1. Introduction
Oral Nicotine Pouches (ONPs) are a type of emerging smokeless tobacco product sold by some large tobacco companies [
1]. Starting from Scandinavia, the selling of ONPs quickly spread to many other countries including the European Union, the UK, the US, and Japan [
1,
2,
3]. The US Food and Drug Administration (FDA) closely describes these oral pouches as “ground, cut, leaf or powdered tobacco regularly available in moist or chew gum snuff often packed in a pre-portioned pouch [
1,
4]. The modern ONPs differ from traditional smokeless tobacco called snus, these newer ONPs are tobacco-free with no tobacco leaf material, instead are constructed of nicotine mined from tobacco leaf and marketed in various forms, including, chewable tablets, nicotine pouches, gums, and lozenges [
5,
6]. The appearance of these tobacco-free pouches is similar to that of snus and similarly, the ONPs are placed in a user’s anterior maxillary vestibule (between the gum and the lip) for chemicals to be released and absorbed [
2,
3]. Since 2016 the US [
2,
7] market consists of nicotine pouches that do not contain tobacco leaves in the final product, and since 2018 in Europe [
2,
8,
9].
In spite of smokeless tobacco is prohibited in some countries, a relatively higher intake of these products has been reported in Sweden and US [
10], as 1800s subjects with traditional usage of snus has been reported in Sweden [
10]. Prime components of snus pouches noted are air or sun-cured tobacco, salt, water, and food-grade flavorings [
6]. Whereas, the ONPs are plant-based fibers boosted by flavorings, nicotine, and other ingredients [
11,
12]. In ONPs, the flavor is marked as an important aspect since the availability of flavors is believed to be the reason why ONPs attracted youth and are used broadly [
13].
In the current scenario, the usage of ONPs serves as a gateway to quitting cigarette smoking. Referring to this fact, evidence states that the first Nicotine Replacement Therapy (NRT) was reported in the form of chewing gum, which was manifested to reduce or prevent symptoms from smoking abstinence [
14,
15]. NRTs are considered to be a short-term intervention with a motive to assist individuals to switch cigarette smoking substituting nicotine supplied by cigarettes [
14]. In this context, a study of 133 trials on various NRTs stated that smokeless tobacco products like ONPs, lozenges, chewing gums, sublingual tablets, nasal sprays, etc. exhibited an increase of 50%–60% rate of successful halting among smokers who are influenced to quit smoking [
14,
16]. However, the rate of successful cessation is not elevated for maximum smokers who just attempt the NRTs [
12].
Since December 2020 in US, none of the oral nicotine products is regulated as the FDA has not granted any authorization for ONPs to be sold as altered-risk tobacco products [
17]. The FDA carries full authority to limit the usage of wrong or deceiving claims in advertising of ONPs that may encourage users that intake of ONPs is harmless [
18]. To prevent addiction and related health issues in individuals due to tobacco products, the FDA finalized the “Deeming Tobacco Products to Be Subject to the Federal Food, Drug, and Cosmetic Act” (the “Deeming Rule”) in 2016 [
19]. This rule established the regulatory authority of the FDA on all tobacco products that include tobacco-derived nicotine (TDN) so that manufacturers of these products need to have the products undergo pre-market assessments, submit product information, and obey the restrictions [
19,
20]. However, the “Deeming Rule” did not include tobacco-free nicotine (TFN) products until the FDA amended the official definition of tobacco products into “any product made or derived from tobacco or containing nicotine from any source, that is intended for human consumption” to include TFN products [
21]. As a result, ONPs are completely under the regulation of the FDA now and all ONPs without a pre-market application (PMTA) would be removed from the market regardless of whether the nicotine content is TDN or TFN [
19,
21].
Over time, marketing of ONPs has increased substantially in the United States, as per reports the commercial market share of ONPs in the US jumped from 0.9% in 2018, to 4.0% in 2019 [
22,
23]. Reports also state that adolescents display high interest in the latest newer smokeless non-tobacco ONPs due to their resemblance with the preferred food products such as chewing gums and their accessibility in appealing flavors [
13,
22]. An illustrative check over among adolescents and Dutch adults has recognized about 0.06% present and 0.56% ever customers of ONPs [
24]. In the United Kingdom (UK) among past and present smokers or e-cigarette users, 15.9% of participants were aware of ONPs among which 2.7% were present users and 4.4% were ever consumers [
25].
Various prime tobacco companies, including Altria, Swedish Match, and RJ Reynolds, are presently marketing ONPs and lozenges, offering flavors within various ranges of nicotine content [
13,
26]. There are various local vendors involved similar to Snus, ONPs are also available in a multitude of flavors like fruit, dessert, citrus, mint, coffee, berry, and wintergreen, this contributes to the prevalence of ONP utilization in the US. [
27]. Modern non-tobacco ONPs are also involved in enrolling advertising and marketing perspectives, digital marketing campaigns, and marketing themes projecting minimal harm, especially in attracting the youth population [
28].
Social behavioral aspects of oral nicotine pouches
The sociological result of oral nicotine products including vaping and pouches (smokeless products) through habitualization, commercialization, and normalization is a
socially accepted illusion [
29]. According to Berger and Luckmann (1966), a person’s sense of reality is socially constructed through human interactions, and those interactions involve repeated exposures and engagements with other habitual participants in the same or similar habit. As a result, a behavioral pattern or set of patterns becomes validated, thus starting a process called habitualization [
30,
31]. In a recent study, Clarke et al. (2021) stated that these products including “e-cigarette devices and vaping fluids demonstrably contain a series of both definite and probable oncogenic responses by nicotine derivatives”. This includes benzo(a)pyrene and nitrosamines for oral, gastric, and liver effects [
32]. Similar aspect can be attributed to flavoring compounds. However, this type of information does not usually reach public communities, especially where teenagers, adolescents and other vulnerable populations congregate [
22,
33,
34]. In fact, the half-truth and deceiving statements specifically the message that harmlessness (non-toxic) of pouches/vaping exist in comparison to the effects of both cigarette/tobacco smoking [
35] Over a span of time, those half-truths and deliberate lies about addiction risks, and the physical effects of pouches/vaping and e-cigarettes, become realities among individuals and collective groups in society. Nitzkin (2014) emphasized the fact that “the tobacco-control movement is now the party deceiving the public through unfounded speculation and outright lies as to the risk posed by nicotine addictiveness to teen non-smokers (or withdrawal of nicotine polyproduts) due to perceptions behavioral effects [
22,
33,
34,
35]. Therefore, the beginning of a socially accepted untruth became a firmly held norm. Once an untruth becomes normalized, a shared illusion develops and spreads through continued interactions containing the same or similar messages.
Commercialization, perceptions, and illusions of nicotine products
The commercialization of the illusion strengthens its influence through a constant buying-in by consumers to the false claims stated by producers and advocates of nicotine addiction through pouches and vaping. According to Boyer et al. (2020), “Vaping has been marketed as a safer alternative than smoking cigarettes, but safety data are lacking”, which can be extrapolated to ONPs [
1,
31]. In other words, inflated claims about the supposed innocence of nicotine influence the choices made by end users currently. As a result, demoralization occurs in which realizations of untruths arouse moral outrage that breeds distrust among community members and toward the organizations that participate in the spreading of deceptive or misleading messages about these products use by the deception of fruit and mint flavors [
1,
31]. For example, Boyer et al. (2020) emphasized the fact that “Addiction is central to the JUUL business model”. In other words, an intention to deceive candidates into becoming future nicotine users, and current ENDS users, exists and continues to influence public communities through marketing strategies and de-emphasizing of harmful effects [
31]. The most likely reason for the intention to deceive others in this way involves the profit motive including the gateway to new users by assuming that these products are not harmful.
Once an untruth becomes socially accepted and validated through positive reinforcement, the process of social normalization sets in allowing the untruth behind the illusion to
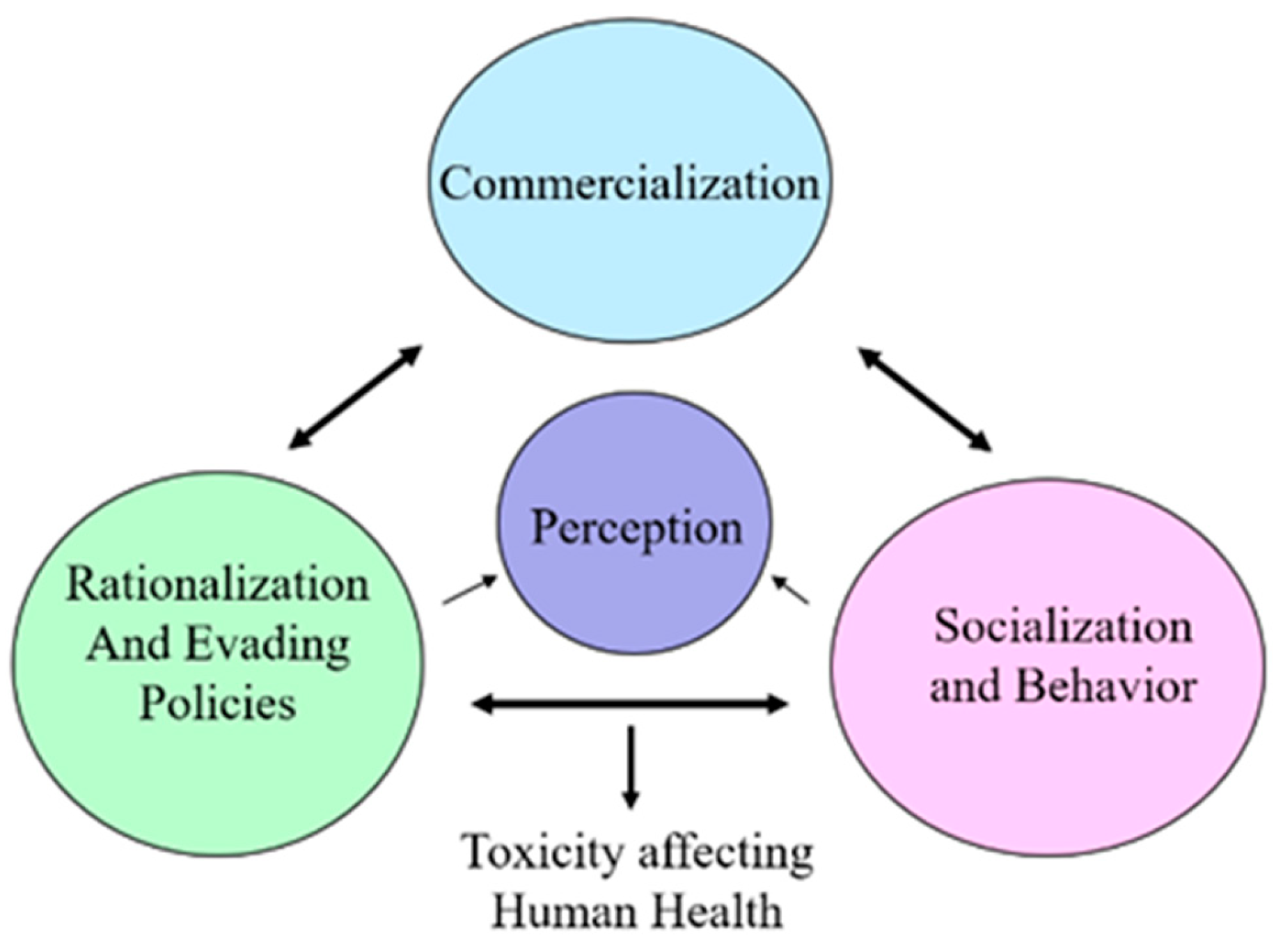
become truthful itself. At a point in time, the pattern of deception becomes embedded into the organizational culture of the companies that produce or derive benefits from advocating vape use, thus leading to further demoralization once the untruth is revealed and moral outrage erupts. Ashforth and Anand (2003) provided a pyramid that illustrated three aspects of organizational normalization [
29].
It may be surmised based on these aspects of this concept based Ashforth and Anand, 2003 as projected in
Figure 1.
- (1)
Commercialization is the process by which unregulated policies are enacted as a matter of routine, often without conscious thought about their propriety or based on scientific knowledge;
- (2)
Rationalization, or evading policies, involves the process by which individuals who engage in usage accept socially constructed accounts that artificially legitimate the acts in their own eyes and perceptions; and
- (3)
Socialization, or behavior changes, involve the process by which newcomers are thought to perform and accept the usage as a norm or alternative to smoking.
The problems created by the socially accepted illusion of perceived harmlessness toward nicotine products deserve significant consideration in future studies on ethical responsibility, organizational functioning, leadership accountability, and health concerns for these users. Researchers in toxicology and medicine continue to intellectually fight for better delivery of all information to these consumers so that all affected by this phenomenon can make more informed decisions about nicotine products' social and bodily impacts on individuals and societal members. The untruth behind the use of illusory advertising to influence others into nicotine product consumption requires an undoing of its framework by regulatory agencies.
In order to examine the ONPs marketplace and enforce FDA as well as local flavor restrictions, we previously tried to classify and categorize snus and non-tobacco (Synthetic) ONPS using the flavor wheel [
27]. The current study is an effort to communicate several research gaps and address regulatory challenges for the usage of ONPs. The aim was the achieve the following goals:1) expanding and enhancing the existing flavor wheel of snus and non-tobacco (synthetic) flavor wheel distributing them among the availability of flavors and various brands in US and Europe. 2) to encounter the availability of common flavors among snus and synthetic ONPs and then utilize use this semantic database to classify and identify various ONPs flavors sold online. 3) Given that both the ONP market and the regulatory environment have been rapidly progressing, our ONP flavor semantic database could be a very convenient tool to identify and classify flavors in existing-era and could also be useful for policymakers and researchers to survey the marketplace and check consent for flavor restrictions. Thus, the findings of the present study could be utilized not only to notify potential policies but also to crystalize future research directions regarding ONPs flavors.
2. Materials and Methods
Utilizing the semantic database, we classified over 152 snus and 228 synthetic ONPs that we gathered from an online store. Considering that each store may characterize or outline ONP flavors differently, we accessed the flavor information employing different methods like : (1) The source from which the flavor database is built includes Snusdirect, which is the website that provides access to consumers in North America and has the widest selection of products, (2) extracting and distributing the flavors of both snus and synthetic ONPs directly from the product website, which is presumably the most accurate, (3) extracting flavor categories in the brand website, (4) flavor description in the product website, and (5) consensus made between authors about flavors. Products are usually categorized on brand websites. Location distribution analysis for the brands producing ONPs is conducted by referencing the following two sources: (1) the brand website domain locations, which represent the target markets for their products; (2) company locations, which indicate the origin of the brands. Numerous companies utilize words that describe possible sensations and experiences for consumers to name their products, and judgment would be made based on the product description in such cases. Previously we constructed two-wheel diagrams, each consisting of one kind of product, constructed with flavors being color-coded, on which the Flavor distribution analysis is done for synthetic and snus ONPs separately and is expanded in the current study.
3. Results
ONPs distributors USA vs Europe
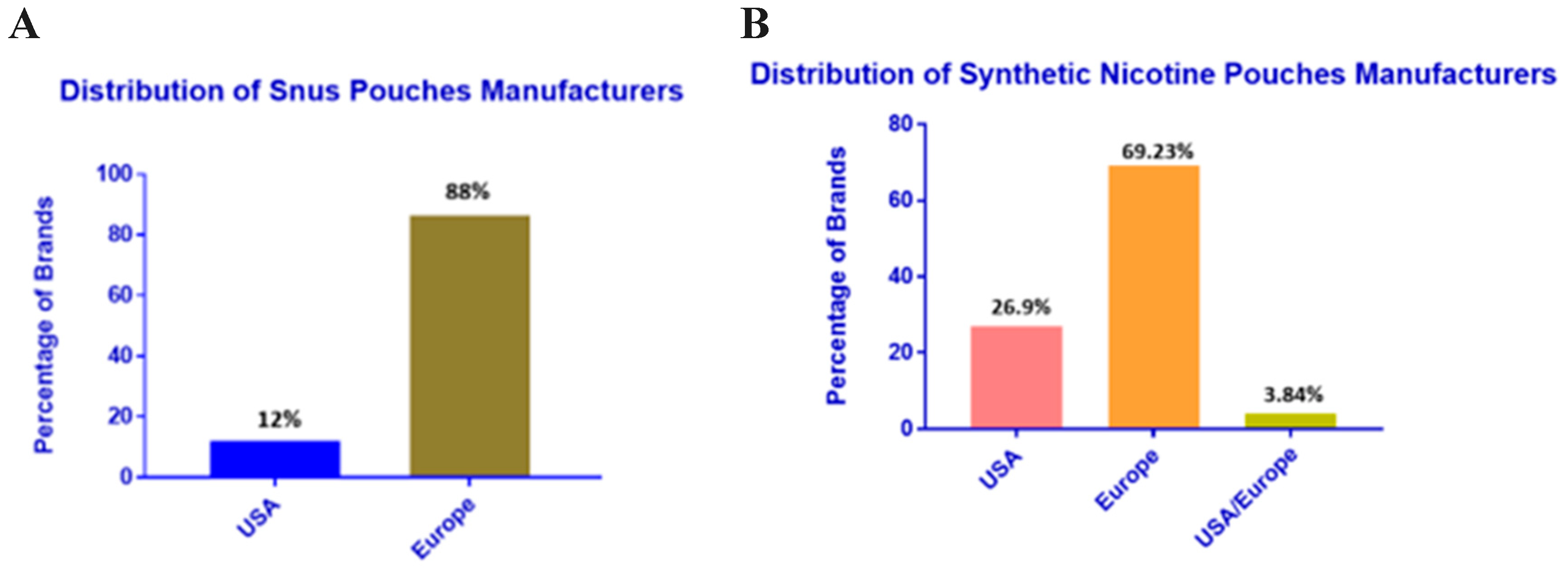
First and foremost, we demonstrate the distributors of the snus ONPs and synthetic ONPs in the USA and European regions based on our collection of ONPs from local and online vendors. European region carries a large share of about 88%of Snus ONPs distributors as compared to the USA which consists of 12% of distributors
Figure 2A. Moreover, the distributors of synthetic ONPs are about 26.9% in the USA 69.23% in Europe and 3.84% are common in USA/Europe
Figure 2B.
Distribution of ONPs on basis of their individual brands Snus vs Synthetic nicotine
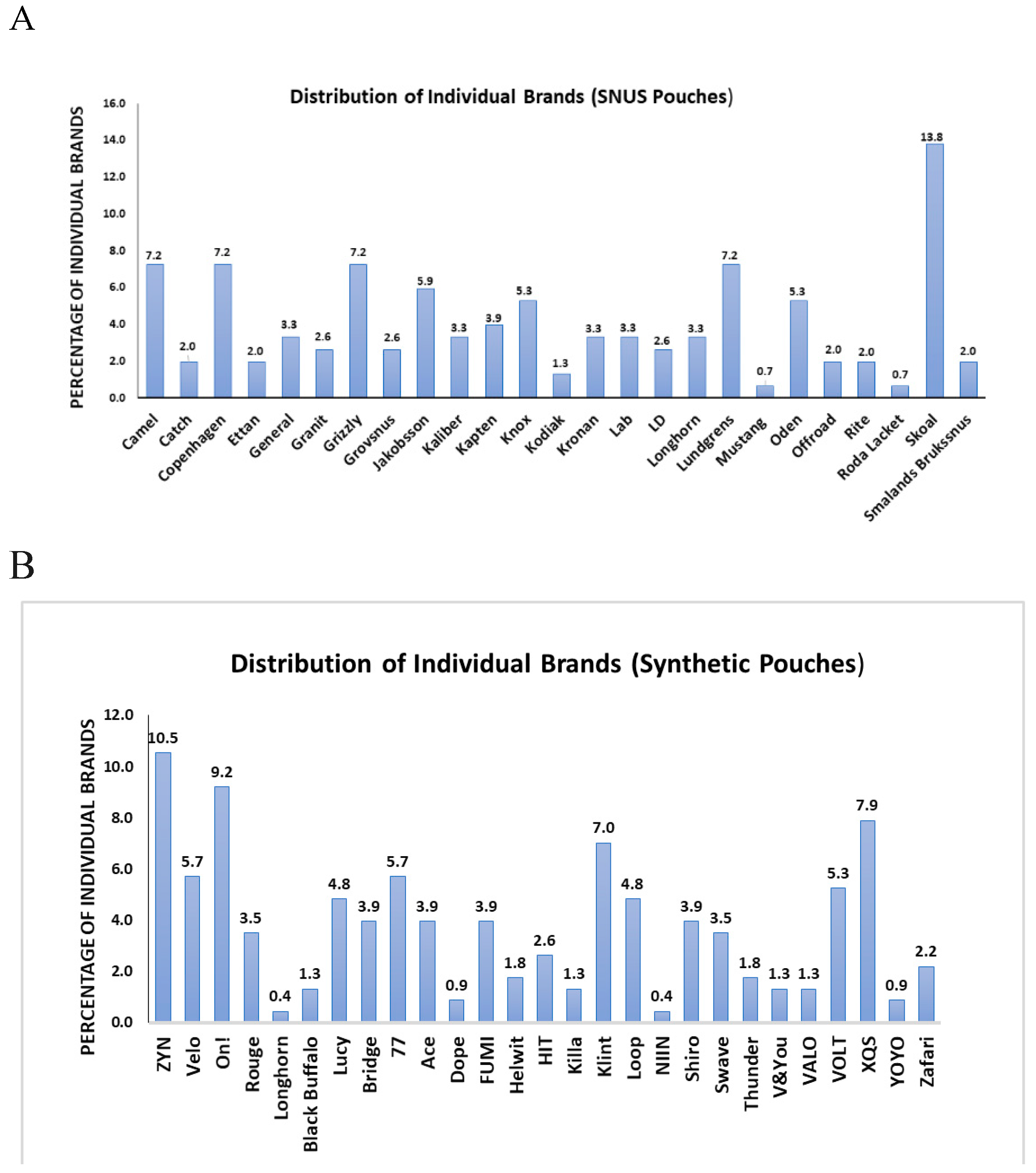
We classified the brands marketing snus and synthetic ONPs brands and were able to via the online ONPs dealers and our samples. We were able to encounter 25 brands marketing snus ONPS and 28 brands marketing synthetic ONPs. The frequency counts of snus ONP brands were
Camel (7.2%), Catch (2.0%), Copenhagen (7.2%), Ethan (2%), General (3.3%), Granit (2.6%), Grizzly (7.2%), Grovsnus (2.6%), Jakobsson (5.9%), Kaliber (3.3%), Kapten (3.9%), Knox (5.3%), Kodiak (1.3%), Kronan (3.3%), Lab (3.3%), LD (2.6%), Longhorn(3.3%), Lundgrens (7.2%), Mustang (0.7%), Oden (5.3%), offroad (2.0%), Rite (2.0%), Roda Lacket (0.7%), Skoal (13.8%) and Smalands Broakssnus (2.0%) as represented in
Figure 2A.
Whereas, the frequency counts of synthetic ONP brands were as follows: ZYN (10.5%), Velo (5.7%), On! (9.2%), Rouge (3.5%), Longhorn (0.4%), Black Bufaalo (1.3%), Lucy (4.8%), Bridge (3.9%), 77 (5.7%), Ace (3.9%), Dope (0.9%), Fumi (3.9%), Helwit (1.8%), HIT (2.6%), Kills (1.3%), Klint (7.0%), Loop (4.8%), NIIN (0.4%), Shiro (3.9%), Swave (3.5%), Thunder (1.8%), V&You (1.3%), Valo (1.3%), Volt (5.3%), XQS (7.9%) Yoyo (0.9%) and Zafari (2.2%) as represented in
Figure 2B. Skoal, Camel, Copenhagen, Grizzly, and Lundgrens were the top marketed brand of snus ONPs on other hand, the top most brands from which synthetic ONPs are marketed are ZYN, On!, XQS and Klint.
Figure 3.
A: Frequencies of Snus ONP Brands N=152; B: Frequencies of Synthetic ONP Brands N=228.
Figure 3.
A: Frequencies of Snus ONP Brands N=152; B: Frequencies of Synthetic ONP Brands N=228.
Availability and distribution of flavors Snus vs Synthetic nicotine
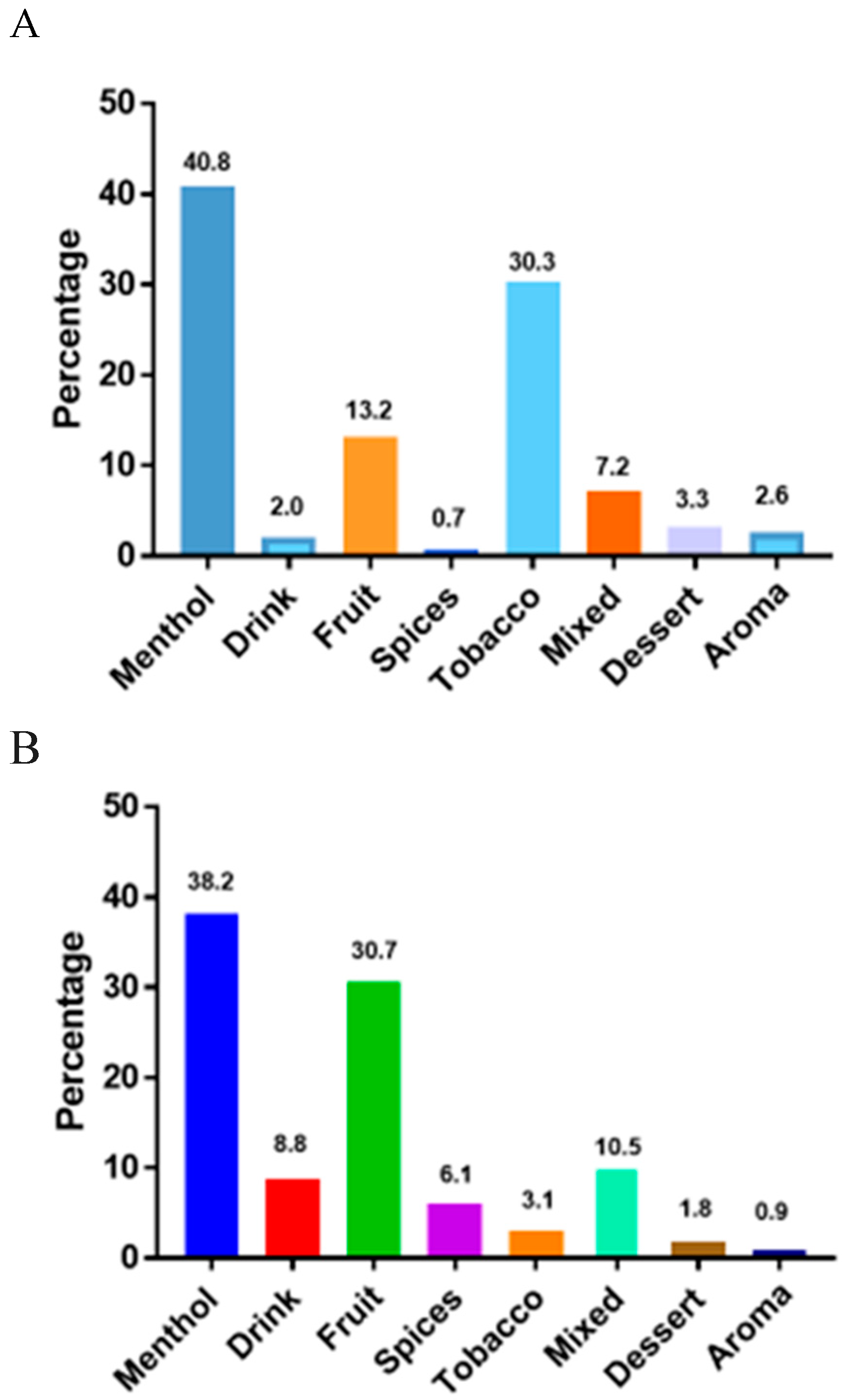
In
Figure 4A,B, we present a frequency plot of the 228 snus ONPs and 152 synthetic ONPs in our sample, by classifying their flavor description into one of the following: (1) Tobacco flavor, i.e., the product contains natural tobacco
(30.3%) in case of snus ONPs flavors like Natural, Strong, Original, Straight, Classic Original, Extra Strong. Synthetic ONPs the tobacco flavor frequency is
(3.1%) flavors usually are smooth, original, and straight. (2) Menthol flavors only, i.e., the product contains mint, wintergreen, spearmint, cool mint. Eucalyptus, classic mint, smooth mint, frosted flavors for snus ONPs
40.8% and synthetic ONPs
38.2% of menthol flavors like cold mint, iced mint, soft mint, peppermint and many more (3) fruity flavor, the product contains one or more fruity flavors like citrus, berry, dragon fruit, mango, and no supplemented flavor from any of the other principal categories; 13.2 % fruit flavors for snus ONPs and
30.7% synthetic ONPs (4) dessert/ candy /candy/other sugary flavors, snus ONPs has
3.2% and synthetic ONPs has
1.8% desert flavors (5) Aroma flavors for snus is
2.6% and synthetic pouches are
0.9% (6) Spices flavors like cinnamon, (7) Drink flavors like coffee (8) Other flavors namely Rasberry/Liquorice, Original Portion were classified as mixed flavors frequency for snus ONPs is
7.2% and synthetic ONPs is
10.5%. Menthol and tobacco was the most prevalent flavor among snus ONPs, on other hand
Menthol and Fruit flavors were most prevalent flavors among synthetic ONPs.
Comparing the common flavor distribution of Snus vs Synthetic ONPs
Based on the availability of ONPs flavors, we next sought to perceive common flavors available in each category like tobacco, menthol, fruit, dessert, spice, aroma, and the one classified as mixed flavors in the case of the Snus and Synthetic ONPs on bases of online shop and our samples.
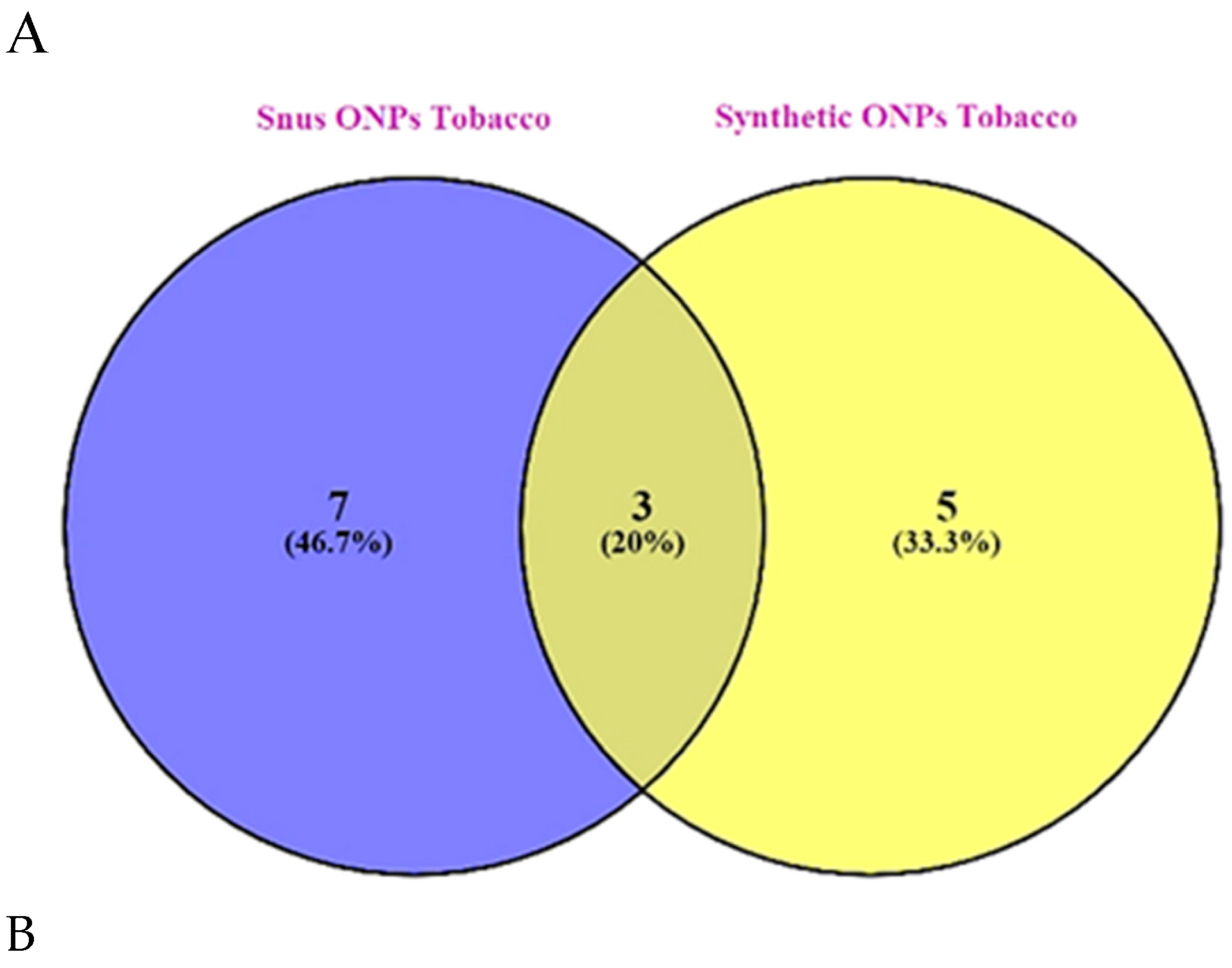
Among tobacco flavors, the frequency of common flavors was 20% in snus vs synthetic ONPs
(Figure 5A).
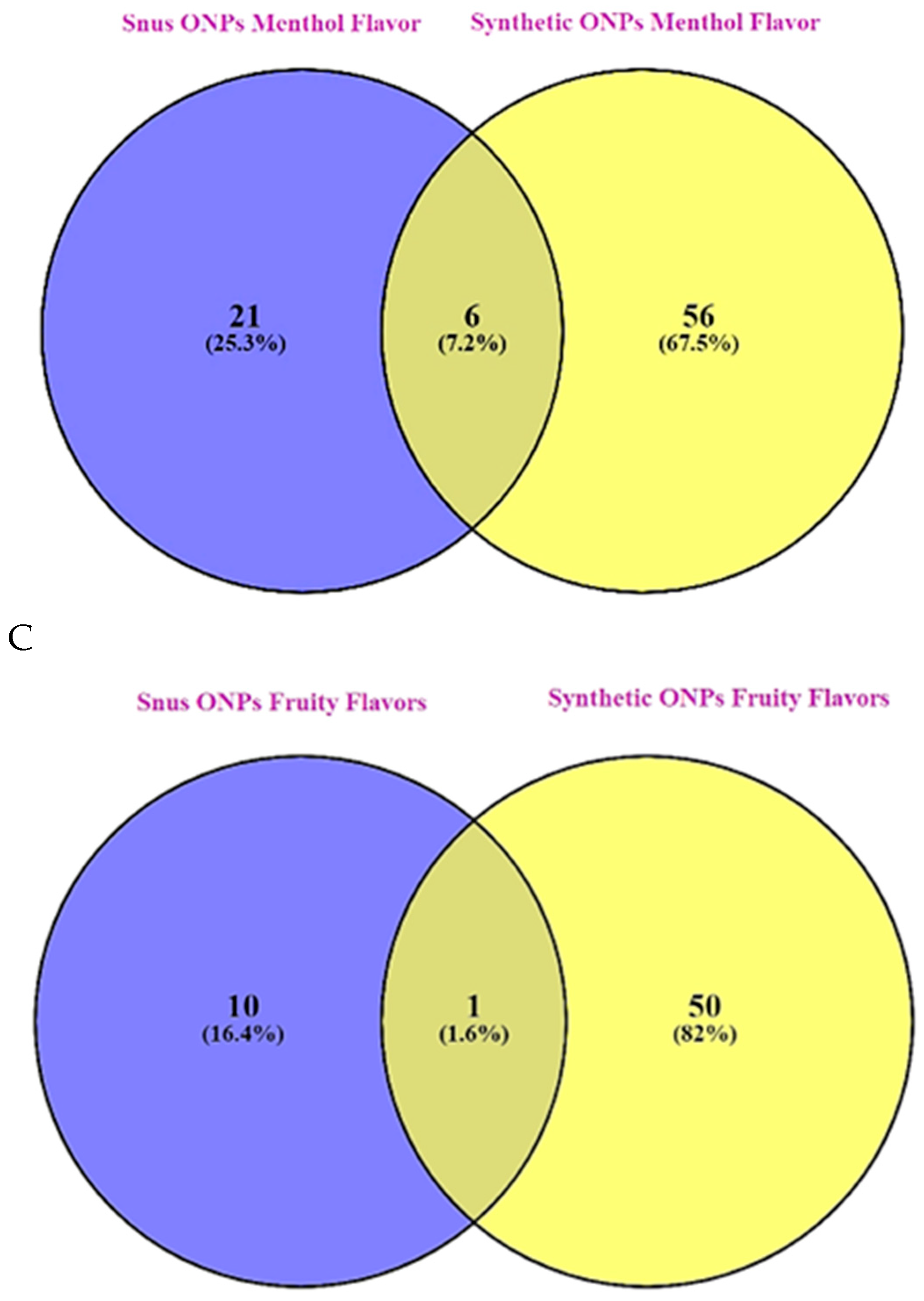
Among menthol flavors, about 7.2% are common among snus vs synthetic ONPs (
Figure 5B).
In the category fruit flavors which is the most diverse category, interestingly only about 1.6% of common fruit flavors are available among snus vs synthetic ONPs (
Figure 5C) which indicates that synthetic ONPs have attractive fruit-flavored pouches available unlike snus pouches.
No common flavors were available in the aroma, spices, and mixed flavor ONPs category.
Application of ONPs in research studies
Within a minimal time, ONPs have emerged in the research field. Various research studies and case reports using ONPs in various perspective are published. The name does imply oral pouches but it does tend to affect the other human organs (systemically) as well. Moreover, studies demonstrated that the regular utilization of smokeless nicotine products is related to a higher risk for diseases as such cancers, Parkinson's disease, birth defects, oral submucosal fibrosis, cardiovascular disease, and type 2 diabetes [
27,
37,
38]. As both Snus and ONPs, are not directly inhaled through the lungs, the flavoring chemicals, nicotine and the byproducts within these products might be secreted across the membrane of the buccal cavity into the systemic circulation; these byproducts can then act locally on various tissues within the human body; responses of these are associated with the cardiopulmonary system via, kidneys, liver, microvasculature, esophagus and the pancreas, [
39,
40,
41]. These commercial products may contain varying concentrations of nicotine from 3mg to 32 mg per pouch with other flavoring agents including triacetin, benzyl alcohol, menthol, and cooling agent WS-23. Studies also depict the high possibility of the absorption of byproducts/chemicals of this oral smokeless to merge with the lung microvasculature along with the airways [
27]. An
in vitro study investigated the oral nicotine pouch products in terms of oral irritation in the EpiGingival™ 3D tissue model and artificial saliva. This study reported oral pouches, as non-genotoxic, and non-cytotoxic, and non-mutagenic [
42]. An interesting study by Dawler et al. studied the diversity among oral tobacco products and reported the ability of these products to convey a high range of carcinogenic and nicotine, Tobacco-specific nitrosamines (TSNAs) which could be very harmful to the users [
43]. Concerning the additional approaches of the ONPs, studies also report the impact of ONPs on lung as case studies put forward that pulmonary aspirations of smokeless tobacco products activate recurrent pulmonary infiltrations and multifocal airway obstructions in the lungs of the users probably causing aspiration pneumonia [
44]. Recent evidence investigated the pharmacokinetic parameters of ONPs and reported that oral nicotine pouches carry the potential to be an acceptable substitute for adult smokers as the users can achieve adequate nicotine levels to deliver into the body [
45]. Our recent
in vitro investigation focused on oral-pulmonary health effects of Snus and ONPs indicating that the flavored ONPs are risky and likely to cause local and systematic toxicological responses during chronic consumption [
27].
Potential molecular targets due to exposure to oral nicotine products triggering possible signaling cascades
One of the prime components of cigarette smoking is nicotine, which can modulate cell proliferation and trigger apoptosis both in normal cells as well as various human cancer cell lines derived from several organs [
46]. Previously, various research studies disclosed the involvement of nicotine in activating several signaling cascades. Yuge et al., 2015, demonstrated that nicotine exposure decreased the reduction of T24 cells via elevating pAkt and pS6 expressions
in vitro and
in vivo via stimulating the PI3K/Akt/mTOR signaling in bladder cancer [
47]. Another study showed that the involvement of nicotine in the progression and development of colon cancer is responsible for cell proliferation regulation and suppression of apoptosis [
46]. Evidence also reports that we found that nicotine stimulates the levels of apoptotic markers like cleaved caspase-3 via increasing oxidative stress and enhancing the number of apoptotic cells upon on podocyte injury [
48]. Nicotine in E-Cigarettes also activate EMT process causing lung cancer [
49].
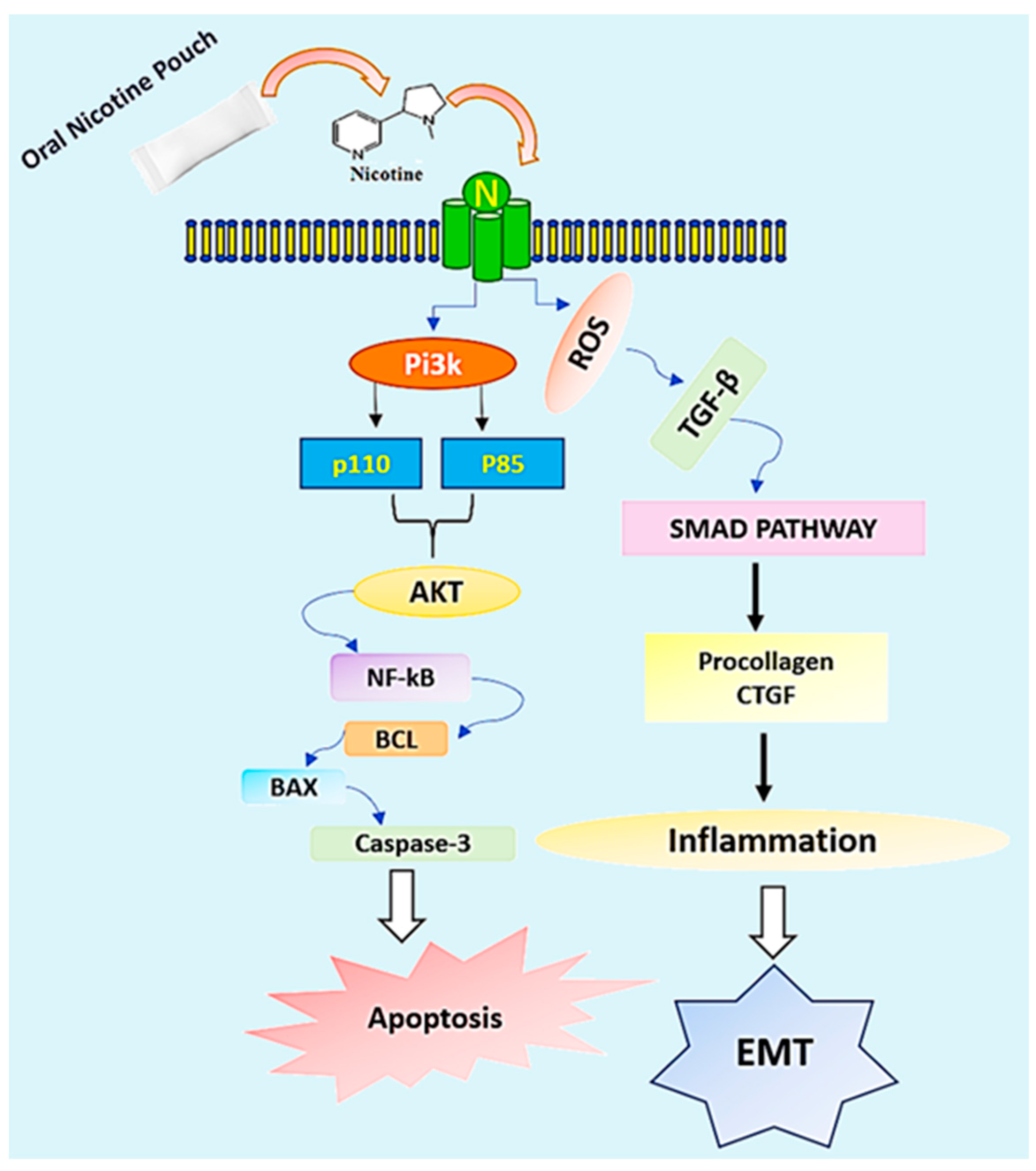
With regard to our previous findings ONPs interacting and activating signaling molecules, here we predict interesting interactions of ONPs by studying their role in targeting the apoptosis and epithelial-mesenchymal transition signaling cascades. Nicotine in ONPs could possibly engage the class-Ia PI3K, which stands for heterodimer constructed of the p110 catalytic and p85 regulatory subunits. Further leads to the recruitment AKT and NF-kB further activating the apoptotic proteins Bcl, Bax, and Caspase-3 triggering the apoptosis process. Additionally, ONPs elevate the Reactive Oxygen Species, ROS [
27], which can stimulate the levels of TGF-β1 triggering the activation of SMAD pathway via modulating the procollagen CTGF augmenting inflammation and ultimately activating the process of EMT (
Figure 6).
Recent literature indicates the involvement of menthol flavor in E-Cigarette in upregulating the cytosolic calcium thereby stimulating the TRPV1 receptor and leading to the activation of certain kinases and cytokines. [
50]. With reference to this, we presume the involvement of Menthol flavored ONPs in modulating TRPV1 receptor via increasing the cytosolic calcium might be responsible for the recruitment of cytokines and kinases.
4. Discussion
The present study provides a timely and instructive insight that could be helpful for researchers working on ONPs to classify and analyze available flavors of ONP sold locally and online, which can be marked with various composite flavor profiles like menthol+fruit, desert+fruit, fruit+aroma, and spice+fruit. Given that these fascinating flavors are most likely the major attributes to attract young and adolescents to try ONPs [
13,
22,
23]. Here we expand the existing ONP flavor wheel of snus (natural) and non-tobacco (synthetic) flavor wheel dividing them among the availability of their flavors and discrete brands marketing ONP in the US and Europe. ONPs has been marketed in U.S. and Europe as a substitute for tobacco smoking [
1,
2,
3]. We could review that the European region demonstrates a large share of about 88% of Snus ONPs distributors whereas USA depicts about 12% of Snus distributors. While synthetic ONPs displays 26.9% distributors in the USA, 69.23% in Europe, and 3.84% combined in USA/Europe. Studies report that Velo, on! and Zyn as the most emphasized brands of ONPs [
51]. Here we tried to analyze from our samples ONPs the most marketed brands of the categories of ONPs. Our analysis evaluating the top marketed U.S. and Europe brands of ONPs implied Skoal, Camel, Copenhagen, Grizzly, and Lundgrens as the top marketed snus ONPs and the top brands marketing synthetic ONPs were ZYN, On!, XQS and Klint. In 2020 it was reported that the most commonly sold flavor group of ONPs were mint flavors (including mint, wintergreens, and spearmint), followed by fruit flavors, cinnamon flavors, and coffee flavors [
52]. In agreement with this, our analysis found that menthol along with tobacco were the most prominent flavors among snus or natural ONPs, and menthol along with fruity flavors was found to be the most dominant flavor in the synthetic ONPs category. This includes various flavoring chemicals including triacetin, benzyl alcohol, menthol, and cooling agent WS-23. Next, we sought to encounter what could be the possibility of common flavors available among both snus and synthetic ONPs. Interestingly, about 20% of common flavors are available among tobacco in snus versus synthetic ONPs, 7.4% in menthol, and least being the fruit which depicted a frequency of 1.6% in snus versus synthetic ONPs.
ONPs have also shown lower cellular toxicity in vitro in human bronchial epithelial cells (H292), human oral fibroblasts (HGF), human lung epithelial cells (BEAS-2B), and human liver epithelial cells (HepG2) [
27,
53,
54]. Moreover, lower mutagenicity was found for ONPs in Salmonella typhimurium and lower genotoxicity was found in V79 hamster lung cells [
54]. It is also shown that ONPs have lower pharmacokinetics and addictive potential compared to traditional tobacco products [
55]. Although thought to have lower cytotoxicity, ONPs can still cause injuries in cells and trigger inflammatory responses, we previously reported Higher levels of lactate dehydrogenase (LDH), reactive oxygen species (ROS), and inflammatory cytokines (TNF-α, IL-6, and IL-8) in human gingival epithelial cells (HGEPp), human lung epithelial cells (BEAS-2B), and human bronchial epithelial cells (16-HBE) after treatment with ONPs [
27]. Studies have also indicated an association between ONPs usage and risks for various diseases including Parkinson’s disease, cancer, birth defects, type II diabetes, oral submucosal fibrosis, and cardiovascular diseases [
55,
56]. Here, we also present an insight into the research perspectives of ONPs based on perception, behavior, and toxicology. Reporting that ONPs can also participate in modulating signaling cascades via enhancing apoptosis and EMT process. Present findings could be useful for putative notification policies as well as for clearing future research approaches utilizing flavoring ONPs.
5. Future Perspectives
For better understanding and to be up to date with a variety of emerging available ONP flavors in the local and online markets, socio-economical aspect, behavioral, chemistry, toxicity/harmful effects, and creativity in data sciences should be applied urgently, especially for classifying and identifying ONP flavors/flavoring chemicals, nicotine strengths, and other descriptions that would be important in conducting surveillance at ONP brand websites as well at social media. Monitoring ONP flavor profiles should be implied, as it might be critical for recognition of the comparative nicotine and tobacco products appeals in the marketplace.
6. Conclusion
Overall, the marketing and usage of ONP products with various flavor profiles, and most of these products containing tobacco, menthol, and fruit flavor in US and Europe. These are associated with behavioral changes amongst the users leading to addiction with nicotine products. Further, due to toxicity of these products on oral cells, it may culminate in to oral mucosal complications including suppression of mucosal immunity. Hence, it is imperative to foster its regulation and marketing based on current policies on flavor restrictions by the regulatory agencies.
Author Contributions
Conceptualization, SS, I.R.; methodology, I.R.; assay performance: SS, WT, YS, software, SS; validation, SS and WC.; formal analysis, SS and WC.; investigation, SS, DL and WC; resources, IR. DO, DL; data curation, SS.; writing—original draft preparation, SS, and I.R.; writing—review and editing, SS, DL, CN, DO; and preparation of schematics and conceptual diagrams; visualization, SS, IR; supervision, I.R.; project administration, I.R.; funding acquisition, I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the TCORS Grant: CRoFT 1 U54 CA228110-01. Informed Consent Statement: Not applicable; no human subjects were involved.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We declare that we have provided all the data in figures.
Acknowledgments
Thanks to Mr. Shaiesh Yogeswaran for his useful scientific and technical expertise.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Czaplicki L, Patel M, Rahman B, Yoon S, Schillo B, Rose SW. Oral nicotine marketing claims in direct-mail advertising. Tobacco control. 2022 Sep 1;31(5):663-6.
- Mallock N, Schulz T, Malke S, Dreiack N, Laux P, Luch A. Levels of nicotine and tobacco-specific nitrosamines in oral nicotine pouches. Tobacco Control. 2022 Aug 5.
- Keogh, A. Nicotine Pouches. British Dental Journal 2021, 230, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Smokeless tobacco products, including dip, snuff, Snus, and chewing tobacco, 2020. Available Online: https://www.fda.gov/tobacco-products/ products-ingredients-components/smokeless-tobacco-products-including-dip-snuffsnus-and-chewing-tobacco.
- Food and Drug Administration. Dissolvable tobacco products, 2018. Available Online: https://www.fda.gov/tobacco-products/products-ingredients components/dissolvable tobacco-products.
- Robichaud MO, Seidenberg AB, Byron MJ. Tobacco companies introduce ‘tobacco-free’nicotine pouches. Tobacco Control. 2020 Dec 1;29(e1):e145-6.
- Delnevo CD, Hrywna M, Miller Lo EJ, et al. Examining market trends in smokeless tobacco sales in the United States: 2011-2019. Nicotine Tob Res 2021;23:1420–4.
- European Commission. Meeting of the group of experts on tobacco policy; 2019. https://ec.europa.eu/transparency/expert-groups-register/screen/meetings/consult? do=groupDetail.groupMeeting&meetingId=16984 [Accessed 07 Jul 2022].
- Tactics, T. Nicotine pouches. University of Bath, 2021. Available: https://tobaccotactics. org/wiki/nicotine-pouches/ [Accessed 07 Jul 2022].
- Bishop E, East N, Bozhilova S, Santopietro S, Smart D, Taylor M, Meredith S, Baxter A, Breheny D, Thorne D, Gaca M. An approach for the extract generation and toxicological assessment of tobacco-free ‘modern’oral nicotine pouches. Food and Chemical Toxicology. 2020 Nov 1;145:111713.
- Rutqvist LE, Curvall M, Hassler T, Ringberger T, Wahlberg I. Swedish snus and the GothiaTek® standard. Harm reduction journal. 2011 Dec;8(1):1-9.
- Coggins CR, Ballantyne M, Curvall M, Rutqvist LE. The in vitro toxicology of Swedish snus. Critical reviews in toxicology. 2012 Apr 1;42(4):304-13.
- Plurphanswat N, Hughes JR, Fagerström K, Rodu B. Initial information on a novel nicotine product. The American Journal on Addictions. 2020 Jul;29(4):279-86.
- Azzopardi D, Ebajemito J, McEwan M, Camacho OM, Thissen J, Hardie G, Voisine R, Mullard G, Cohen Z, Murphy J. A randomised study to assess the nicotine pharmacokinetics of an oral nicotine pouch and two nicotine replacement therapy products. Scientific Reports. 2022 Apr 28;12(1):1-2.
- Schneider NG, Popek P, Jarvik ME, Gritz ER. The use of nicotine gum during cessation of smoking. The American Journal of Psychiatry. 1977 Apr.
- Hartmann-Boyce J, Chepkin SC, Ye W. Minerva-Bondige besprekingen-17/12/2018 Hebben nicotinevervangers nog nut bij rookstop?
- United States Code. Title 21: food and drugs, chapter 9 federal food, drug, and cosmetic act, subchapter IX tobacco products, section 387k – modified risk tobacco products. Available: https://www.govinfo.gov/content/pkg/USCODE-2012-title21/ html/USCODE-2012-title21-chap9-subchapIX-sec387k.htm.
- Food and Drug Administration. Tobacco-Related health fraud. food and drug administration, 2019. Available: https://www.fda.gov/tobacco-products/healthinformation/health-fraud.
- Yu, S.; Escobedo, P.; Garcia, R.; Cruz, T.B.; Unger, J.B.; Baezconde-Garbanati, L.; Meza, L.; Sussman, S. A Descriptive Longitudinal Study of Changes in Vape Shop Characteristics and Store Policies in Anticipation of the 2016 FDA Regulations of Tobacco Products, Including E-cigarettes. Int. J. Environ. Res. Public Health 2018, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.G.; Plunkett, S.; Campbell, P.; Hilldrup, J.; Coffa, B.G.; Gilliland, S., III; Eckard, S. Analysis and Differentiation of Tobacco-derived and Synthetic Nicotine Products: Addressing an Urgent Regulatory Issue. PLoS ONE 2022, 17, e0267049. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J. FDA Gains Power to Regulate Synthetic Nicotine in E-cigarettes. JAMA Health 2022, 3, e221140. [Google Scholar] [CrossRef] [PubMed]
- Harlow AF, Vogel EA, Tackett AP, Cho J, Han DH, Wong M, Cockburn MG, Sussman SY, Unger JB, Leventhal AM, Barrington-Trimis JL. Adolescent use of flavored non-tobacco oral nicotine products. Pediatrics. 2022 Aug 1;150(3).
- Dai HD, Leventhal AM. Use of Traditional Smokeless, Snus, and Dissolvable Tobacco Among US Youth. American Journal of Preventive Medicine. 2022 Nov 3.
- Havermans A, Pennings JL, Hegger I, Elling JM, de Vries H, Pauwels CG, Talhout R. Awareness, use and perceptions of cigarillos, heated tobacco products and nicotine pouches: A survey among Dutch adolescents and adults. Drug and Alcohol Dependence. 2021 Dec 1;229:109136.
- Brose LS, McDermott MS, McNeill A. Heated tobacco products and nicotine pouches: A survey of people with experience of smoking and/or vaping in the UK. International Journal of Environmental Research and Public Health. 2021 Aug 22;18(16):8852.
- Kary T, Gretler C. Big tobacco hopes oral nicotine pouches fill the Vaping void. Bloomberg Businessweek, 2020. Available: https://www.bloomberg.com/news/ articles/2020-07-17/big-tobacco-hopes-oral-nicotine-pouches-fill-the-vaping-void.
- Shaikh SB, Tung WC, Pang C, Lucas J, Li D, Rahman I. Flavor Classification/Categorization and Differential Toxicity of Oral Nicotine Pouches (ONPs) in Oral Gingival Epithelial Cells and Bronchial Epithelial Cells. Toxics. 2022 Nov;10(11):660.
- Hatsukami DK, Carroll DM. Tobacco harm reduction: past history, current controversies and a proposed approach for the future. Preventive medicine. 2020 Nov 1;140:106099.
- Ashforth, B.E. & Anand, V. (2003). The normalization of corruption in organizations. Research in Organizational Behavior, 25(1), 1-52.
- Berger, P. & Luckmann, T. (1966). The social construction of reality. First Anchor.
- Boyer, E.W., Levy, S., Smelson, D., Vargas, S., & Casey, A. (2020). The clinical assessment of vaping exposure. J Addict Med, 14(6), 446-450.
- Clarke, D.B, Kapoor, D., Baird, A.M., Buchanan, P.J., Gately, K., Cuffe, S., & Finn, S.P. (2021), Vaping and lung cancer – A review of current data and recommendations. Lung Cancer, 153 (1), 11-20. [CrossRef]
- Vogel EA, Barrington-Trimis JL, Harlow AF, Wong M, Cho J, Han DH, Leventhal AM, Tackett AP. Prevalence of and disparities in adolescents' susceptibility to novel oral nicotine products marketed as “tobacco-free”. Preventive Medicine. 2022 Dec 9:107387.
- Vogel EA, Barrington-Trimis JL, Kechter A, Tackett AP, Liu F, Sussman S, Lerman C, Unger JB, Hughes Halbert C, Chaffee BW, Leventhal AM. Differences in young adults’ perceptions of and willingness to use nicotine pouches by tobacco use status. International Journal of Environmental Research and Public Health. 2022 Feb 25;19(5):2685.
- Nitzkin, J.L. (2014). E-cigarette primer for state and local lawmakers. RStreet, 25(1), 1-13.
- Ashforth BE, Anand V. The normalization of corruption in organizations. Research in organizational behavior. 2003 Jan 1;25:1-52.
- Vist, G.E.; Grimsrud, T.K.; Valen, H.; Becher, R.; Brinchmann, B.C.; Elvsaas, I.K.Ø.; Alexander, J. Are the health risks of moist oral snuff (snus) underestimated?. Tidsskrift for Den norske legeforening, 2020. 140(9).
- Panta, P.; Dhopathi, S.R.; Gilligan, G.; Seshadri, M. Invasive oral squamous cell carcinoma induced by concurrent smokeless tobacco and creamy snuff use: A case report. Oral Oncol, 2021,.
- Gupta, A.; Goyal, K.; and Gupta, R.K. Pulmonary Functions in Smokeless Tobacco Users in Haryana. Int j health sci res, 2016, 6, 106-112.
- Shukla, A.K.; Khaitan, T.; Gupta, P.; Naik, S.R.; 2019. Smokeless Tobacco and Its Adverse Effects on Hematological Parameters: A Cross-Sectional Study. advances in preventive medicine, Adv Prev Med, 2019, 3182946.
- Thacher, J.D.; Schultz, E.S.; Hallberg, J.; Hellberg, U.; Kull, I.; Thunqvist, P.; Pershagen, G.; Gustafsson, P.M.; Melén, E.; Bergström, A. Tobacco smoke exposure in early life and adolescence in relation to lung function. Eur Respir J, 2018, 51.
- Miller-Holt J, Baskerville-Abraham I, Sakimura M, Fukushima T, Puglisi A, Gafner J. In vitro evaluation of mutagenic, cytotoxic, genotoxic and oral irritation potential of nicotine pouch products. Toxicology Reports. 2022 Jan 1;9:1316-24.
- Lawler TS, Stanfill SB, Zhang L, Ashley DL, Watson CH. Chemical characterization of domestic oral tobacco products: total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food and chemical toxicology. 2013 Jul 1;57:380-6.
- Gupta, A.; Goyal, K.; and Gupta, R.K. Pulmonary Functions in Smokeless Tobacco Users in Haryana. Int j health sci res, 2016, 6, 106-112.
- Chapman F, McDermott S, Rudd K, Taverner V, Stevenson M, Chaudhary N, Reichmann K, Thompson J, Nahde T, O’Connell G. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic, pharmacodynamic and safety and tolerability profiles of tobacco-free oral nicotine pouches relative to cigarettes. Psychopharmacology. 2022 Sep;239(9):2931-43.
- Cucina A, Dinicola S, Coluccia P, Proietti S, D'Anselmi F, Pasqualato A, Bizzarri M. Nicotine stimulates proliferation and inhibits apoptosis in colon cancer cell lines through activation of survival pathways. journal of surgical research. 2012 Nov 1;178(1):233-41.
- Yuge K, Kikuchi E, Hagiwara M, Yasumizu Y, Tanaka N, Kosaka T, Miyajima A, Oya M. Nicotine Induces Tumor Growth and Chemoresistance through Activation of the PI3K/Akt/mTOR Pathway.
- Lan X, Lederman R, Eng JM, Shoshtari SS, Saleem MA, Malhotra A, Singhal PC. Nicotine induces podocyte apoptosis through increasing oxidative stress. PloS one. 2016 Dec 1;11(12):e0167071.in Bladder CancerNicotine and the PI3K/Akt/mTOR Pathway in Bladder Cancer. Molecular cancer therapeutics. 2015 Sep 1;14(9):2112-20.
- Vu T, Jin L, Datta PK. Effect of cigarette smoking on epithelial to mesenchymal transition (EMT) in lung cancer. Journal of clinical medicine. 2016 Apr 11;5(4):44.
- Tarran R, Barr RG, Benowitz NL, Bhatnagar A, Chu HW, Dalton P, Doerschuk CM, Drummond MB, Gold DR, Goniewicz ML, Gross ER. E-cigarettes and cardiopulmonary health. Function. 2021;2(2):zqab004.
- Duan Z, Henriksen L, Vallone D, Rath JM, Evans WD, Romm KF, Wysota C, Berg CJ. Nicotine pouch marketing strategies in the USA: an analysis of Zyn, On! and Velo. Tobacco control. 2022 Jul 11.
- Marynak KL, Wang X, Borowiecki M, Kim Y, Tynan MA, Emery S, King BA. Nicotine pouch unit sales in the US, 2016-2020. JAMA. 2021 Aug 10;326(6):566-8.
- Bishop, E.; East, N; Bozhilova, S.; Santopietro, S.; Smart, D.; Taylor, M.; Meredith, S.; Baxter, A.; Breheny, D.; Throne, D; Gaca, M. An Approach for the Extract Generation and Toxicological Assessment of Tobacco-free “Modern” Oral Nicotine Pouches. Food and Chemical Toxicology 2020, 145, 111713.
- Yu, F.; Rudd, K.; Pour, S. J.; Sticken, E. T.; Dethloff, O.; Wieczorek, R.; Nahde, T.; Simms, L.; Chapman, F.; Czekala, L.; Stevenson, M.; O’Connell, G. Preclinical Assessment of Tobacco-Free Nicotine Pouches Demonstrates Reduced In Vitro Toxicity Compared with Tobacco Snus and Combustible Cigarette Smoke. Applied In Vitro Toxicology 2022, 8(1), 24–35. [Google Scholar] [CrossRef]
- Chapman, F.; McDermott, S.; Rudd, K.; Taverner, V.; Stevenson, M.; Chaudhary, N.; Reichmann, K.; Thompson, J.; Nahde, T.; O’Connell, G. A Randomised, Open-label, Cross-over Clinical Study to Evaluate the Pharmacokinetic, Pharmacodynamic and Safety and Tolerability Profiles of Tobacco-free Oral Nicotine Pouches Relative to Cigarettes. Psychopharmacology 2022, 239, 2931–2943. [Google Scholar] [CrossRef] [PubMed]
- Panta, P.; Dhopathi, S. R.; Gilligan, G.; Seshadri, M. Invasive Oral Squamous Cell Carcinoma Induced by Concurrent Smokeless Tobacco and Creamy Snuff Use: A Case Report. Oral Oncology 2021, 118, 105354. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).