1. Introduction
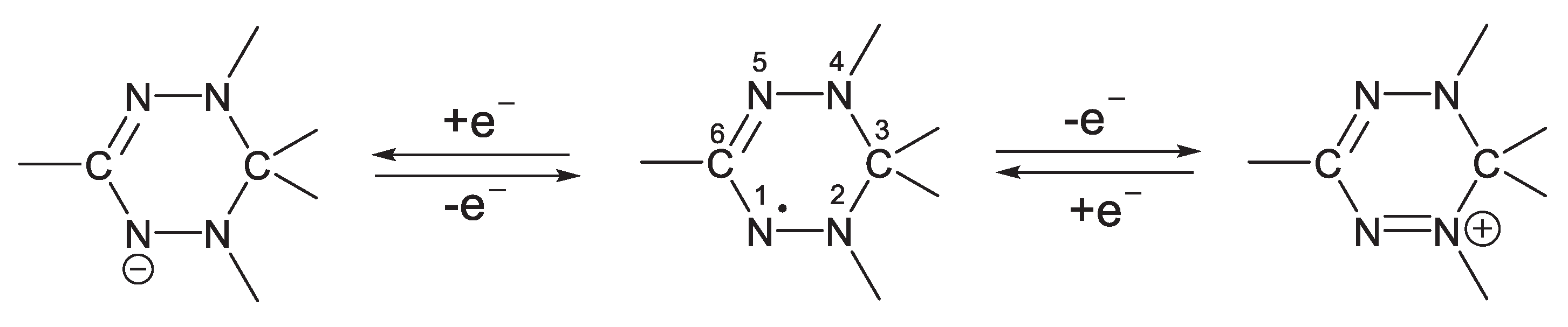
Tetrazinyl radicals (verdazyls), first reported in 1964 by Kuhn and Trishman, are "electronamphoteric," that is, they can be oxidized and restored to form with stable anions cations (
Scheme 1).
Precursors in erdazilny x radicals are typically salts of 5,6-dihydro-1,2,4,5-tetrazine I, from which neutral leuco base is formed, which oxidizes tsya to radicals. Researchers are interested in verdazyl radicals because of their remarkable chemical stability, magnetic characteristics, structural diversity, and capacity to form complexes with metals. The electrochemical properties of these radicals have received far less attention; for instance, the electrochemical behavior of the triphenylverdazyl radical in DMF in the absence of carboxylic acids has been investigated (Alkhawaldeh, 2020).
The cyclic voltammetry method was used to investigate the redox properties of a series of triaryl verdazyl radicals with varied substituents in aromatic fragments, and a correlation between both the electron spin distribution and redox properties was discovered. The researchers discovered that the boundary orbitals that contribute to the oxidation and recovery processes are localized in different parts of the molecule: the HOMO atom and nitrogen in position 2 contribute the most to the oxidation process, while the LUMO atom and carbon in position 6 contribute the most to the recovery process (Alkhawaldeh, 2022).
In this context, new organometallic (aliphatic, cyclic and heterocyclic) compounds are being searched and used with a variety of functional groups of most transition metals. Iron is necessary for the ecosystem and is non-toxic. Iron, as we all know, plays a critical role in the ecosystem. This element is also very important in the biosphere. Iron is required for photosynthesis (cytochromes, ferrodoxins), as well as a variety of enzymes. In the absence of iron, the formation of chlorophyll is delayed. Chlorosis (yellowing) and loss of leaf color are possible, especially when young. With prolonged iron deficiency, dense grasses die off at the edges of the leaf blade, tree shoots die off, overall productivity decreases, and plant resistance to diseases decreases ( Alkhawaldeh, 2021).
It should also be highlighted that iron compounds are useful in the petrochemical industry. Many compounds have been investigated in automobiles to replace the environmentally toxic tetraethyl lead as a fuel additive. Although great effort has been done to improve the effectiveness of fuel additives in this area, more effective ways are still required, and development is now continuing (Alkhawaldeh, 2020).
It is well known that the reaction of alyphatic ketones with ferrocene is simpler and yields more than that of cyclic ketones. It is worth noting that there are numerous uncertainties around the manufacturing of ferrocenylcyclokarbonyls. Reactions between alyphatic ketones and ferrocene were carried out in the presence of a catalyst H2SO4/DEAN (diethylammonium naphthenate) (2:1), and matching ferrocenylcarbonyls were produced. The reaction between cyclic ketones and ferrocene has been studied as a follow-up to previous studies. (Alkhawaldeh, 2020).
The use of 3-phenyl-1,5-di-n -olilverdazila in a symmetrically second nonaqueous second redox th battery is discussed. Describes the synthesis of 6-oxoverdazyl radical polymers with customizable electrochemical characteristics. Verdazyl radicals also have profluorescent characteristics. Thus, the chemistry of verdazil nyh radicals is fascinating and diversified, but the salts of 1,3,5-triaryl-5,6-dihydro-1,2,4,5-tetrazine derivatives of I, which are precursors Kuhn radicals, have received far less attention. Our research focused on the synthesis of a series of 3,3'- (1,4-phenylene) perchlorates as probable precursors of verdazyl biradicals (Alkhawaldeh, 2020).
The reactivity of hydrogen sulfide and various mercaptanes with aliphatic and aromatic compounds has been investigated. This method yielded noxious-smelling ferrocenylthioethers. These chemicals dissolve in almost all organic solvents, particularly benzene. This feature can be used to purify some oils that include mercaptane or its fractions. Given this, hazardous compounds from exhaust gases, particularly unburned residues, are currently the most significant and urgent environmental issues for diesel and non-standard motor fuels that contaminate air. It is widely known that the substitution of ferrocene for the aromatic core in organic compounds results in products with features that are not characteristic of or are less exhibited in the starting compounds.
2. Experimental
Before usage, the solvents were dried and distilled. All reagents were acquired from Sigma-Aldrich Rus LLC / Merck LLC and used in their original form. NMR spectra of 1 H and 13C of compound solutions were recorded on an ECX400 spectrometer JNM (JEOL, Japan; 400.1 and 100.6 MHz, respectively) for the substance solutions in DMSO, and chemical shifts relative to SiMe4 were determined. The constants of spin-spin coupling were measured in hertz. On an Infralum FT-02 Fourier spectrometer, IR spectra of KBr pellets were acquired (Russia). Elemental analysis was carried out using a Vario MICRO CHNS analyzer (Germany).
TLC conditions for analysis: adsorbent - Silufol UV-245, eluents - benzene; benzene - ethyl acetate (2: 1), development in an iodine chamber. Using an MP-50 melting point analyzer, the melting points of the compounds were evaluated in sealed glass capillaries (Mettler Toledo, Switzerland). Cyclic voltammetry was used to collect electrochemical data in a solution of acetonitrile (0, 1 M supporting electrolyte Bu4NBF) utilizing a Gamry (Canada) potentiostat in a 5 ml electrochemical cell. As a working electrode, a glassy carbon (CU) electrode S2 = 0, 125 cm2 is utilized. Before taking measurements, the electrode was meticulously polished and rinsed. A platinum auxiliary electrode was used, as well as a typical silver chloride electrode as a reference electrode (E 0 = 0.33 B, Me, CN and Fc). By blowing, all solutions were totally deaerated.
By blowing argon through all of the solutions, they were entirely deaerated. To a solution of 6.07 g (0.042 mole) phenylhydrazine hydrochloride and 2.88 g anhydrous sodium acetate in 70 ml of water, add tiny amounts of 2.68 g (0.02 mole) terephthalic aldehyde in 25 ml of dioxane. The reaction mixture was stirred at room temperature for 60 minutes after the addition was completed. The precipitate was filtered off and the filter was cleaned with water. Recrystallized from an ethanol/DMSO combination after being dried in the air.
t-BuONO was added to the resultant mixture after it had been cooled to 0 °C. The reaction mixture was agitated at 0 °C for 30 minutes, then at room temperature for another 30 minutes. The arendiazonium tosylates produced were used without further purification. This reaction mixture turned a deep dark cherry color. The reaction mixture was kept at a temperature of around 0°C for 24 hours. The reaction mixture was then given 2 ml of water, and the precipitated precipitate was filtered off and washed on the filter with methanol, water, methanol, and diethyl ether in that order.
3. Results and Discussions
Starting from ter eftalevogo aldehyde, a straightforward three-stage synthesis of 3,3'- (1,4-phenylene) perchlorate s was achieved. Their electrochemical characteristics were investigated using cyclic voltammetry. In three phases, symmetric tetrazinium perchlorates (R: MeO, H, CO, Me, Me, Br, NO
2) were synthesized from terephthalic aldehyde (
Figure 1). Terephthalaldehyde phenylhydrazone was obtained in the first step, formazans in the second, and suitable perchlorates in the third. The method proposed by Katritsky was utilized to synthesize perchlorates.
Formazans were created by combining phenylhydrazone and terephthalaldehyde 3 with tosylates ap ene diazonium, which were derived from steam ameschennyh anilines. The decision to employ tosylates arenediazonium was taken since getting arenediazonium chlorides in the case of some para-substituted anilines is challenging due to limited solubility in water and low reactivity. Because of the solubility of arenediazonium tosylates in organic solvents, we were able to streamline the purifying technique and employ arenediazonium chlorides. Almost all formazans are dark red crystalline compounds that are highly pigmented. However, unlike the remaining compounds, compound f is orange in hue. Cyclization of formazans was carried out in dioxane solution using a 37 percent formalin solution in the presence of perchloric acid.
Formazans were synthesized by mixing phenylhydrazone and terephthalaldehyde 3 with tosylates ap ene diazonium obtained from steam-s ameschennyh anilines. Because arenediazonium chlorides are difficult to obtain in the case of some para-substituted anilines due to restricted solubility in water and low reactivity, the choice to use tosylates arenediazonium was made. We were able to expedite the purification procedure and use arenediazonium chlorides due to the solubility of arenediazonium tosylates in organic solvents. Almost all formazans are intensely colored dark red crystalline molecules. Compound f, on the other hand, is orange in color, unlike the other compounds. Formazans were cyclized in dioxane solution with a 37 percent formalin solution in the presence of perchloric acid (
Figure 2).
According to the literature second technique, fenilgidrazon was generated in 90% yield by condensation of terephthalic aldehyde and phenylhydrazine; its constants correspond to the literature data. Tosylates ap ene diazonium were synthesized by adding claim -toluolsulfokisloty and tert -butyl nitrite to aniline solutions in a combination of THF - CH3COOH. Without additional purification, oli diazonium was used to drive the reaction with the chemical. The reactions of tosylates arendiazo n and I with phenylhydrazone were carried out in a combination of DMF and pyridine in a 2:1 ratio. Frormazan was acquired in the form of CV spectra that were independently analyzed.
Thus, there are three absorption maxima in the UV spectra of all formazans: 240-260 nm, 305-330 nm, and 380-420 nm. The absorption bands in the IR spectra are typical of the bonds C = N (1400 - 1490 cm
-1), N-H (3400 - 3300 cm
-1) and N = N (1500-1480 cm
-1) formazan fragment. When working on formazans 37 percent formalin solution in the presence of perchloric acid, perchlorates triarilverdaziliya was produced in dioxane. UV spectra were used to confirm the structure of perchlorates. The UV spectra show absorption maxima at 250-260 nm and 370-420 nm, as well as a maloin intensity peak at 610-720 nm. The absorption bands in the IR spectra of salts are indicative of the bonds C = N (1600-1590 cm
-1), N = N (1480-1550 cm
-1) and the perchlorate anion (1200 - 1250 cm
-1). Verdazil perchlorates are crystalline compounds that range in color from dark blue to dark brown, depending on the type of the substituent (
Figure 3).
The absorption bands in the IR spectra of salts are indicative of the bonds C = N (1700- 1800 cm
-1), N = N (1800-1950 cm
-1) and the perchlorate anion (1300- 1400 cm
-1). Verdazil perchlorates are crystalline compounds that range in color from dark blue to dark brown, depending on the type of the substituent. Previously, using triarylverdazyl radicals as an example, the DFT approach was utilized to investigate the effect of various substituents on the electron density distribution for border orbitals. The presence of donor substituents facilitates the oxidation of radicals to cations, whereas acceptor substituents inhibit the oxidation of the radical (
Figure 4).
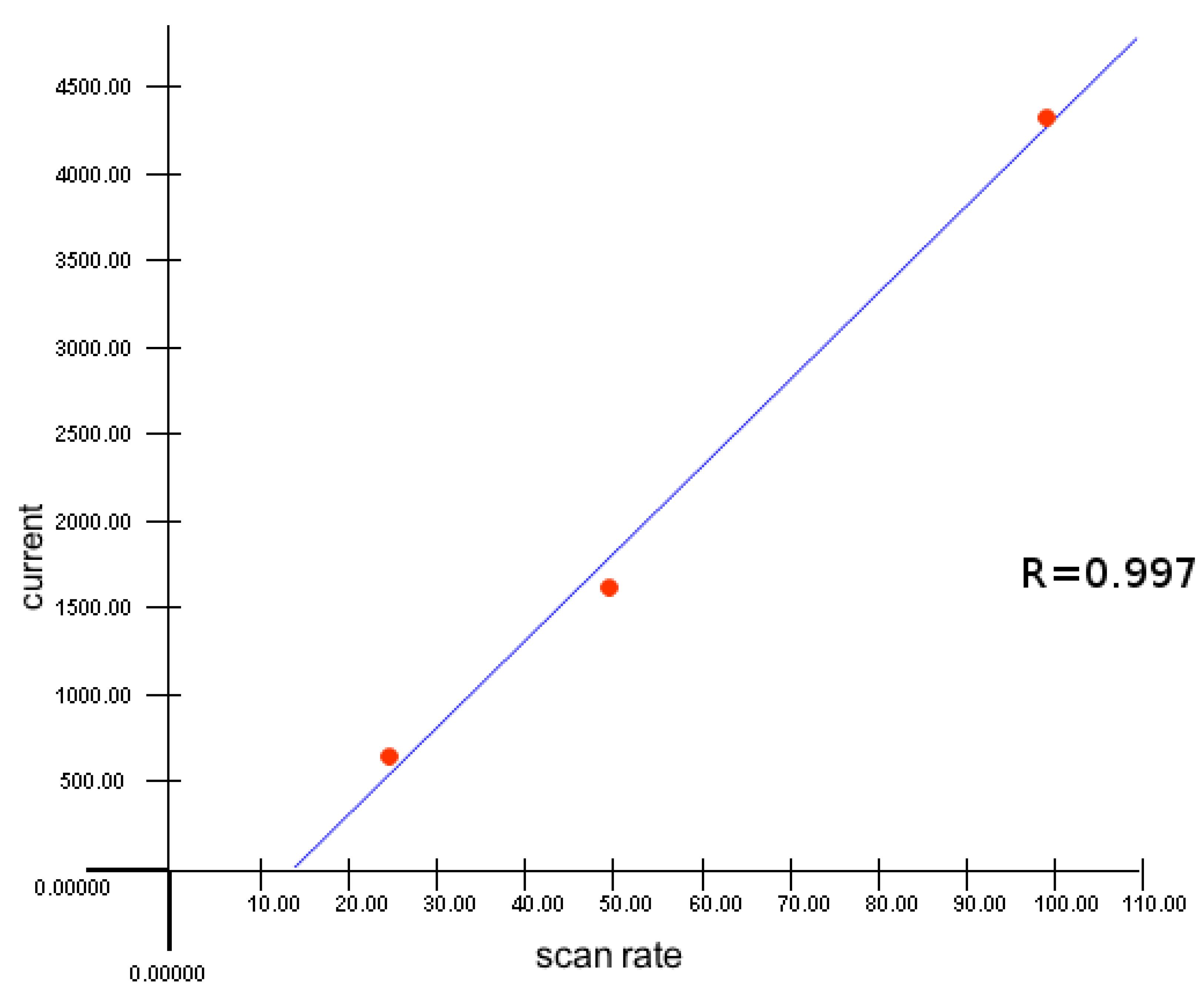
Without a doubt, the nature of the substituent will affect the electron density distribution and, therefore, the ability of verdazylium cations to be reduced to create the corresponding verdazyl radicals in the case of verdazilium perchlorates. We previously studied the electrochemical properties of triarilverdazilnyh radicals using the method of cyclic voltammetry (CV) and found that substituents in the 2-position tetrazinilnogo fragment affect the value of the radical oxidation potential as substituents and in the 6-position affect the radical reduction potentials values.
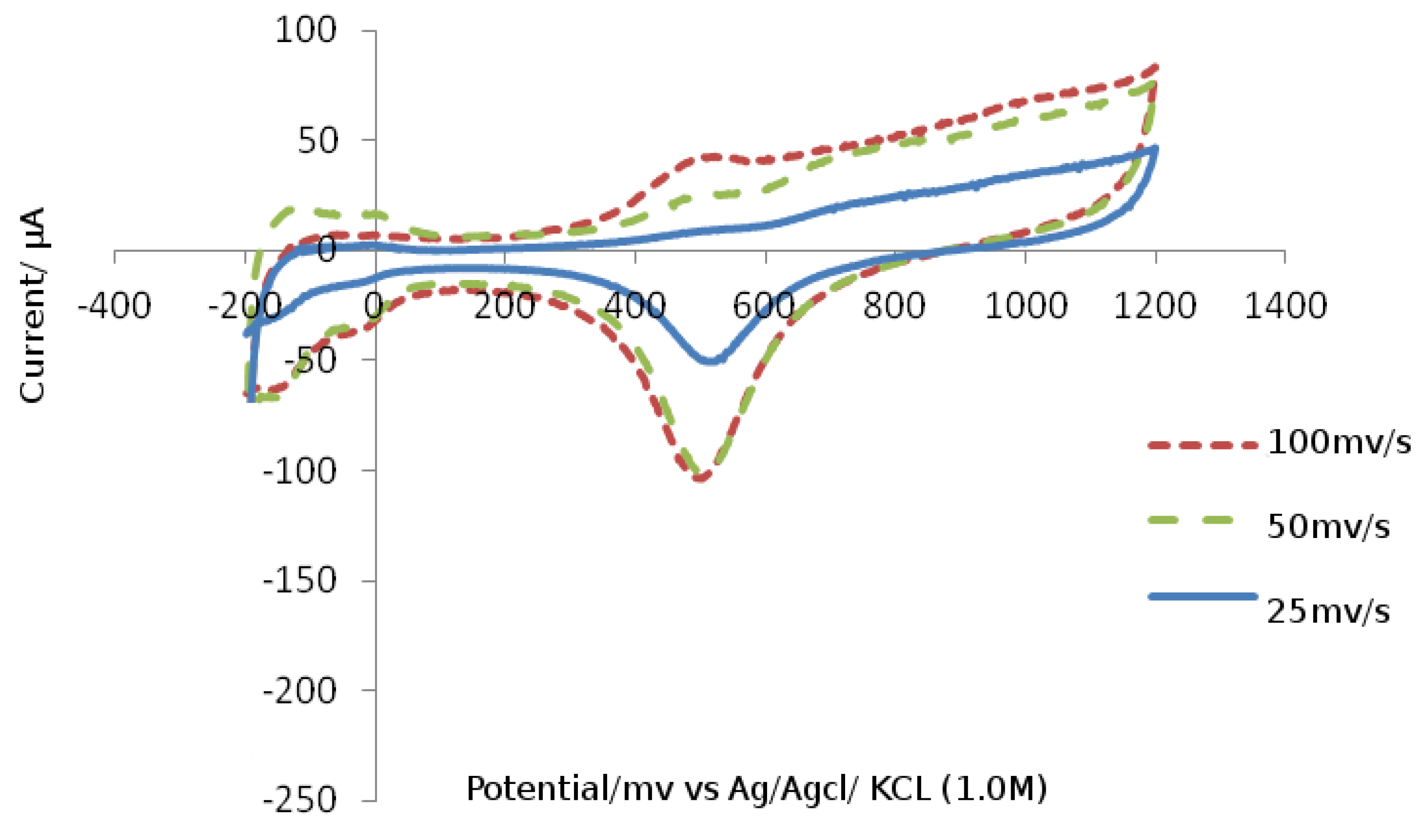
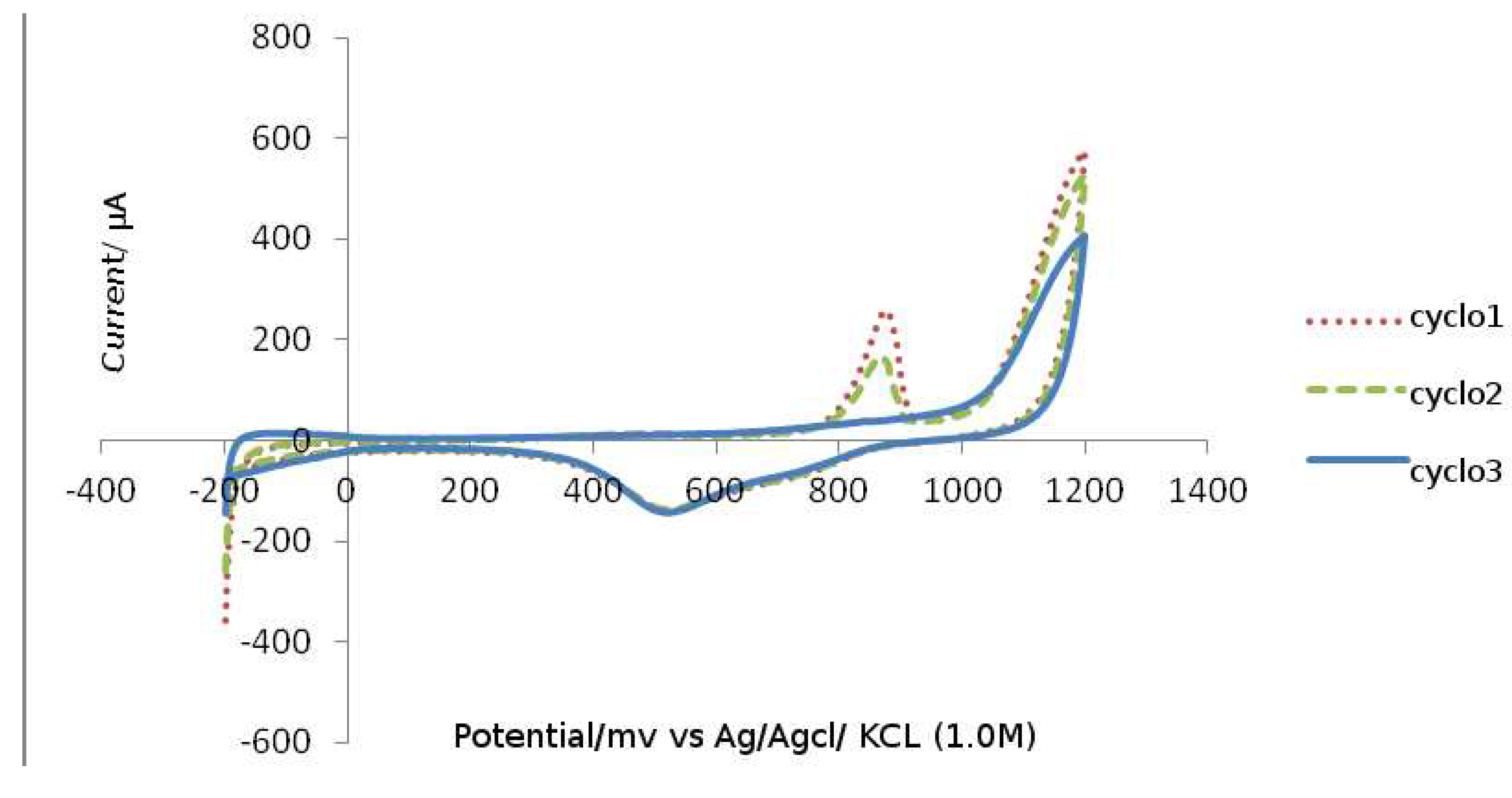
As a result, the type of the substituents in tetrazine Eve's salts is thought to alter the ability to renew cation radicals. To test this idea and investigate the possibilityY and preparation of salts verdazilnyh. platinum was employed as a working electrode, platinum as an auxiliary electrode, and standard as a reference electrode. In desalt IME, whether vinyl CVA two -electron reduction peak in the range of – 0.30 V to -1.00 V (Ag/AgCl/KCl), which we suppose is related to the recovery processes dictating first to the radical cation and then to the biradial. The type of the substituent in the vapor-position and an aromatic ring has an effect on the ability of verbals cations to recover, as shown in the
Figure 5.
Cyclic voltammograms (CV) of chemicals (R: H, OMe and NO
2) are shown in
Figure 5. CVs were recorded at a scanning rate of 200 mV/s for solutions in MeCN in the presence of a supporting electrolyte of 0.1 M Bu 4 NBF 4 (CVs are displayed with an offset relative to the Y axis). Cyclic voltammograms (CV) of chemicals (R: H, OMe and NO
2) are shown in
Figure 5. CVs for solutions in MeCN in the presence of 0.1M Bu4NBF background electrolyte were obtained at a scan rate of 200 mV/s.
As shown in the figure, under the influence of the methoxy group, the value of the first reduction potential decreased by 0.15 V and the second by 0.18 V, whereas under the influence of the nitro group, the first reduction potential increased by 0.18 V and the second by 0.10 V in comparison to unsubstituted derivatives.
4. Conclusions
Starting from ter eftalevogo aldehyde, a straightforward three-stage synthesis of 3,3'- (1,4-phenylene) perchlorate s was achieved. Their electrochemical characteristics were investigated using cyclic voltammetry. Curves in the cathode area are present on the two-electron reduction peak 1 in cyclic voltammetry, linked to sequential reduction th dication in a radical cation and further in radical. It was discovered that donor substituents R = Me, OMe accelerate verdazyl cation reduction, but acceptor substituents R: NO2, and COMe, hinder the process. Based on these findings, it was hypothesized that the corresponding bis-verdazyl radicals would develop easily in the presence of donor substituents in the aromatic ring. As a result, verdazylium perchlorates can function as precursors of the equivalent bis-verdazyl radicals.
References
- Alshamaileh, E.; Al-Sulaibi, M.; Al-Khawaldeh, A.; Almatarneh, M.; El-Sabawi, D.; Al-Rawajfeh, A. Current status of nanotechnology in Jordan, World Journal of Science, Technology and Sustainable Development, 2016, 13, 66-81. [CrossRef]
- Hourani, M. K. and Alkawaldeh A. (2016). Synergistic Effects of Bismuth Adatoms on Electrocatalytic Properties of Electrodeposited Nanostructured Platinum Electrodes. International Journal of Electrochemical Science, 3555–3566. [CrossRef]
- Altwaiq, A. Jawad, I Aljalab, T. Abu alhaj, O. Muwalla, M. and Alkhawaldeh, A. (2019) The Determination of Some Heavy Metals in Different Selected Diets, Eurasian Journal of Analytical Chemistry, 14 (4), 7-18, emEJAC-00326.
- Ahmad Alkhawaldeh, Mariam Odettalah and Mohammed Hourani, (2019). Electrochemical Reduction of Carbon Dioxide by Application of a Square Wave Potential Regime at Polycrystalline Palladium Electrodes, Jordan Journal of Chemistry. 14(3), 105 – 112.
- Almatarneh, M. H., Elayan, I. A., Al-Sulaibi, M., Khawaldeh, A., Saber, S. O. W., Al-Qaralleh, M., and Altarawneh, M. (2019). Unimolecular Decomposition Reactions of Propylamine and Protonated Propylamine. ACS Omega, 4(2), 3306–3313. [CrossRef]
- Krishan, M. Alkhawaldeh, A. and Soliman, A. (2020). Development of Nitride-Sensors for Monitoring in Control Systems, Journal of Measurements in Engineering, 8 (3): 90-97. [CrossRef]
- Alkhawaldeh, A. K., and Alkhawaldeh, R. (2020). Highly Sensitive copper Heavy Metal Analysis on Nanoparticle Platinum and palladium electrode, International Journal of Engineering and Artificial Intelligence. 1(2): 33-39. [CrossRef]
- Alkhawaldeh, A. K., M.Krishan, M., Altwaiq, A., Dabaibeh, R. N. (2020). Preparation of Nanostructured/ Microplatinum Surfaces by Application of a Square Wave Potential Regime for Methanol Oxidation. Eurasian Journal of Analytical Chemistry, 15(1), emEJAC-00362.
- Alkhawaldeh, A. K., (2020). COVID-19: Simultaneous Surveillance Studies and Case Series, Jordan as a Case Study. International Journal of Multidisciplinary Sciences and Advanced Technology. Special Issue 1: Covid-19: 55-62.
- Alkhawaldeh, A. K., (2020). Analytics of Antimony in Natural Water of Nanoparticle Platinum Electrode by Application Square Wave Voltammetry. International Journal of Multidisciplinary Sciences and Advanced Technology, 1(4): 96-103.
- Alkhawaldeh, A. K., (2020). Electrical Conductivity of Natural Volcanic Tuff Mix by Cyclic Voltammetry Method, International Journal of Multidisciplinary Sciences and Advanced Technology, 1(5): 37-44.
- Alkhawaldeh, A. K., (2020). Platinum nanoparticle electrode modified iodine used cyclic voltammetry and chronoamperometric for determination of ascorbic acid. Analytical and Bioanalytical Electrochemistry, 12 (6): 780-792.
- Alkhawaldeh, A. K., (2020). Platinum Nanoparticle in Tantalum Electrode for the Electrochemical Analysis of Heavy Metal Ions. International Journal of Intelligent Computing and Technology, 4 (1): 25-35.
- Alkhawaldeh, A. K., (2020). Platinum Nanoparticles for the Electrochemical Study of Heavy Metal ions Formed by the Sputtering Deposition of the ion beam Electrode. International Journal of Engineering and Artificial Intelligence. 1(3): 1-8. [CrossRef]
- Almatarneh, M. Al Omari, R. Omeir, R. AlKhawaldeh, A. Afaneh, A. Sinnokrot, M. Akhras, A. Marashdeh, A. (2020) Computational Study of the Unimolecular and Bimolecular Decomposition Mechanisms of Propylamine, Sci Rep 10, 11698. [CrossRef]
- Altwaiq, A. , Khouri, S. , Abdel-Rahem, R. and Alkhawaldeh, A. (2020) Conductivity Method as a New Monitoring Technique for Corrosion and Corrosion Inhibition Processes of Zinc Metal. American Journal of Analytical Chemistry, 11, 349-361. [CrossRef]
- Alkhawaldeh, A. K., Alzawahreh, A., Alkhawaldeh, R., (2021). Electrochemical Sensors and Determination for Heavy Metal by Rotating Disk Platinum Electrode and Chronoamperometric Method. International Journal of Engineering and Artificial Intelligence, 2 (1): 17-26. [CrossRef]
- Alkhawaldeh, A. K., (2021). Electrochemical Analysis of Heavy metal by Cyclic Voltammetry Method. International Journal of Engineering and Artificial Intelligence, 2 (2): 27-33.
- Alkhawaldeh, A. K., (2021). Platinum nanoparticle electrode electrochemical lead (II) determination with square wave voltammetry modified with iodine. AIP Conference Proceedings, 2339 (1): 020221 (2021); [CrossRef]
- HudaAlhasan, Zhraa H. Obaid, Ahmad AlKhawaldeh, (2021). The extent of citizens’ knowledge of preservatives and their health effects, International Journal of Psychosocial Rehabilitation. 25 (2) (2021): 908-931. [CrossRef]
- Alkhawaldeh, A. K., Abdel Hadi Al Jafari (2021). Electrochemical Sensors and Determination for silver ion by Cyclic Voltammetry at iodine-coated Platinum nanoparticles electrode. Annals of the Romanian Society for Cell Biology, 25 (6): 20280 – 20291.
- Abdullah Mohammed Al-Dhuraibi, Wadah Mohammed Al-Dhuraibi, Ahmad khalaf alkhawaldeh, Vladislav Soldatov, Mikhail Vladimirovich pokrovskiy (2021). Safety and Efficacy of Eltrombopag in Patients with Chronic Immune Thrombocytopenia: Meta-Analysis of Randomized Controlled Trials. Annals of the Romanian Society for Cell Biology, 25 (6): 20617 – 20634.
- Malek Khalaf Albzeirat, Kadhim H. Suffer, Nik Noriman Zulkepli, Ahmad Khalaf Alkhawaldeh. A Vision Future for Application Artificial Intelligent in Solar Energy. International Journal of Engineering and Artificial Intelligence, 2 (1). (2021): 60-70. [CrossRef]
- Ahmad Khalaf Alkhawaldeh. Technology patterns in Nanochemistry Based on GII Indicator. International Journal of Engineering and Artificial Intelligence, 2 (3). (2021): 28–32.
- Ahmad Khalaf Alkhawaldeh. Cyclic voltammogram analysis of the environmental aspects of the use of ferrocenyl carbinols. International Journal of Engineering and Artificial Intelligence, 2 (4). (2021): 7–12. [CrossRef]
- Abdullah Mohammed AL-Dhuraibi, Mikhail Vladimirovich Pokrovskiy, Ahmad khalaf alkhawaldeh, Wadah Mohammed AL-Dhuraibi (2021). Evaluation of Eltrombopag Efficacy in Patients with Hepatitis C-induced Thrombocytopenia: Systematic Reviews of Meta-Analysis. Natural Volatiles and Essential Oils, 8 (6): 5453 – 5471.
- Abdullah Mohammed AL-Dhuraibi, Ahmad khalaf alkhawaldeh, Iman Hassan Abdoon, Wadah Mohammed AL-Dhuraibi (2021). A comparative quantitative study of selected drugs commercialized in Yemen with HPLC. Natural Volatiles and Essential Oils, 8 (6): 5472 – 5483.
- Abdel Hadi Al Jafari, 29. Alkhawaldeh, A. K, (2022). The stability study of ginger exhaustive extraction using HPLC. Plant Cell Biotechnology and Molecular Biology, 23 (14): 55 – 60.
- Alkhawaldeh, A. K, Abdel Hadi Al Jafari, (2022). Cyclic voltammogram analysis of the 3,3´- (1,4-phenylene) perchlorates. International Journal of Engineering and Artificial Intelligence, 3 (2): 8–14.
- Ibtisam Jasim Sodani, Nadhum H. Safir, Ahmad Khalaf Alkawaldeh (2021). Potential pharmacological Therapeutics options for COVID-19: Review. International Journal of Natural and Human Sciences, 2 (2): 9-22.
- Ahmad khalaf alkawaldeh. (2021). Smart Care Business Uses the Internet of Things. J. New Medical Innovations and Research, 2 (3): 1–17. [CrossRef]
- Ahmad khalaf alkawaldeh, Malek Albzeirat. (2022). The Perception of Misuse of Antibiotics in Jordanian People. International Journal of Natural and Human Sciences, 3(1): 18–23.
- Malek Albzeirat, Osama Alkhawaldeh, Nadhum Safir, Nazih Bin-Abdun, Ahmad Khalaf Alkawaldeh, Kadhim Suffer. (2022). Development of a Hierarchical Model of Criteria for Construction of Nuclear Power Plants in the light of Nuclear Accidents from the World War to the Ukrainian-Russian War perception of misuse of antibiotics in Jordanian people. International Journal of Natural and Human Sciences, 3(1): 24–31.
- Ahmad khalaf alkawaldeh. Abdullah Mohammed AL-Dhuraibi, (2022). Nanotechnology of Status in Jordan. International Journal of Pharmaceutical and Nanoscience, 1 (1): 13-19.
- Ahmad khalaf alkawaldeh. Abdel Hadi Al Jafari, Abdullah Mohammed AL-Dhuraibi. (2022). Trends in contemporary culture in chemistry and pharmaceutical. International Journal of Pharmaceutical and Nanoscience, 1(1): 21-30.
- Ahmad khalaf alkawaldeh. Abdullah Mohammed AL-Dhuraibi. (2022). Cyclic voltammetry of heavy metal/amino acid in Jordanian water. International Journal of Pharmaceutical and Nanoscience, 1(1): 31-36.
- Mohammad Amer, Ahmad khalaf alkawaldeh. (2022). The ideal treatment for heart failer. International Journal of Pharmaceutical and Nanoscience, 1(1): 1-6.
- Ahmad khalaf alkawaldeh. Mohammed Hourani. (2020). Electrochemical reduction from carbon dioxide to urea through the application of a polycrystalline palladium electrode potential Square Wave Regime. J. Indian Chem. Soc., Vol. 97, No. 11a, pp. 2321-2328. [CrossRef]
- Ahmad khalaf alkawaldeh. (2022). Electrochemical study of Bismuth heavy metals using the cyclic voltammetry method in anti-protozoal. International Journal of Engineering and Artificial Intelligence, 3 (4): 1–7. [CrossRef]
- 40. Ahmad khalaf alkawaldeh. (2022). Electrocatalytic activities of a platinum nanostructured electrode modified by gold adatom toward methanol and glycerol electrooxidation in acid and alkaline media. Journal of Oleo Science,.
- Ahmad khalaf alkawaldeh. (2022). Photocatalytic degradation of platinum nanostructure in tantalum electrode. Journal of Pharmaceutical Negative Results, Vol 13, Special Issue 9, 6264–6272.
- Ahmad khalaf alkawaldeh. Alhasan, Huda S. (2022). Enhanced electrochemical efficiency of lithium-ion battery using titanium and rhenium adatoms by the application of square wave potential regime. Egyptian Journal of Chemistry. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).