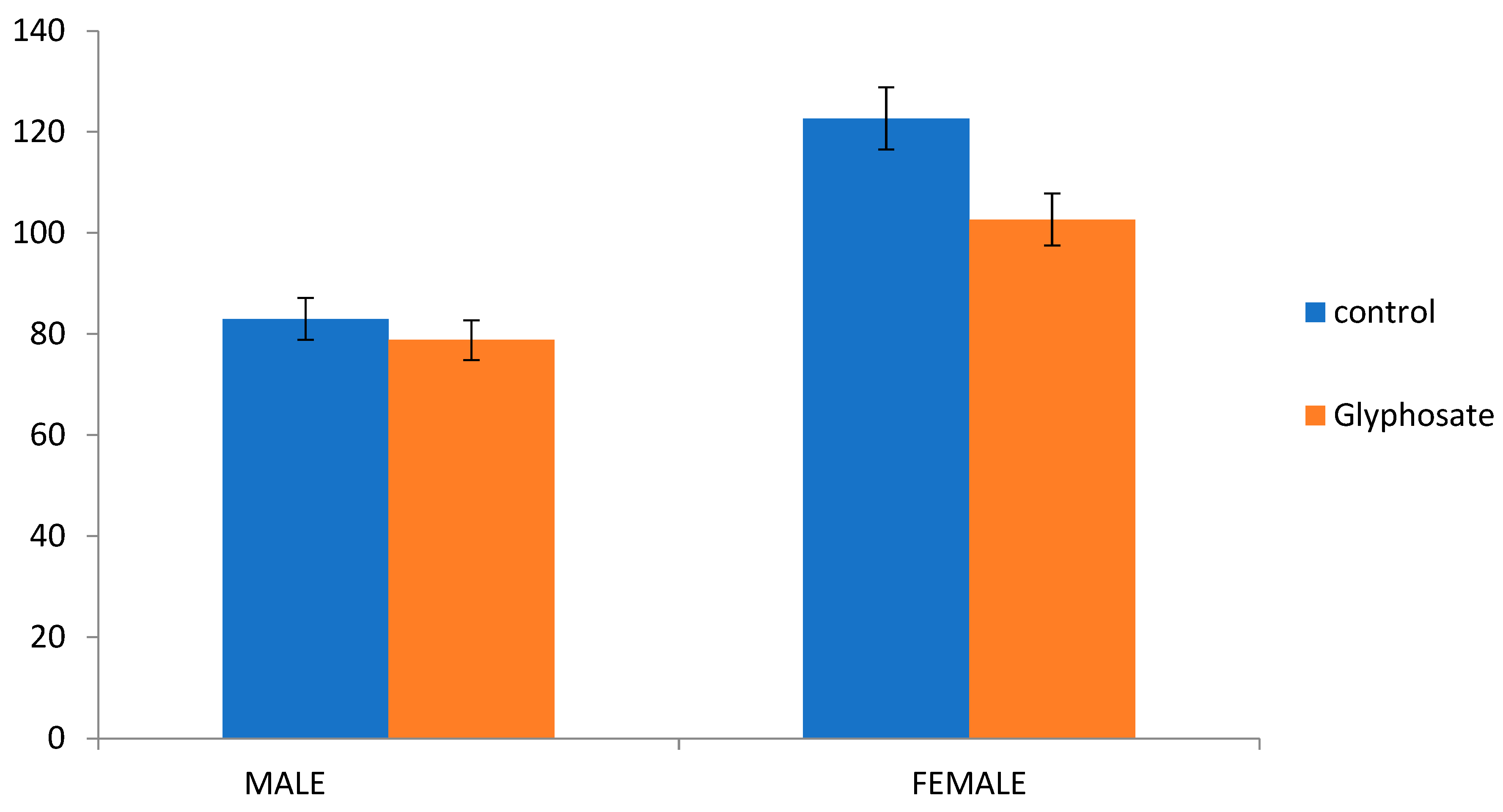Carrot grass (Parthenium hysterophorus L.) is known worldwide as one of the most obnoxious weed of family Asteraceae, cosmopolitan in nature and exhibits erect and ephemeral growing habit. This weed is most accountable for triggering several ailments in human beings and animals; in addition to reduced crop yield and loss of biological diversity (Kumar, 2012). In India, Parthenium weed made its first presence in Pune during 1955 (Rao, 1956) and currently found in the whole country, and occupying an area of approximate 35 million ha in India (Singh et al., 2008). Several efforts were made to effectively bring this weed under control in abroad including India. Biological control of Parthenium hysterophorus through insects viz Smicronyx lutulentus Dietz, Zygogramma bicolorata Pallister, and Epiblema strenuana (Walker) has been reported by some workers (Jayanath and Bali, 1994; Gupta and Bali, 2004), an effective and promising strategy, alternative to chemicals that are environmental unsafe (Singh, 1997). Among several insect, a leaf feeding Zygogramma bicolorata Pallister, host-specific beetle of Coleoptera order and family Chrysomelidae is most effective for successful biological control agents against this weed. This beetle was introduced as a biological control from Mexico to India as a host-specific leaf feeding insect of Parthenium by Indian Institute of Horticultural Research, Bangalore (Jayanth, 1987), and became abundant with in period of 3 years (Jayanth and Visalakshy, 1996). The augmentative release of biocontrol Z. bicolorata was made in India during 1984 and in Jammu and Kashmir in 1989 for effective and successful biological control of Parthenium weed (Gupta et al., 2002). The larvae and adults stages of Zygogramma beetle feed vigorously on leaves and flower of Parthenium (Hasan and Ansari, 2016). However, it was observed that neonatal stages feed voraciously on fresh and young tender leaves. The grubs undergo four moults and, complete development within period of 11 to 14 days.
The integration and simultaneous use of herbicides and biocontrol agents could be an effective and important aspect of integrated pest management (IPM). However, this approach could only be accomplished when applied herbicides are non-toxic to the biological control agent. Only temporary results can be achieved by the use of herbicides against this weed (Sushilkumar et al., 2005). Extensive application of herbicides has become a chief concern in current period, and with excessive negative impact on non-target organisms, and further, we are unable to switch our dependence on these chemicals towards other eco-friendly alternatives for control of weed populations (Stark et al., 2007). These herbicides have induced a series of pollution in all parts of ecosystem. Effect has been observed like increased mortality, reduced fecundity and direct exposure to toxic ingredient may results in multiple lethal and sub lethal effects viz., deformity in developing stages, shortened life span, genetic mutations in offspring's, loss of weight, extent in development period, variations in oviposition behaviour and diminished viability of egg. Thus, it can be pointed out that harmful effects of herbicide application on biocontrol agents can disrupt successful integrated weed management (IWM) programs (Benamu et al., 2010). Therefore, proper selection of right pesticides and correct dose determination would be a key factor for maintaining the optimum natural populations of biocontrol agent and their services render towards ecosystem (Biondi et al., 2015).
Previously, ecotoxicological studies were used previously to assess the toxicity of synthetic chemicals on biocontrol agents. Earlier studies have revealed that some herbicide applications at small doses are much compatible with biological control of weeds (Benamu et al., 2010). For example, glyphosate was found comparatively harmless to Eccritotarsus catarinensis of water hyacinth, and Neochetina eichhorniae (Hill et al., 2012). However, Jayanth and Bali (1993) reported the lethal effect on Z. bicolorata population by commonly used herbicides of Parthenium.
To the best of our knowledge, less literature is available that demonstrate the negative effects on biology attributes of insects as well as on their ecology by herbicides application. However, contrary results on the non-target and target effects of herbicides are also existed in literature (Schneider et al., 2009; Benamu et al., 2010). Thus, the hypothesis of study framed was: herbicides affect development of other inscets; it will also exert negative effect on biological attributes of Z. bicolorata. Thus, the current study was carried out at the laboratory, Division of Entomology, FoA, SKUAST-Jammu, Chatha, India, with the objective to evaluate the detrimental-effects of glyphosate on biological attributes viz., development, fertility of Z. bicolorata as biocontrol agent of Parthenium.
Material and Methods
Collection, Maintenance and Experimental Conditions for Z. bicolorata
A natural population of Zygogramma bicolorata adults were collected from different field’s location during kharif season 2020, and were reared in laboratory, Division of Entomology, SKUAST-J, FoA, Chatha. The Z. bicolorata adults were put in rearing glass jars (50x30cm) containing freshly leaves and apical stems of Parthenium plant in a glass vial filled with sterile water and enclosed with para film, to maintain the leaf freshness for feeding and to lay eggs. Muslin cloth was used to cover the glass jars and incubated consecutively at 26±2◦C, RH of 66±7% and 10 hours of light for 15 Days. The newly hatched grubs were transferred to a fresh vial containing apical Parthenium stems. In order to maintain hygienic conditions, newly developed grubs were transferred into fresh vial on alternate days until they reached to pre-pupal stage. The last instars were shifted to a glass jar of size 10 × 12cm, containing sterilized soil for pupation. The hatched adults from pupation were transferred to Petri dishes (15cm dai) containing fresh leaves of Parthenium plant as nourishment and oviposition. The leaves of Parthenium were kept fresh by distilled water soaked cotton swab wrapping around leaf petiole. Mass culture of Zygogramma bicolorata was kept up to completion of experiments.
Preparation of Herbicides Concentration
Commercial available formulation of glyphosate (Roundup, 41% SL) used for the control of weed Parthenium, procured from the FoA, SKUAST-Jammu, Chatha, India, and was evaluated against the Z. bicolorata in bioassay experiment with lowest range of their field recommended dose (0.44 per cent), as per the guideline issued by Directorate of Plant Protection, Quarantine and Storage, Faridabad, India and Central Insecticide Board and Registration Committee, New Delhi (Anon, 2009). The concentrated form of glyphosate was diluted by slowly adding in distilled water, and thoroughly stirring for half an hour at room temperature. In control only sterilized distilled water was used.
Bioassay of Glyphosate on Z. bicolorata
Fresh Parthenium leaves as well as twigs were dipped in glyphosate @ 0.44 per cent concentration for 2-3 minutes and then dried in air at 25◦C for next 30 min. In control only sterile distilled water was used. Glyphosate treated leaves were kept into vials (200 ml) with water, covered with paraffin film and then kept in glass jars (50x30 cm diameter) after drying. Twenty new moulted Z. bicolorata grubs were taken from the stock culture and released into jar contain glyphosate treated leaves and were replicated 15 times. The glass jars were covered by muslin cloth for proper aeration and were incubated at 26±2◦C, RH of 66±7% and 10 hours of light for 15 Days. After 24 hrs, treated grubs were shifted to clean glass jars (50x30cm diameter) containing healthy leaves until pupation so that the actual mortality of treated grubs was measured.
Indirect Effects of Glyphosate on Development of Z. bicolorata
Development of Immature
Z. bicolorata third instars treated with glyphosate in direct exposure were obtained and kept in Petri plates (15 cm dia) containing fresh leaves of Parthenium. Rearing of Z. bicolorata eggs and experimental conditions were the same as mentioned above. Daily visual observation for hatching of eggs and newly developed larvae were provided with fresh leaves of Parthenium weed regularly, prior to pupation. For determination of instars mortality, live and dead larvae were recorded by observing the exuviae or head capsule. The pre-pupae were transferred moist sterilized sand filled in glass vial (20×10cm) for pupation. Development time of Z. bicolorata, larval stages, prepupae, and pupae was determined in treated and control. Each treatment was replicated 15 times.
Fecundity and Reproductive Attributes
Emerged adults of Z. bicolorata obtained from above experiment were grouped. Each pair of male and female was kept in a glass vials (20×10 cm) and made a total set of 10 pairs and each replicated 15 times. Each pair was fed with leaves of Parthenium for oviposition. Whenever a male died, new male was introduced in glass vial and thus each Z. bicolorata female during its life period had only single male available for mating. Daily observation on eggs laid by female from the first day of oviposition until death of each female was recorded. In order to get the total number of female births, eggs were divided on base of sex ration of 1:1 ratio. Other parameters viz., Pre-oviposition, oviposition adult emergence, post-oviposition of newly emerged adults and egg viability was also checked.
Statistical Analysis
Results obtained from the bioassay study were subjected to ANOVA and post-hoc test Tukey's HSD to compare biological attributes. Statistical analysis was carried through SPSS software.
Result
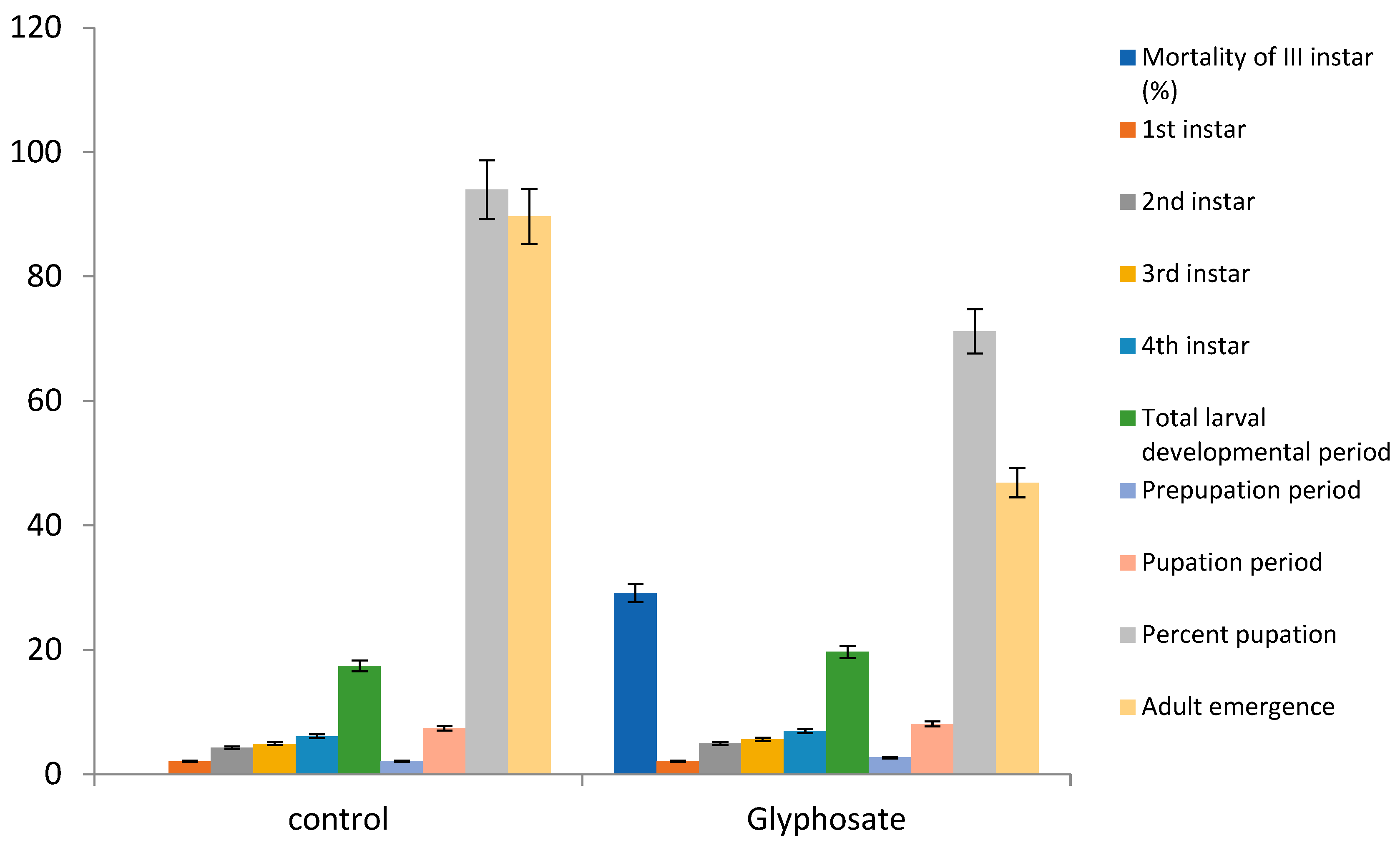
Direct Effect of Glyphosate on Mortality and Larval Development of Zygogramma bicolorata
Results in
Table 1 and
Figure 1 revealed that application of glyphosate significantly affect the performance and development of larval stages of
Zygogramma bicolorata. Glyphosate causes significant (F=0.055; P < 0.33) mortality of 3
rd instar than the control. A total days of 19.70 were required for hatching of eggs when exposed to glyphosate, which was found to be significantly prolonged when compared to non-treated control which took only 17.44 days (F=29.09; df=2,15;
p < 0.00). Shortening of development period of I instars from eggs were observed with the application of glyphosate (2.12 days) but non-significant in comparison to non-treated control that took 2.10 days (F=0.008; df =2, 15;
p> 0.997). Effect of glyphosate herbicide on the developmental stages from first instar to 4
th instar was found non-significant with control. Further, it was observed that application of glyphosate showed considerable effect on “hatching day” and was toxic to egg hatching since it causes delay in total larval developmental period (19.70) (F=29.09; df=2,26;
p <0.001) as compared to the control (17.44 days). The mean prepupation development period decreased significantly with the application of herbicides (F=33.35; df=2, 15;
p <0.008). The average values of glyphosate and control treatments for effect of herbicide on the pupation were 2.72 and 2.13 days, respectively. The pupation period of glyphosate and control treatments was 8.13 and 7.40 days, respectively (F=62.04; df=2,15;
p <0.002 ).The results showed that significant effect of herbicides was observed on the prepupation, pupation and percent pupation stages. Pupation percentage of the treated
Z. bicolorata generation was affected by glyphosate treatment (F=225; df=2, 15;
p<0.004).
Indirect Effects of Glyphosate on Fecundity and Life Indices of Z. bicolorata
Persistent toxicity of the evaluated herbicides with significant effect over untreated control was observed during the bioassay of glyphosate. The maximum effect (minimum adult emergence) was observed due to toxicity of glyphosate (46.88 per cent) in comparison to control (58.68). Results (
Table 1 and
Figure 2) clearly revealed that sex ratio (male proportion) is greatly influenced by glyphosate. Longevity of adult females treated with glyphosate herbicides increased significantly (F=133; df=2, 15;
p <0.000) with mean longevity of 102.67±1.47 compared to control 93.66±1.14. Similar, effect was observed with male longevity which significantly increases (F=24.69; df=2, 15;
p <0.001) to 78.83±0.60 compared to 68.33±2.43 with untreated control. It was observed that sex ratio (proportion of male) was considerably more in glyphosate (1:0.47) than control with only 1:0.41. Glyphosate cause significant negative effect on the longevity of male and females of
Z. bicolorata.
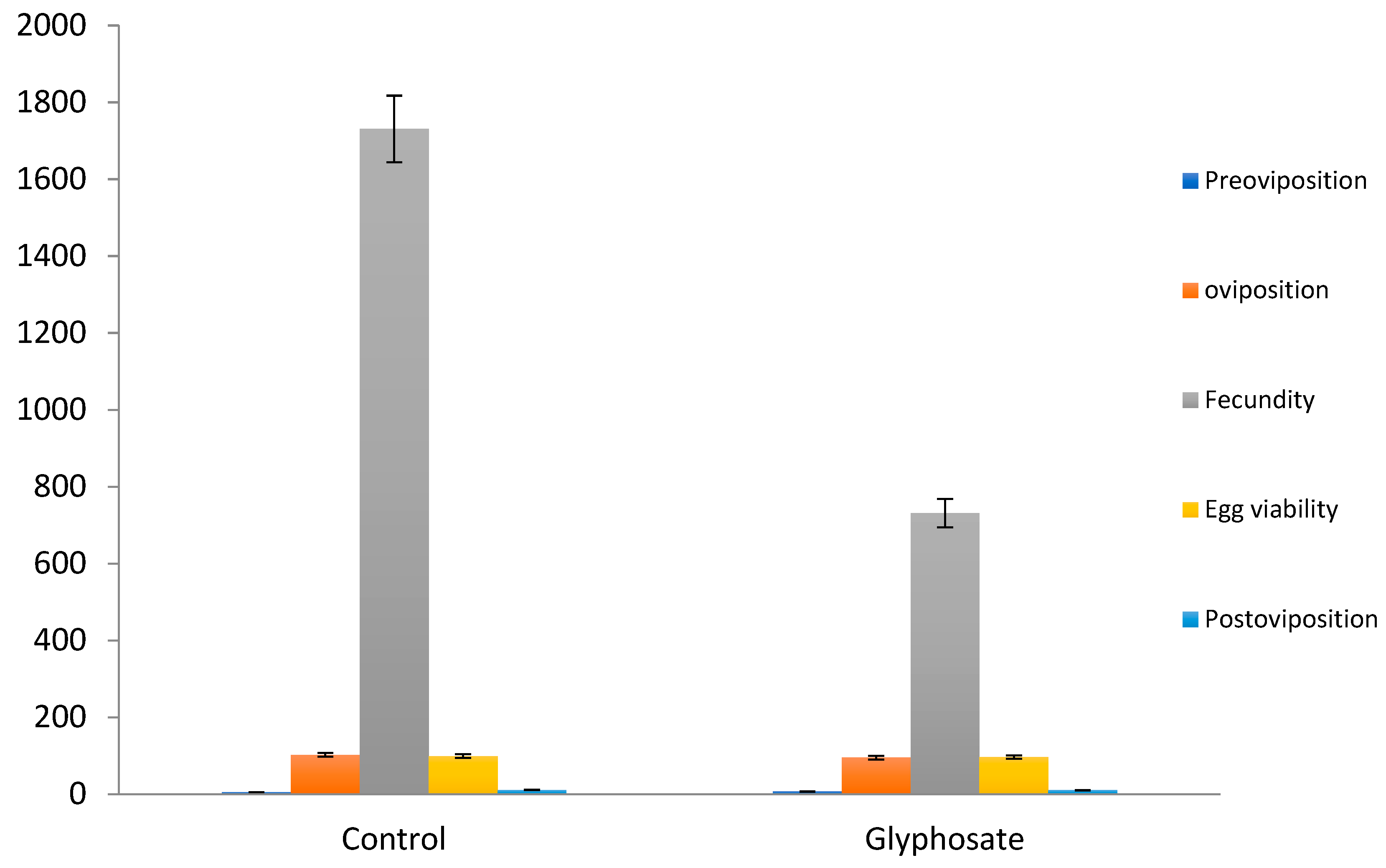
Glyphosate herbicide bioassay showed a significant variation in preoviposition period (F=44.59; df =2, 15;
p< 0.00-) than control non treated group (
Table 2 and
Figure 3). Preoviposition period was prolonged in glyphosate (7.20 days) than control with 5.75 days. Oviposition rate was significantly decreased in females by exposure of glyphosate (F=118; df=2,15;
p <0.000 ). Further, the data revealed that a significant reduction in oviposition resulted in slowdown in immature development when treated with glyphosate. Results also demonstrated that herbicides caused negative effects on fecundity (F=5195; df=2, 15;
p < 0.000).
Average fecundity was highest in untreated control (1730.7) followed by fecundity rate of 731.67. Sever negative effect was observed on fecundity and life indices of Z. bicolorata.
Discussion
Application of glyphosate demonstrated the ecotoxic effects on biology of Z. bicolorata and showed that Z. bicolorata was found susceptible to glyphosate in both direct as well as in indirect exposure experiments, however, susceptibility varied among developmental stages. Further, adverse effects on biological attributes of insect caused by the application of herbicides are limited (Manzoni et al., 2006; Benamu et al., 2010). The results showed that herbicides caused sever negative impact on adult longevity, larval survivorship and, reproduction fittness of Z. bicolorata.
Herbicidal treatment of glyphosate considerably affected larval development of Z. bicolorata. A significant effect was observed in total larval developmental period when exposed to glyphosate (19.70) treatment in comparison to control (17.44 days). However, the developmental period from 1st instar to 4th instar was found non-significant when exposed to glyphosate group including control. The results further reveals herbicide glyphosate were toxic to egg hatching, delay in total larval developmental period as compared to the control. Maximum dosage of herbicides significantly prolonged the duration of T. evanescens development (Sebai and Tawil, 2012). The present findings are also in agreement with other studies which reported that herbicide formulations cause adverse effect on developmental time of biocotrol agents of water hyacinth (Grodowitz and Pellessier, 1990 and Jianqing et al., 1999). The possible effect of prolonged larval development of Z. bicolorata may be due to the indirect effect of herbicides caused on insects by altering the plant growth or destroy the food supplies, which were less favourable to larval development of Z. bicolorata (Hassan,1993). Both prepupaion and pupational period enhanced significantly increased with application of glyphosate than control, however decreased percent pupation and adult emergence was observed with application of glyphosate application. Similarly, in case of indirect effect considerable negative effect were observed in preoviposition, oviposition, fecundity, egg viability and postoviposition with application of herbicides with more pronounced effect in glyphosate. The present findings clearly demonstrate that detrimental effects of glyphosate vary based on various factors like type of herbicide, chemical composition, direct vs. indirect exposure and response towards a chemical at individual genetic level (Center, 1994). Numerous studies have also demonstrated no adverse glyphosate treatment effect on insect herbivores which are used as biological control of several terrestrial weeds (Nelson and Lym, 2003). The possible reason may be low direct toxicity to insects biology due to commercial herbicides including glyphosate, because of its active ingredient and more precisely selected to act as photosynthetic inhibitors (Lindgren et al., 1998). Several workers also reports that herbicides causes numerous sub-lethal effects such as, prolonged development (Schneider et al., 2009), reduced fecundity and alteration in fertility rates (Hassan, 1993, Paoletti and Pimentel, 2000), and fluctuations in oviposition behaviour (Schneider et al., 2009; Stark et al., 2007). The current study also revealed that glyphosate treatment significantly reduced the average fecundity of Z. bicolorata compared to control. These findings are in agreement with other studies which also reported harmful effects on biocontrol agents at different exposure levels of herbicide (Osama et al., 2012).
Conclusions
The present investigation concluded that glyphosate induces a range of lethal and sub-lethal effects on biological attributes of Z. bicolorata, even in minimum recommended field dose. The study revealed that glyphosate induces mortality of 3rd instars, prolongs the development stages of larvae, pre pupation, pupation as well as on percent pupation. Significant negative effect was observed on sex ratio, fecundity and on fitness attributes. The present evaluation provides a new insight on herbicides side-effects on biological traits of Z. bicolorata. Thus, it can conclude that suitable selection of right pesticides and accurate dosage would be a crucial factor for maintaining the natural populations of Z. bicolorata and more evaluation of these glyphosate under field-related conditions to comprehend the ecotoxicological hazards on Z. bicolorata.
Acknowledgments
The authors have no competing or conflicting interests. The author is highly thankful to Division of Entomology, FoA, SKUAST-Jammu, Chatha, India for support, and technical assistance.
References
- Anonymous, 2009. Major Uses of Pesticides. Registered under the Insecticides Act, (1968). Govt. of India, Ministry of Agriculture, Department of Agricultural & Cooperation. Directorate of Plant Protection, Quarantine & Storage, Central Insecticide Board & Registration Committee, N.H.
- Benamu M A, Schneider M I, Sanchez N E. Effects of the herbicide glyphosate on biological attributes of Alpaida veniliae (Araneae, Araneidae), in laboratory. Chemosphere 2010, 78, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Biondi A, Campolo O, Desneux N. Life stage-dependent susceptibility of Aphytis melinus DeBach (Hymenoptera: Aphelinidae) to two pesticides commonly used in citrus orchards. Chemosphere 2015, 128, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Center T, D. 1994. In: Rosen, D., Bennett, F. D., Capinera, J. L. (Eds.), Biological Control of Weeds: Water Hyacinth and Water Lettuce in pest Management in the Tropical, Biological Control in Florida, Perspective. Intercept Ltd., UK: 481-521.
- Ding J Q, Wang R, Fu W D. Effects of Roundup on the mortality of eggs, larvae and adults of Neochetina eichhorniae. Chinese Journal of Biological Control 1998, 14, 152–155. [Google Scholar]
- Hasan F, Ansari M S. Temperature dependent development and demography of Zygogramma bicolorata Pallister (Coleoptera: Chrysomelidae) on Parthenium hysterophorus L. Annals of Applied Biology. 2016. [Google Scholar] [CrossRef]
- Hassan S, A. The mass rearing and utilization of Trichogramma to control lepidopterous pests: achievements and outlook. Pesticide Science 1993, 37, 387–391. [Google Scholar] [CrossRef]
- Hill M P, Coetzee J A, Ueckermann C. The acute toxic effect of herbicides used in water hyacinth control on two insect species released for its biological control in South Africa. Biocontrol Science and Technology 2012, 22, 1321–1333. [Google Scholar] [CrossRef]
- Jayanth K, P. Introduction and establishment of Zygogramma bicolorata on Parthenium hysterophorus at Bangalore. 1987; 56, 310–311. [Google Scholar]
- Jayanth K P, Bali G. Biological studies on Zygogramma bicolorata Pallister (Coleoptera: Chrysomelidae), a biocontrol agent of Parthenium hysterophorus L. (Asteraceae). Biological Control 1993, 7, 93–98. [Google Scholar]
- Jayanth K P, Bali G. Life table of the Parthenium beetle Zygogramma bicolorata Pallister (Coleoptera: Chrysomelidae) in Bangalore, India. Insect Science and its Application 1994, 15, 19–23. [Google Scholar] [CrossRef]
- Jayanth K P, Visalakshy G P N. Succession of vegetation after suppression of Parthenium weeds by Zygogramma bicolorata in Bangalore, India. Biological Agriculture and Horticulture 1996, 12, 303–309. [Google Scholar] [CrossRef]
- Jianqing D, Wang R, Zhiqun C. Weidong F. Jianqing D, Wang R, Zhiqun C. Weidong F. 1998. Towards Integrated Management of Water Hyacinth with Insects and Herbicides in Southern China. In Proceedings of the First IOBC Global Working Group Meeting for the Biological and Integrated Control of Water Hyacinth, eds. MP Hill, MH Julien, and TD Center, -19 (p. 42-147). 16 November.
- Kumar S, Nair G, Singh A P. Evaluation of the larvicidal efficiency of stem, roots and leaves of the weed, Parthenium hysterophorus (Family: Asteraceae) against Aedes aegypti L. Asian Pacific Journal of Tropical Disease, 2012; 2, 395–400. [Google Scholar]
- Lindgren C J, Gabor T S, Murkin H R. Impact of Trichlopyr Amine on Galerucella calamariensis L. (Coleoptera: Chrysomelidae) and a step toward Integrated Management of Purple Loosestrife, Lythrum salicaria. Biological Control, 1998; 12, 14–19. [Google Scholar]
- Manzoni G C, Grutzmacher A D, Giolo P F. Selectivity of pesticides used in integrated apple production for adults of Trichogramma pretiosum. Brazilian Agricultural Research, 2006; 41, 1461–1467. [Google Scholar]
- Nelson J A, Lym R G. Interactive effects of Aphthona nigriscutis and Picloram plus 2, 4-D in Leafy Spurge. Weed Science, 2003; 51, 118–124. [Google Scholar]
- Osama A, Sebai E, Mohamed F. Side-effect of certain herbicides on egg parasitoid, Trichogramma evanescens (West.) (Hymenoptera: Trichogrammatidae). Journal of Applied Entomology, 2012; 5, 1–10. [Google Scholar]
- Paoletti M G, Pimentel D. Environmental risk of pesticides versus genetic engineering for agricultural pest control. Journal of Agriculture and Environmental Ethics, 2000; 12, 279–303. [Google Scholar]
- Rao R, S. Parthenium , a new record for India. Journal of the Bombay Natural History Society, 1956; 54, 218–220. [Google Scholar]
- Schneider M, Sanchez N, Pineda S. Impact of Glyphosate on the Development, Fertility and Demography of Chrysoperla externa (Neuroptera: Chrysopidae): ecological approach. Chemosphere, 2009; 76, 1451–1455. [Google Scholar]
- Sebai O A, Mohamed F, Osama A E. Side-effect of certain herbicides on egg parasitoid Trichogramma evanescens (West.) (Hymenoptera: Trichogrammatidae). Academic Journal of Entomology, 2012; 5, 1–10. [Google Scholar]
- Singh R K, Kumar S, Kumar S. Development of Parthenium based activated carbon and its utilization for adsorptive removal of p-cresol from aqueous solution. Journal of Hazard Material, 2008; 155, 523–535. [Google Scholar]
- Stark J D, Sugayama R L, Kovaleski A. Why demographic and modelling approaches should be adopted for estimating the effects of pesticides on biocontrol agents. Biological Control, 2007; 52, 365–374. [Google Scholar]
- Sushilkumar. Current spread, impact and management of Parthenium weed in India. Int. Parthenium News, 2012; 5, 1–13. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).