-4, while interferon γ fails to elicit such a change [
23,
24], i.e., FXIII-A content of the cells is drastically different in polarized M1 and M2 phenotypes. As the up-take of oxLDL also induces macrophage differentiation and activation toward M2 phenotype [
25], we were interested how the cellular FXIII-A content is influenced by the transformation of macrophages into foam cells. In our experiments the FXIII-A level of the non-polarized macrophages became more than double following the ingestion of oxLDL particles. Although longer and more robust stimulation by IL-4 induced considerably higher increase of cell-associated FXIII-A [
23], it would be interesting to study how polarizing agents would influence FXIII-A level in foam cells.
In tissue sections a rather abundant cell population was stained for FXIII-A. Most of the FXIII-A+ cells appear as macrophages derived foam cells; the empty part of the cells suggests the solubilization of lipid particless during fixation/staining. Indeed, combination of staining for FXIII-A with the lipid stain ORO clearly demonstrated that FXIII-A and lipid droplets could be found within the same cell population. In the cryosections a considerable non-cellular area of the atherosclerotic plaque also showed intensive staining for FXIII-A. FXIII-A in the sclerotic core are very likely derived from foam cells that lost their integrity and participate in building up the necrotic core. A further question was if FXIII present in the atherosclerotic plaque is in an active form, i.e., is it functional and cross-links substrate proteins. The finding that FXIII is upregulated on the surface of human monocytes in response to stimulation by IL-4 and IL-10 suggests that it might be present in a surface associated form as an active transglutaminase in the plaque [
15]. It is also very likely that cFXIII becomes released from disintegrating cFXIII containing cells in an active form. The main extracellular function of FXIII in tissues is the cross-linking of substrate proteins through iso-peptide bonds. Using an antibody that specifically detects iso-peptide bonds we were able to detect cross-linked protein structures in the atherosclerotic plaque that shows that FXIIIa exerts its transglutaminase activity in the extracellular compartment. Further studies exploring the location and pathological consequences of these cross-linked structures are required to shed light on the role of FXIII and its cross-linking activity in the pathological mechanism of atherosclerosis.
4. Materials and Methods
4.1. Culturing of Macrophages and Induction of Foam Cell Formation
Human buffy coat from healthy donors was obtained from the Hungarian National Blood Transfusion Service. Its use for macrophage and macrophage derived foam cell preparation was approved by the Ethics Review Board of the University of Debrecen, Faculty of Medicine in accordance with the Helsinki Declaration. It was diluted with an equal volume of sterile phosphate-buffered saline containing 5 mM EDTA (PBS-EDTA). 30 mL of the cell suspension was layered onto 15 mL of Histopaque-1077® (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged at 400 × g for 30 min at 25 °C. Peripheral blood mononuclear cells (PBMCs) were then aspirated and washed twice with PBS-EDTA. Monocytes were isolated by negative selection based magnetic cell sorting (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) according to the manufacturer’s protocol. To differentiate into macrophages, monocytes were cultured in RPMI 1640 medium containing 2 mM L-glutamine and 25 mM HEPES (Life Technologies, Waltham, MA, USA), 10 µg/mL gentamycin (Krka, d. d., Novo mesto, Novo mesto, Slovenia), 10 % fetal bovine serum (FBS, Life Technologies, Grand Island, NY, USA) and 50 ng/mL granulocyte macrophages-colony stimulating factor (GM-CSF, Life Technologies, Carlsbad, CA, USA) for 3 days at 37 °C in 5% CO2 humidified air. Foam cells were then generated from macrophages under the same conditions by incubation with 50 µg/mL oxLDL (Life Technologies, Eugene, OR, USA) for 3 days. Cells were cultured at concentration of 106 cells/mL in Teflon dishes to keep cells in suspension and used for the detection/measurement of cFXIII by enzyme-linked immunosorbent assay (ELISA) and Western blotting techniques.
4.2. Immunofluorescent Analysis of Macrophage Derived Foam Cells
For immunofluorescent studies cells were seeded onto non-treated, 8-well Lab-Tek™ chamber slides (Nunc™, Thermo Fisher Scientific, Rochester, NY, USA) at 5 x 10
5 cells/well density. RPMI 1640 medium was removed, and cells were washed three times with PBS. Adherent cells were fixed in 3.7% paraformaldehyde for 30 min followed by rinsing with PBS. Non-specific IgG binding was blocked by normal human serum (EMD Millipore Corporation, Burlington, MA, USA) for 15 min. Foam cells were stained with rabbit anti-human FXIII-A antibody [
26] for 60 min and DyLight 488-labeled goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA, USA) was used as a secondary antibody for 45 min. Then, slides were washed, and staining with ORO (Sigma-Aldrich) was performed for 15 min, followed by washing with distilled water three times. To counterstain nuclei, Vectashield® mounting medium with DAPI (Vector Laboratories) was used. Staining steps were carried out at room temperature in the dark. Slides were investigated with Zeiss LSM 700 confocal microscope (Zeiss, Oberkochen, Germany) and solid-state diode lasers (405 nm, 488 nm, and 555 nm). Detection of the fluorescence signals was performed by selective laser excitation coupled to efficient splitting of the emitted light using variable secondary dichroic (VSD) beam-splitter.
4.3. Preparation of Enzyme-Modified LDL (eLDL)
A slight modification of the method described by Bhakdi et al. [
27] was used for the generation of eLDL. Briefly, human native LDL (density 4 mg/mL) from plasma of healthy donors was isolated by ultracentrifugation [
28]. LDL was diluted to 2 mg/ml in 20 mM HEPES, 150 mM NaCl, 2 mM CaCl
2, pH 7.0. For enzymatic modification, LDL was digested with 4 µg/mL trypsin from bovine pancreas (Sigma-Aldrich) at 37 °C for 6 hours and with 24 µg/ml cholesterol-esterase from Pseudomonas sp. (Sigma-Aldrich) for an additional 6 hours at 37 °C. Then another 4 µg/ml trypsin and 36 µg/ml cholesterol-esterase were added, and the mixture was incubated at 37 °C for 24 hours. Finally, trypsin activity was blocked by 10 µg/mL soybean trypsin inhibitor (Roche Diagnostics, Mannheim, Germany) for 60 min at 37 °C and modified LDL was dialyzed against PBS.
4.4. Human Aortic Smooth Muscle Cell (HAoSMC) Derived Foam Cell Formation
HAoSMCs were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). Naive cells (donor: 38 years old healthy Caucasian male) were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Gibco™, Thermo Fisher Scientific) containing 1 mM sodium-pyruvate, 2 mM L-glutamine, 10 % FBS and 1 % gentamycin in T75 flask at 37 °C in 5% CO2 humidified air. Cells were grown to 90% confluence and used at passage 8 then, seeded onto non-treated 4-well chamber slides (Nunc™, Thermo Fisher Scientific) under the same conditions for 24 hours. At the end of the incubation, adherent HAoSMCs were washed three times with PBS and treated with 75 µg/mL eLDL or with native LDL in DMEM medium for further 24 hours.
4.5. Immunofluorescent Analysis of HAoSMCs Derived Foam Cells
After a 24-hour treatment by eLDL, DMEM medium was removed and slides were washed three times with PBS. Cells were fixed by a 58:2 mixture of methanol and acetic acid for 15 min. Cells were washed again and then blocking with normal human serum was performed for 15 min. HAoSMCs were stained by rabbit anti-human FXIII-A antibody (1:200 dilution) or mouse anti-human alpha-smooth muscle actin antibody (1:250 dilution, Thermo Fisher Scientific) for 60 min. After washing with PBS, DyLight 488-labeled goat anti-rabbit or DyLight 488-labeled goat anti-mouse secondary antibodies were added for 45 min. To visualize eLDL uptake by HAoSMCs, ORO staining was carried out for 15 min, then cells were washed with distillated water. Finally, slides were mounted by Vectashield® mounting medium with DAPI and investigated using Zeiss LSM 700 confocal microscope.
4.6. Quantification of FXIII-A by ELISA in Macrophage Derived Foam Cells
For the detection of FXIII-A, cells from macrophage and macrophage derived foam cells cultures were removed every day and centrifuged at 200 ×
g for 10 min at 25 °C. Pellets were resuspended in the mixture of PBS, 1% Triton, 120 µg/mL 2-methyl-4-isothiazolin-3-one (MIT, Sigma-Aldrich), 1x SIGMAFAST™ Protease inhibitor (Sigma-Aldrich). Samples were stored at +4 °C until measurement. FXIII-A antigen concentration was determined by sandwich ELISA [
29]. Results were adjusted to 10
6 cells. Statistical analysis was carried out by GraphPad Prism 8.01. Distribution of the results was investigated by the Kolmogorov-Smirnov test and paired
t-test was used for calculating the level of significance.
p < 0.05 was considered statistically significant. Means represent the results of 5 measurements.
4.7. Western Blotting
Cultured cells were collected daily and centrifuged at 200 × g for 10 min at 25 °C. Cell pellets were resuspended in SDS-PAGE sample buffer containing 8 M urea, then denatured in boiling water for 5 min. After reduction by adding 5% 2-mercaptoethanol proteins of equal number of lysed cells were separated by SDS-PAGE (7.5% gel), followed by transfer to PVDF membrane. Affinity purified sheep anti-FXIII-A antibody (1:3000 dilution, Affinity Biologicals, Ancaster, Canada), biotinylated anti-sheep IgG (1:1000 dilution, Vector Laboratories), avidin-biotinylated peroxidase complex (Vector Laboratories) and ECL chemiluminescent reagent (ECL Plus+, Amersham, Little Chalfont, UK) were used for the immune reaction detecting FXIII-A in the cell lysates. Results were compared to that obtained with 100 ng of recombinant FXIII-A (Novo Nordisk A/S, Bagsvaerd, Denmark). Biotinylated SDS-PAGE standard (Bio-Rad, Hercules, CA, USA) was used as molecular weight standard.
4.8. Investigation of Atherosclerotic Plaque by Immunohistochemistry
Tissue fragments harvested by conventional transluminal angioplasty from patients diagnosed with symptomatic carotid artery (CA) atherosclerosis were fixed in 4% formaldehyde and embedded in paraffin. Histological features of carotid plaques were examined in 5 µm sections stained with hematoxylin and eosin. Using the criterion of the American Heart Association [
30], clinically relevant type IV plaques (known also as “atheroma”) were selected for further investigations. Macrophages were visualized by immunohistochemistry using anti-CD68 mouse monoclonal antibody, clone KP1 (Immunologic, Duiven, The Netherlands). Anti-FXIII-A rabbit polyclonal antibody was used (Thermo Fisher Scientific, Fermont, CA, USA) for the detection of cellular and extracellular localization of FXIII-A. In parallel experiments Nε-(𝛾-L-glutamyl)-L-lysyl isopeptide bonds were detected by an antibody purchased from Covalab (Villeurbanne, France). EnVision FLEX/HRP (Agilent, Dako Santa Clara, CA, USA) was used as secondary antibody in combination with 3,3'-diaminobenzidine chromogen (DAB) substrate to give the reaction product a brown color. Nuclei were counterstained with hematoxylin. For negative control, normal serum was substituted for the primary antibody.
4.9. Lipids in FXIII-A Positive Cells of the Atherosclerotic Plaque
Neutral lipids were visualized in cryosections according to the protocol for ORO staining solution. Anti-FXIII-A primary antibody in combination with ORO was used for demonstrating the intracellular presence of FXIII-A in foam cells. Briefly, after fixation in isopropyl alcohol and endogenous peroxidase blocking, polyclonal antibody against FXIII-A was added and incubated overnight in thermostat at 56 ○C. After subsequent washing, secondary antibody was added (30 min), which was followed by immersion in ready to use ORO solution for 5 min. The slides were then rinsed with running tap water, and color development was carried out by PolyDetector HRP Green (Bio SB, Santa Barbara, CA, USA) following the manufacturer’s instructions. FXIII-A positive macrophages shown in green were considered positive also for ORO when red intracytoplasmic vacuoles were present.
Author Contributions
Conceptualization, L.S., N.J.M., L.M.; data curation, H.B., É.K. and J.B.; methodology, L.S., E.H., H.B., B.B., D.P. and É.K.; investigation, L.S., E.H., H.B., B.B., D.P., É.K. and L.M.; writing-original draft preparation, L.S. and L.M.; writing-review and editing, E.H., H.B., J.B., N.J.M. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by grants from the National Research, Development and Innovation Office (NKFIH) (K129287), by the GINOP 2.3.2-15-2016-00050 project co-financed by the European Union and the European Regional Development Fund and by the Hungarian Academy of Science (11014 project). JB and DP were supported by Eötvös Loránd Research Network (11003). The work was also supported by the University of Aberdeen Development Trust and by project grants from Friend of Anchor (RS2015 006), the British Heart Foundation (PG/15/82/31721) and a British Heart Foundation Fellowship (FS/11/2/28579) awarded to N.J.M.
Institutional Review Board Statement
This study on tissue sections was conducted according to the principles of the Helsinki Declaration and was approved by the Institutional Review Board of County Emergency Clinical Hospital of Targu Mures, Romania (no. 29496/2019).
Informed Consent Statement
Written informed consent was obtained from each patient involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are indebted to Gizella Haramura (Faculty of Medicine, University of Debrecen, Debrecen, Hungary), Ágnes Bana (Faculty of Medicine, University of Debrecen, Debrecen, Hungary) and Dr. István Szász (Faculty of Medicine, University of Debrecen, Debrecen, Hungary) for the expert assistance. We also thank to Andrea Kovács-Paluska (Faculty of Medicine, University of Debrecen, Debrecen, Hungary) for her technical help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Komaromi, I.; Bagoly, Z.; Muszbek, L. Factor XIII: novel structural and functional aspects. J Thromb Haemost 2011, 9, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Komaromi, I.; Katona, E. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev 2011, 91, 931–972. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, F.S.M.; Whyte, C.S.; Mutch, N.J. Factor XIII-A: An Indispensable "Factor" in Haemostasis and Wound Healing. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.L.; Mutch, N.J. Let's cross-link: diverse functions of the promiscuous cellular transglutaminase factor XIII-A. J Thromb Haemost 2019, 17, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, V.; Kohler, H.P. Factor XIII: Structure and Function. Semin Thromb Hemost 2016, 42, 422–428. [Google Scholar] [PubMed]
- Byrnes, J.R.; Wolberg, A.S. Newly-Recognized Roles of Factor XIII in Thrombosis. Semin Thromb Hemost 2016, 42, 445–454. [Google Scholar] [CrossRef]
- Muszbek, L.; Adany, R.; Szegedi, G.; Polgar, J.; Kavai, M. Factor XIII of blood coagulation in human monocytes. Thromb Res 1985, 37, 401–410. [Google Scholar] [CrossRef]
- Adany, R.; Belkin, A.; Vasilevskaya, T.; Muszbek, L. Identification of blood coagulation factor XIII in human peritoneal macrophages. Eur J Cell Biol 1985, 38, 171–173. [Google Scholar]
- Henriksson, P.; Becker, S.; Lynch, G.; McDonagh, J. Identification of intracellular factor XIII in human monocytes and macrophages. J Clin Invest 1985, 76, 528–534. [Google Scholar] [CrossRef]
- Sun, H.; Kaartinen, M.T. Transglutaminases in Monocytes and Macrophages. Med Sci (Basel) 2018, 6. [Google Scholar] [CrossRef]
- Muszbek, L.; Adany, R.; Kavai, M.; Boda, Z.; Lopaciuk, S. Monocytes of patients congenitally deficient in plasma factor XIII lack factor XIII subunit a antigen and transglutaminase activity. Thromb Haemost 1988, 59, 231–235. [Google Scholar] [CrossRef]
- Murtaugh, M.P.; Arend, W.P.; Davies, P.J. Induction of tissue transglutaminase in human peripheral blood monocytes. J Exp Med 1984, 159, 114–125. [Google Scholar] [CrossRef]
- Sarvary, A.; Szucs, S.; Balogh, I.; Becsky, A.; Bardos, H.; Kavai, M.; Seligsohn, U.; Egbring, R.; Lopaciuk, S.; Muszbek, L.; Adany, R. Possible role of factor XIII subunit A in Fcgamma and complement receptor-mediated phagocytosis. Cell Immunol 2004, 228, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Cordell, P.A.; Kile, B.T.; Standeven, K.F.; Josefsson, E.C.; Pease, R.J.; Grant, P.J. Association of coagulation factor XIII-A with Golgi proteins within monocyte-macrophages: implications for subcellular trafficking and secretion. Blood 2010, 115, 2674–2681. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, F.S.M.; Whyte, C.S.; Tuncay, A.; Williams, M.L.; Wilson, H.M.; Mutch, N.J. Monocytes Expose Factor XIII-A and Stabilize Thrombi against Fibrinolytic Degradation. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.F.; Lappalainen, J.; Lee-Rueckert, M.; Kovanen, P.T. Conversion of human M-CSF macrophages into foam cells reduces their proinflammatory responses to classical M1-polarizing activation. Atherosclerosis 2016, 248, 170–178. [Google Scholar] [CrossRef]
- Nagenborg, J.; Goossens, P.; Biessen, E.A.L.; Donners, M. Heterogeneity of atherosclerotic plaque macrophage origin, phenotype and functions: Implications for treatment. Eur J Pharmacol 2017, 816, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front Cardiovasc Med 2022, 9, 845942. [Google Scholar] [CrossRef] [PubMed]
- Lee-Rueckert, M.; Lappalainen, J.; Kovanen, P.T.; Escola-Gil, J.C. Lipid-Laden Macrophages and Inflammation in Atherosclerosis and Cancer: An Integrative View. Front Cardiovasc Med 2022, 9, 777822. [Google Scholar] [CrossRef]
- Gomez, D.; Owens, G.K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 2012, 95, 156–164. [Google Scholar] [CrossRef]
- Tang, J.; Wang, H.; Huang, X.; Li, F.; Zhu, H.; Li, Y.; He, L.; Zhang, H.; Pu, W.; Liu, K.; Zhao, H.; Bentzon, J.F.; Yu, Y.; Ji, Y.; Nie, Y.; Tian, X.; Zhang, L.; Gao, D.; Zhou, B. Arterial Sca1(+) Vascular Stem Cells Generate De Novo Smooth Muscle for Artery Repair and Regeneration. Cell Stem Cell 2020, 26, 81–96 e4. [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef]
- Torocsik, D.; Bardos, H.; Nagy, L.; Adany, R. Identification of factor XIII-A as a marker of alternative macrophage activation. Cell Mol Life Sci 2005, 62, 2132–2139. [Google Scholar] [CrossRef]
- Torocsik, D.; Szeles, L.; Paragh, G., Jr.; Rakosy, Z.; Bardos, H.; Nagy, L.; Balazs, M.; Inbal, A.; Adany, R. Factor XIII-A is involved in the regulation of gene expression in alternatively activated human macrophages. Thromb Haemost 2010, 104, 709–717. [Google Scholar] [CrossRef]
- Rios, F.J.; Koga, M.M.; Pecenin, M.; Ferracini, M.; Gidlund, M.; Jancar, S. Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediators Inflamm 2013, 2013, 198193. [Google Scholar] [CrossRef]
- Adany, R.; Bardos, H.; Antal, M.; Modis, L.; Sarvary, A.; Szucs, S.; Balogh, I. Factor XIII of blood coagulation as a nuclear crosslinking enzyme. Thromb Haemost 2001, 85, 845–851. [Google Scholar] [PubMed]
- Bhakdi, S.; Torzewski, M.; Klouche, M.; Hemmes, M. Complement and atherogenesis: binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler Thromb Vasc Biol 1999, 19, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Petho, D.; Gall, T.; Hendrik, Z.; Nagy, A.; Beke, L.; Gergely, A.P.; Mehes, G.; Toth, C.; Gram, M.; Akerstrom, B.; Balla, G.; Balla, J. Ferryl Hemoglobin and Heme Induce A(1)-Microglobulin in Hemorrhaged Atherosclerotic Lesions with Inhibitory Function against Hemoglobin and Lipid Oxidation. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Katona, E.E.; Ajzner, E.; Toth, K.; Karpati, L.; Muszbek, L. Enzyme-linked immunosorbent assay for the determination of blood coagulation factor XIII A-subunit in plasma and in cell lysates. J Immunol Methods 2001, 258, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).