1. Introduction
Mangroves form a distal continental fringe along the tropical/subtropical coasts (
Figure 1), which marks the extent of normal tides. Mangrove forests protect coasts and other coastal ecosystems (corals, seagrasses, salt marshes) from erosion and develop a complex physical structure that favors habitat and niche diversification, which allows many terrestrial and aquatic species to thrive, thus enhancing biodiversity and ecological complexity (Laegdsgaard & Johnson 2001; Saenger 2002; Nagelkerken et al., 2008). Mangroves not only provide ecological and cultural services (fisheries, cultivation, aquaculture, timber, fuel, aesthetics, ecotourism) but also contribute to the mitigation of global warming. These communities, along with seagrasses and salt marshes, are among the most important blue-carbon ecosystems – i.e., marine ecosystems that sequester and immobilize carbon, thus removing it from the global cycle – acting as efficient carbon sinks and contributing to the alleviation of atmospheric CO
2 increase (Nellemann et al., 2009; Mcleod et al., 2011; Fest et al. 2022). Presently, mangrove forests are among the world’s most threatened ecosystems (Worthington et al. 2020). According to the latest estimates, the global mangrove extent was reduced by ~25% in only three decades (1980-2010) due to natural and anthropogenic deforestation (Bunting et al. 2022). If these rates are maintained, these ecosystems will be severely reduced during this century and could disappear from the face of the Earth within the next century (Duke et al. 2017).
The Neotropical Caribbean region is one of the main mangrove hotspots (Duke, 2017; Bryan-Brown et al., 2020), and the most relevant threats are urbanization, damming, agriculture, forestry, tourism, fisheries, salt production and shrimp farming (Lacerda et al., 2019). A significant amount of basic ecological information is still needed to properly address the conservation and restoration of Caribbean mangroves. Part of this information may be retrieved from paleoecological and evolutionary records, which provide a natural laboratory where to study the responses of Neotropical ecosystems to long-term natural and anthropogenic drivers of ecological change (Vegas-Vilarrúbia et al., 2011). In the last couple of years, the paleoecological and evolutionary study of Caribbean mangroves has experienced a significant burst with the compilation of a nearly exhaustive database of >140 sites (CARMA, for CARibbean MAngroves) encompassing the whole history of these communities from their origin, ~50 million years ago (Ma), to their anthropization during the last millennia (
Figure 2). The detailed analysis of this dataset has provided new clues on the origin, evolution and biogeography of Caribbean mangroves that challenge classical views and have been published in a collection of recent papers, sorted chronologically (Rull, 2022a, b, c; 2023a, b). A synthetic update of this new information is still unavailable and is the main target of this paper, which highlights the most relevant findings for understanding the shaping of these ecosystems and for informing their conservation.
2. Extant mangroves
The extant Caribbean mangroves are characterized by three main tree genera, known as mangrove-forming trees:
Rhizophora (Rhizophoraceae),
Avicennia (Acanthaceae) and
Laguncularia (Combretaceae) (
Figure 1). The first is the most abundant and widespread and is represented by two species (
R. mangle, R. racemosa), whereas the second has three Caribbean species (
A. germinans, A. bicolor, A. shaueriana) and the third is monospecific (
L. racemosa) (
Table 1). These trees are known as major true-mangrove elements, as they are restricted to mangrove ecosystems, play a major structural role and are able to develop pure stands, possess special morphological adaptations to tidal environments and bear physiological mechanisms of salt exclusion (Tomlinson, 2016). Minor true-mangrove elements share the same features but occupy peripheral habitats, rarely form pure stands and are not major structural elements; they are the tree
Pelliciera rhizophorae (Tetrameristaceae), the herb
Crenea patentinervis (Lythraceae) and the ferns
Acrostichum aureum and
A. danaefolium (Pteridaceae). Other 25 species (
Table 1) are known as mangrove associates, as they are characteristic of these communities but are not restricted to them and lack the adaptations that characterize true-mangrove elements (Tomlinson, 2016). Some important mangrove associates are
Conocarpus erectus (Combretaceae),
Mora oleifera and
Muellera moniliformis (Fabaceae),
Pavonia paludicola and
P. rhizophorae (Malvaceae), and
Tabebuia palustris (Bignoniaceae) (Duke, 2017).
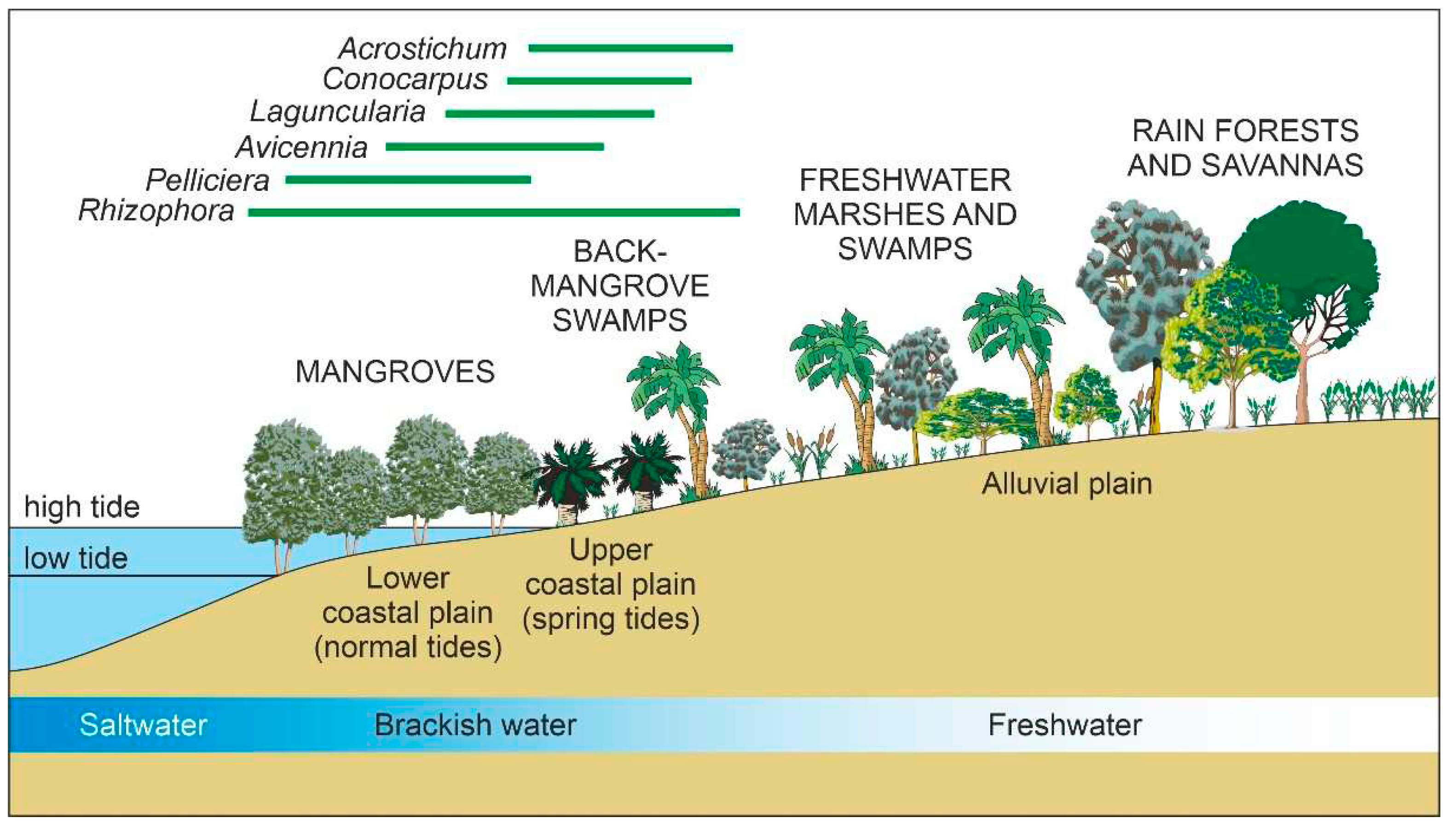
The Neotropical mangrove forests show a characteristic sea-inland zonal pattern with no herbaceous understory, characterized by the sequence
Rhizophora-
Avicennia-
Laguncularia in the more saline zone dominated by normal tides,
Acrostichum and
Conocarpus in the brackish back-mangrove swamps and elevated areas under the influence of spring tides, and
Mauritia and other palms in the more inland freshwater swamps, which mark the transition to the interior savannas and rainforests (
Figure 3). Mangrove zonation is influenced by a diversity of biotic and abiotic factors, notably geomorphology, inundation frequency/intensity, salinity, propagule sorting and competition (Tomlinson, 2016). Biogeographically, Caribbean mangroves belong to the Atlantic-East Pacific (AEP) region (
Figure 1), which is significantly less diverse (17 typical mangrove species belonging to 11 genera and 9 families) than the Indo-West Pacific region, with 54 species (24 genera and 17 families) characteristic of mangroves (Duke, 2017). Among mangrove-forming trees, the genera
Rhizophora and
Avicennia occur in both the AEP and IWP but are represented by different species, whereas
Laguncularia and
Pelliciera occur only in the AEP, with the second restricted to a relictual patch around the Central American Panama Isthmus (Duke, 2020).
3. Evolution
3.1. Eocene origin
According to the classical and more accepted view, the Neotropical mangroves would have originated by regional differentiation from a hypothetical Late Cretaceous (>65 Ma) pantropical mangrove belt along the coasts of the Tethys Sea after the formation of the African barrier by continental drift (
Figure 1). This idea was based on qualitative evidence, usually the presence of pollen and other fossils from assumed mangrove elements, notably
Spinizonocolpites (the fossil representative of the palm
Nypa, characteristic of the IWP mangroves) and
Deltoidospora (the fossil representative of
Acrostichum) (Srivastava & Prasad, 2018). However, a recent analysis of the quantitative palynological evidence has shown that Neotropical mangroves, as ecosystems, likely originated much later, between 50 and 40 Ma (Lutetian, Middle Eocene) (Rull, 2022a). Rather than the evolutionary descendants of a former hypothetical pantropical belt, the Caribbean mangroves signified an evolutionary innovation that emerged de novo, thanks to the evolutionary appearance of
Pelliciera, the oldest known mangrove-forming tree, represented in the fossil record by the pollen morphospecies
Lanagiopollis crassa (also
Psilatricolporites crassus). This tree dominated the Eocene mangrove communities, with
Nypa in the understory,
Acrostichum in the back-mangrove belt and
Mauritia (represented by the fossil pollen
Mauritiidites franciscoi) in the inland freshwater swamps. The communities quickly dispersed across the region and were distributed across the Caribbean area by the Middle/Late Eocene. According to the updated Caribbean database, evidence for mangrove communities before the Middle Eocene is lacking, and the scattered individual appearances of fossil pollen from nonmangrove-forming elements, such as the palm
Nypa or the fern
Acrostichum, are insufficient to support the occurrence of mangrove forests (Rull, 2022a).
3.2. Oligocene revolution
The newly assembled Caribbean database also allowed the identification of a major evolutionary shift that occurred in the Eocene/Oligocene transition, hereafter EOT (~34 Ma), characterized by the replacement of ancient
Pelliciera mangroves by modern-like
Rhizophora mangroves (
Figure 1).
Rhizophora (represented by the fossil pollen
Zonocostites ramonae) was absent from the Neotropics during the Eocene (Graham, 1995) and reached the Caribbean region in the EOT, likely by trans-Atlantic dispersal from the IWP, where it originated (Takayama et al., 2021). Quantitative pollen records showed that the dominance shifted abruptly from
Pelliciera to
Rhizophora in the EOT, coinciding with global cooling and sea-level fall, along with an intense biotic turnover, although not as catastrophic as the Big Five mass extinctions, characterized by enhanced Eocene extinction and Oligocene radiation rates (Coxall & Pearson, 2007; Hutchinson et al., 2021). Noteworthy,
Nypa disappeared from the EAP region during the EOT (
Figure 1). This major community turnover did not signify the disappearance of
Pelliciera, which remained a minor component of the new
Rhizophora mangroves since the Oligocene and expanded its range to the whole Neotropcis in the Miocene, always as a subordinate component represented by small and diffuse populations (Rull, 2023b). It has been suggested that the continuity and further expansion of
Pelliciera, a stenothermic taxon with low dispersal power, was facilitated by the protection offered by
Rhizophora, a more eurthermic and vagile element, whose canopy would have created a microhabitat for the first to endure the new less favorable environments created by the EOT global disruption, as occurs today (Dangremond et al., 2015). After the Miocene,
Pelliciera underwent a significant reduction in its range to an area similar to the Middle Eocene equatorial distribution, which led to its present-day residual distribution (Rull, 2023b).
3.3. Neogene diversification
The main diversification trend of Caribbean mangroves occurred in the Neogene (Mio-Pliocene), when the remaining true mangrove elements (
Crenea,
Avicennia,
Laguncularia) and most of the associated taxa (>20 genera) emerged (Rull, 2023a), thus conforming the present-day richness patterns (
Figure 1). This represented a diversity increase of almost 80% with respect to the Paleogene (Eocene‒Oligocene), when only half of the present-day true-mangrove elements (
Rhizophora, Pelliciera, Acrostichum) were present. No extinctions have been recorded since the Miocene in Caribbean mangroves at the genus level (Graham, 1995). The potential influence of climatic and sea-level fluctuations on the Neogene-Quaternary diversification trend (NQDT) remains unclear.
4. Paleoecology
4.1. Pleistocene reorganization
A significant gap exists in the Caribbean mangrove record for most of the Pleistocene (the last 2.6 Ma), the oldest records dating from ~130,000 years before present (yr BP), which corresponds to the Eemian Interglacial or the Marine Isotopic Stage (MIS) 5e, occurred just before the Last Glaciation (Weichselian). This has been attributed to the lack of full Pleistocene records for the Caribbean region, and the need for developing extensive coring campaigns, especially in marine environments, has been emphasized (Rull, 2022b). In the Caribbean, the Eemian interstadial was characterized by sea-surface temperatures (SST) a few degrees above the present ones and sea levels at least 3 m (maximum estimates of 20 m) higher than today. During the Last Glacial Maximum (LGM), which occurred ~21,000 yr BP, SSTs were 2-4 °C lower than today, and sea levels were up to 120 m below their present position (Schmidt et al., 2006; Hearthy et al., 2007). The extrapolation of these trends to former Pleistocene glacial-interglacial cycles suggests that full Pleistocene records should be sought in deep (>120 m) marine environments beyond the present continental shelf, where the Caribbean coasts were located during glacial maxima. Otherwise, glacial records would be lost due to coastal erosion. The available records suggest that all extant mangrove elements (true and associate) were already present at the beginning of the Pleistocene, and this period was characterized by spatial and community reorganization driven by climatic and eustatic fluctuations (Rull, 2022b), but more studies are needed to test this hypothesis.
4.2. Holocene anthropization
A new external environmental driver, anthropogenic pressure, was added in the Holocene. Although Paleoindian settlements as old as ~13,000 yr BP (Lateglacial) are known for the southern Caribbean coasts (Bryan et al., 1978), the first significant disturbances on mangrove ecosystems did not occur until the Middle Holocene (~6000 yr BP), when Mesoamerican Maya societies cleared these forests using fire, mainly for maize and squash cultivation (Neff et al., 2006; López-Angarita et al., 2016). The Greater and Lesser Antilles (
Figure 1) were colonized by humans between approximately 6000 and 2000 yr BP (Napolitano et al., 2019), and therefore, mangrove disturbance was posterior. Superimposed on the growing Holocene human influence were the maintained temperature and sea level increases and the nondirectional moisture variability (
Figure 1), along with the corresponding feedbacks and synergies among these drivers (Rull, 2020b). Rising sea levels were a major influence on mangrove communities, which responded in different ways, according to the particular features of each locality. The balance between sediment input from the continent and sea-level rise seems to have been crucial for mangrove dynamics. When this balance was biased toward continental terrigenous input, coastal progradation overcame sea-leevel rising and favored seaward mangrove migration of mangrove communities. Conversely, when sea-level rise was dominant, landward migration was favored. Regional moisture declines and increases in drought frequency/intensity were especially important during the Late Holocene, causing significant mangrove reductions by salinity stress caused by increasing evaporation and reduced freshwater input from the continent (Rull, 2022b). Human disturbance has grown during the last millennia, as shown in paleoecological records documenting increased mangrove deforestation for wood extraction, fisheries, coconut plantations and rice crops (González et al., 2010; Urrego et al., 2019).
5. Historical decline
In recent decades, the Caribbean mangrove extent has been drastically reduced by natural and anthropogenic deforestation. According to the most updated data, the total mangrove cover of this region dropped from ~21,000 km2 in 1980 to ~14,000 km2 in 2010 (Table 3). This represents a one-third reduction in three decades, at an average rate of >2300 km2 per decade. If these deforestation rates were maintained, the Caribbean mangroves would totally disappear within the next 60 years. Warming and aridification driven by ongoing anthropogenic climate change, along with the associated acceleration of sea-level rise, can aggravate the situation and exacerbate mangrove loss in the Caribbean and the Neotropics, in general (Ellison & Farnsworth, 1996, Godoy & Lacerda, 2015). In addition to removal, fragmentation is also a threat for Caribbean mangroves, as it increases exposure to environmental stresses and reduces the capacity of these ecosystems to provide ecological services such as coastal protection and carbon sequestration (Bryan-Brown et al., 2020).
Table 2.
Mangrove extent (km
2) by country/island in the Caribbean region, comparing the most updated estimates (2010) with those of 1980 (Rull, 2022b). Country/island codes correspond to
Figure 2.
Table 2.
Mangrove extent (km
2) by country/island in the Caribbean region, comparing the most updated estimates (2010) with those of 1980 (Rull, 2022b). Country/island codes correspond to
Figure 2.
| Country/island |
Code |
1980 (FAO, 2007) |
2010 (Bunting et al., 2022) |
| Anguilla (UK) |
An |
0.90 |
1.00 |
| Antigua & Barbuda |
AB |
15.70 |
8.63 |
| Aruba |
Ar |
4.20 |
0.26 |
| Barbados |
Bd |
0.30 |
0.14 |
| Belize |
Bz |
785.00 |
445.07 |
| Cayman Islands (UK) |
Cy |
10.10 |
41.48 |
| Colombia |
Co |
4400.00 |
2622.12 |
| Costa Rica |
CR |
634.00 |
364.75 |
| Cuba |
Cu |
5374.00 |
3328.16 |
| Dominican Republic |
DR |
344.00 |
187.41 |
| El Salvador |
ES |
467.00 |
375.89 |
| Grenada |
Gr |
2.95 |
1.90 |
| Guadeloupe (France) |
Gp |
30.00 |
37.13 |
| Guatemala |
Gu |
186.00 |
235.23 |
| Guyana |
Gy |
910.00 |
286.40 |
| Haiti |
Ht |
178.00 |
144.32 |
| Honduras |
Ho |
1525.00 |
597.32 |
| Jamaica |
Ja |
120.00 |
94.11 |
| Martinique (France) |
Mr |
19.00 |
20.52 |
| Nicaragua |
Ni |
1034.00 |
739.88 |
| Panama |
Pa |
2500.00 |
1533.37 |
| Puerto Rico |
PR |
76.50 |
86.85 |
| Saint Kitts & Nevis |
SK |
0.85 |
0.28 |
| Saint Lucia |
SL |
2.00 |
16.40 |
| Saint Vincent & The Grenadines |
VG |
0.55 |
0.31 |
| Trinidad & Tobago |
TT |
75.00 |
76.96 |
| Venezuela |
Ve |
2600.00 |
2753.25 |
| Virgin Islands (US and UK) |
VI |
10.10 |
20.53 |
| Caribbean Total |
|
21,295.05 |
13,999.14 |
6. Conservation insights
It is often said that what evolution has taken millions of years to develop might be lost in just centuries. In the case of Caribbean mangroves, this assessment may be quantified, as these ecosystems originated ~50 million years ago and, if current loss rates are maintained, they may disappear in barely half a century. Given the importance of mangroves for terrestrial and marine biodiversity and ecology, as well as for climate change mitigation, the preservation of these ecosystems has been considered a priority in the Caribbean region and a number of local and regional conservation and restoration initiatives have been undertaken that have contributed to alleviate the situation, although they have been insufficient to revert the regional trend toward mangrove loss and fragmentation (Barker, 2002; Polidoro et al., 2010; Lacerda et al., 2019; Bryan-Brown et al., 2020; Grimm et al., 2022; Walker et al., 2022). According to Lacerda et al. (2019), regional assessments and politically coordinated initiatives including the Latin America and Caribbean (LAC) mangrove-bearing countries are fundamental for mangrove conservation and sustainable use. In this framework, empirically-based ecological knowledge is essential for establishing the suitable conservation and restoration baselines. Part of this knowledge may emerge from past paleoecological and evolutionary studies like those summarized above. The below points could contribute to set suitable and realistic conservation/restoration targets and to guarantee their success.
6.1. Evolutionary hints
The Caribbean mangroves were dominated by single mangrove-forming tree species for tens of million years – during the Mid-Late Eocene, when Pelliciera was the dominant tree, and the Oligocene/Early Miocene, under the dominion of Rhizophora. The remaining structural mangrove trees, Avicennia and Laguncularia, did not appear until the Middle and Late Miocene, respectively. This means that preserving the dominant species would be enough to guarantee the continuity of mangroves, as communities, and the preservation and/or restoration of other species may be addressed gradually. An important lesson of the fossil record is that ecological communities do not emerge instantaneously as a whole but by progressive enrichment due to evolutionary innovations and community assembly. Any new species successfully incorporates to the community when the ecological conditions are suitable for them. This would especially useful in restoration practices where the short-term reconstruction of a whole community is difficult or unfeasible and the reproduction of the natural process of community assembly would seem more suitable.
Long-term range expansions and contractions may also be informative for conservation purposes. A characteristic example is that of Pelliciera, which dominated the primeval Eocene mangroves and today is endemic of a small relict equatorial patch. The long-term range shifts of this taxon have been considered to be an example of taxon cycle (Wilson, 1961), characterized by an expansion from its original equatorial range (Eocene) to the whole Neotropics (Miocene) and a further contraction to its extant area of distribution. According to the taxon cycle predictions, Pelliciera could be in its last steps before natural extinction (Rull, 2023b), which could be accelerated by current urban expansion (Blanco-Libreros et al., 2021). Currently, this species is listed as “Vulnerable” in the IUCN Red List of Threatened Species (Polidoro et al., 2010) but it has been suggested that it could be transferred to the “Critically Endangered” category if the predictions of the taxon cycle are considered (Rull, 2023b).
6.2. Paleoecological contributions
Another clue identified in Quaternary studies is the importance of the balance between sedimentation and sea-level shifts, which controls coastal dynamics, with the corresponding lateral mangrove migrations and spatial ecological reorganizations. This point is especially important today, as the Caribbean sea level is rising at rates of 1.8-2.5 mm per year (Palanisamy et al., 2012; Torres & Tsimplis, 2013). In these conditions, in situ mangrove conservation is largely dependent on sedimentation, which is a function of climate and the features of the local fluvial network. As climate cannot be managed, conservation actions should focus on the maintenance of a regular sediment supply able to overcome sea-level rising. This would be especially important in arid climates and areas with insufficient drainage, which might require engineering solutions. Local records of mangrove colonization or degradation under rising or falling sea levels may also be useful to identify the species involved in each successional stage, which can inform restoration practices.
Paleoecological and evolutionary studies also provide evidence-based past analogs to assess the effects of climatic changes on mangrove communities. This study can be addressed regionally or locally. At a regional level, the Caribbean mangroves have been sensitive to long-term temperature and moisture variations but this is hardly applicable to specific conservation problems, as is more dependent on worldwide policies to mitigate the ongoing anthropogenic global warming. At a local level, studies on particular mangrove communities and their response to climatic shifts are highly informative, as they provide site-specific responses to temperature and hydrological balance shifts. These responses could be used as straightforward inputs for forecasting possible future developments or may be incorporated into more general predictive models of regional, continental or global scope, in the search for potential regularities (Anderson et al., 2006). Numerous examples of particular case studies dealing with mangrove responses to climatic and sea-level shifts can be found in the above-quoted database (
Figure 2 and Supplementary Material).
6.3. Anthropogenic pressure
Paleoecological records of anthropization processes are usual for the Mid-Late Holocene, especially for the last centuries. Human activities affecting the Caribbean mangroves may be local and direct (deforestation) or general and indirect (global warming, sea-level rise). The most effective conservation measures are legislation (including the creation of protected areas) and restoration, which are of more local scope, as well as general policies of global change mitigation. Once more, local assessments and actions may benefit from the known history of each particular site in relation to human disturbance, whereas global initiatives may be able to found past analogs in long-term paleoecological and evolutionary studies, along with global paleoecological databases.
7. Conclusions
The conservation of Caribbean mangroves may benefit from paleoecological and evolutionary studies, which have shown the influence of environmental drivers such as paleogeographical shifts, climate changes, sea-level fluctuations and anthropogenic pressure on the biodiversity, biogeography, ecology and evolution of these ecosystems. The lessons learned from these studies may be useful to inform conservation and restoration actions, as they provide evidence-based past analogs that can be incorporated into predictive models aimed at forecasting the responses of these communities to future environmental changes. The availability of a newly assembled and updated database encompassing virtually all the paleoecological and evolutionary studies developed to date on Caribbean mangroves provides an advantage with respect to other mangrove-bearing tropical/subtropical areas that should not be neglected. It is hoped that contributions such as the present will help raise awareness of the importance of past records for conservation and restoration purposes.
Acknowledgments
The author acknowledges Alan Graham, Jan Muller and Thomas Van der Hammen for their fundamental contributions to Neotropical plant evolution and paleoecology, and Estela Di Giacomo and María Antonieta Lorente for teaching on Caribbean palynostratigraphy, along with constant support and encouragement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Anderson, H.J., Bugmann, H., Dearing, J.A., Gaillard, M.-J. 2006. Linking palaeoenvironmental data and models to understand the past and to predict the future. Trends Ecol. Evol. 21, 696-704. [CrossRef]
- Barker, D.R. 2002. Biodiversity conservation in the wider Caribbean region. Rev. Europ. Comp. Int. Environ. Law 11, 1. [CrossRef]
- Blanco-Libreros, J.F., Ramírez-Ruiz, K. 2021. Threatened mangroves in the Anthropocene: habitat fragmentation in urban coastalscapes of Pelliciera spp. (Tetrameristaceae) in northen South America. Front. Mar. Sci. 8, 670354. [CrossRef]
- Bryan, A.L., Casamiquela, R.M., Cruxent, J.M., Gruhn, R., Ochsenius, C. 1978. A El Jobo mastodon kill at Taima-taima, Venezuela. Science 200, 1275–1277.
- Bryan-Brown, D.N., Connolly, R.M., Richards, D.R., Adame, F., Friess, D.A., Brown, C.J. 2020. Global trends in mangrove forest fragmentation. Sci. Rep. 10, 7117. [CrossRef]
- Bunting. P., Rosenqvist, A., Hilarides, L., Lucas, R.M., Thomas, N. 2022. Global Mangrove Watch: updated 2010 mangrove forest extent (v2.5). Remote Sens. 10, 1669. [CrossRef]
- Coxall, H.K., Pearson, P.N. 2007. The Eocene‒Oligocene transition. In: Williams, M., Haywood, A.M., Gregory, F.J., Schmidt, D.N. (Eds.), Deep-Time Perspectives on Climate Change: Marrrying the Signal from Computer Models and Biological Proxies. The Geological Society, London, pp. 351-387.
- Dangremond, E.M., Feller, J.C., Souza, W.P. 2015. Environmental tolerance of rare and common mangroves along light and salinity gradients. Oecologia 179, 1187-1198.
- Duke, N.C. 2017. Mangrove floristics and biogeography revisited: further deductions from biodiversity hot spots, ancestral discontinuities and common evolutionary processes. In: Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R. (Eds.), Mangrove Ecosystems: a Global Biogeographic Perspective. Springer, Cham, pp. 17-53.
- Duke, N.C. 2020. A systematic revision of the vulnerable mangrove genus Pelliciera (Tetrameristaceae) in equatorial America. Blumea 65, 107-120. [CrossRef]
- Duke, N.C., Meyneccke, J.-O., Dittman, S., Ellison, A.M., Anger, K., Berger, U., et al. 2017. A world without mangroves? Science 317, 41b-42b.
- Ellison, A.M., Farnswworth, E.J. 1996. Anthropogenic disturbance of Caribbean mangrove ecosystems: past impacts, present trends, and future predictions. Biotropica 28, 549-565.
- FAO 2007. The World’s Mangroves 1980–2005. FAO Forestry Paper 153, Rome.
- Fest, B.J., Swearer, S.E., Arndy, S.K. 2022. A review of sediment carbon sampling methods in mangroves and their broader impacts on stock estimates for blue carbon ecosystems. Sci. Total Environ. 816, 151618. [CrossRef]
- Godoy, M.D.P., Lacerda, L.D. 2015. Mangrove responses to climate change: a review of recent findings on mangrove extension and distribution. Ann. Acad. Bras. Ciên. 87, 651-667.
- González, C., Urrego, L.E., Martínez, J.I., Polanía, J., Yokohama, Y. 2010. Mangrove dynamics in the southwestern Caribbean since the ‘Little Ice Age’: A history of human and natural disturbances. Holocene 20, 849–861. [CrossRef]
- Graham, A. 1995. Diversification of Caribbean/Gulf mangrove communities through Cenozoic time. Biotropica 27, 20-27. [CrossRef]
- Grimm, K.E., Archibald, J.L., Axelsson, E.P., Grady, K.C. 2022. Follow the money: understanding the Latin America and Caribbean mangrove restoration funding landscape to assist organizations and funders in improved social-ecological aoutcomes. Cons. Sci. Pract. [CrossRef]
- Haug, G.H., Hughen, K.A., Sigman, D.M., Peterson, L.C., Röhl, U. 2001. Southward migration of the Intertropical Convergence Zone through the Holocene. Science 293, 1304–1308. [CrossRef]
- Hearthy, P.J., Hollin, J.T., Neumann, A.C., O’Leary, M.J., McCulloch, M. 2007. Global sea-level fluctuations during the Last Interglaciation (MIS 5e). Quat. Sci. Rev. 26, 2090–2112. [CrossRef]
- Hutchinson, D.K., Coxall, H.K., Lunt, D.J., Steinthorsdottir, M., de Boer, A.M., Baatsen, M., et al. 2021. The Eocene‒Oligocene transition: a review of marine and terrestrial proxy data, models and model-data comparisons. Clim. Past 17, 269-315.
- Iturralde-Vinent, M.A. 2006. Meso-Cenozoic Caribbean paleogeography: implications for the historical biogeography of the region. Int. Geol. Rev. 48, 791-827. [CrossRef]
- Khan, N.S., Ashe, E., Horton, B.P., Dutton, A., Kopp, R.E., Bocard, G., et al. 2017. Drivers of Holocene sea-level change in the Caribbean. Quat. Sci. Rev. 155, 13–36. [CrossRef]
- Lacerda, L.D., Borges, R., Ferreira, A.C. 2019. Neotropical mangroves: conservation and sustainable use in a scenario of global climatic change. Aquat. Cons. 29, 1347-1364. [CrossRef]
- Laegdsgaard, P., Johnson, C. 2001. Why do juvenile fish utilize mangrove habitats? J. Exp. Mar. Biol. Ecol. 257, 229-253.
- Lea, D.W., Pak, D.K., Peterson, L.C., Hughen, K.A. 2003. Synchroneity of tropical and high-latitude Atlantic temperatures over the Last Glacial Termination. Science 301, 1361–1364. [CrossRef]
- López-Angarita, J., Roberts, C.M., Tilley, A., Hawkins, J.P., Cooke, R.G. 2016. Mangroves and people: lessons from a history of use and abuse in four Latin American countries. Forest Ecol. Manag. 368, 1151-162. [CrossRef]
- Mcleod, E., Chmura, G.L., Bouillon, S., Salm, R., Björk, M., Duarte, C.M., et al. 2011. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552-560. [CrossRef]
- Miller, K.G., Browning, J.V., Schmetz, W.J., Kopp, R.E., Mountain, G.S., Wright, J.D. 2020. Cenozoic sea level and chryospheric evolution from deep-sea geochemical and continental margin records. Sci. Adv. 6, eaaz1346.
- Nagelkerken, I., Blaver, S.J.N., Bouillon, S., Green, P., Haywood, M., Kirton, L.G., et al. 2008. The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat. Bot. 89, 155-185. [CrossRef]
- Napolitano, M.F., DiNapoli, R., Stone, J.H., Levin, M.J., Jew, N.P., Lane, B.G., et al. 2019. Reevaluating human colonization of the Caribbean using chronometric hygiene and Bayesian modeling. Sci. Adv. 5, eaar7806. [CrossRef]
- Neff, H., Pearsall, D.M., Jones, J.G., Arroyo, B., Collins, S.K., Freidel, D.E. 2006. Early Maya adaptive patterns: Mid-Late Holocene paleoenvironmental evidence from Pacific Guatemala. Lat. Am. Antiq. 17, 287–315. [CrossRef]
- Nellemann, C., Corcoran, E., Duarte, C., Valdés, L., De Young, C., Fonseca, L., et al. 2009. Blue Carbon. A Rapid Response Assessment. UNEP, GRID-Arendal.
- Palanisamy, H., Becker, M., Meyssignac, B., Henry, O., Cazenave, A. 2012. A regional sea level change and variability in the Caribbean Sea since 1950. J. Geodet. Sci. 2, 125-133. [CrossRef]
- Polidoro, B.A., Carpenter, K.E., Collins, L., Duke, N.D., Ellison, A.M., Ellison, J.C., et al. 2010. The loss of species: mangrove extinction risk and geographic areas of global concern. PLoS ONE 5, e10095. [CrossRef]
- Rull, V. 2022a. The Caribbean mangroves: an Eocene innovation with no Cretaceous precursors. Earth-Sci. Rev. 231, 104070. [CrossRef]
- Rull, V. 2022b. Responses of Caribbean mangroves to Quaternary climatic, eustatic and anthropogenic drivers of ecological change: a review. Plants 11, 3502.
- Rull, V. 2022c. Eocene/Oligocene global disruption and the revolution of Caribbean mangroves. EarthArxiv. [CrossRef]
- Rull, V. 2023a. The Neogene-Quaternary diversification trend in the shaping of Caribbean mangroves. Quat. Sci. Rev. 300, 107920. [CrossRef]
- Rull, V. 2023b. Taxon cycles in Neotropical mangroves. Plants 12, 244. [CrossRef]
- Saenger, P. 2002. Mangrove Ecology, Silviculture and Conservation. Dordrecht: Kluwer. [CrossRef]
- Schmidt, M.W., Vautravers, M.J., Spero, H.J. 2006. Western Caribbean sea surface temperatures during the late Quaternary. Geochem. Geophys. Geosys. 7, 2. [CrossRef]
- Spalding, M., Kainuma, M., Collins, L. 2010. Wolrd Atlas of Mangroves. London: Routledge.
- Srivastava, J., Prasad, V. 2018. Evolution and paleobiogeography of mangroves. Mar. Ecol. 40, e12571. [CrossRef]
- Takayama, K., Tateishi, Y., Kaijita, T. 2021. Global biogeography of a pantropical mangrove genus Rhizophora. Sci. Rep. 11, 7228.
- Tomlinson, P.B. 2016. The Botany of Mangroves. Cambridge Univ. Press, Cambridge.
- Torres, R.R., Tsimplis, N.M. 2013. Sea-level trends and interannual variability in the Caribbean Sea. J. Geophys. Res. 118, 2934-2947.
- Urrego, L.E., Prado, M.A., Bernal, G., Galeano, A. 2019. Mangrove responses to droughts since the little ice age in the Colombian Caribbean. Est. Coast. Shelf Res. 230, 196432. [CrossRef]
- Vegas-Vilarrúbia, T., Rull, V., Montoya, E., Safont, E. 2011. Quaternary palaeoecology and nautre conservation: a general review with examples from the Neotropics. Quat. Sci. Rev. 30, 2361-2388.
- Walker, J.E., Ankersen, T., Barchiesi, S., Meyer, C.K., Altieri, A.H., Osborne, T.Z., et al. 2022. Governance and the mangrove commons: advancing the cross-scale, nested framework for the global conservation and wise use of mangroves. J. Environ. Manag. 312, 114823. [CrossRef]
- Westerhold, T., Marwan, N., Drury, A.J., Liebrand, D., Agnini, C., Anagnostou, E., Barnet, J.S., et al. 2020. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 369, 1383-1387. [CrossRef]
- Worthington, T.A., zu Ermgassen, P.S.E., Fries, D.A., Krauss, K.W., Lovelock, C.E., Thorley, J., et al. 2020. A global biophysical typology of mangroves and its relevance for ecosystem structure and deforestation. Sci. Rep. 10, 15652.
Figure 1.
Present patterns and past trends of Caribbean mangroves. A) Present worldwide mangrove distribution (green patches) (Spalding et al., 2010) and the two main biogeographical regions (AEP, Atlantic-East Pacific; IWP, Indo-West Pacific). The location of the Caribbean region is indicated by a red box. B) The Caribbean region, as considered in this study (GA, Greater Antilles; LA, Lesser Antilles). C) The main genera of mangrove-forming trees (Rull, 2022a, b). D) Paleogene and Neogene evolution of Caribbean mangroves (Rull, 2022a, c, 2023a) in relation to paleographic (Iturralde-Vinent, 2006), paleoclimatic (Westerhold et al., 2020) and paleoeustatic (Miller et al., 2020) shifts. Chronology: Quat, Quaternary; Pli, Pliocene; E, Early, M. Middle; L, Late. Paleogeography: PI, Panama Isthmus. Paleoclimates: EECO, Early Eocene Climatic Optimum; MECO, Middle Eocene Climatic Optimum; EOT, Eocene‒Oligocene Transition; OMT, Oligocene/Miocene Transition; MCO, Miocene Climatic Optimum; Iceh, Icehouse; NQ, Neogene-Quaternary. Polar Ice Caps (IC): NH, Northern Hemisphere. Richness: NQ, Neogene-Quaternary. E) Holocene paleoclimatic (Haug et al., 2002, Lea et al., 2003), paleoeustatic (Khan et al., 2017) and cultural trends and events (Rull, 2022b). SST, Sea Surface Temperature; Ti, Titaniun concentration as a moisture proxy.
Figure 1.
Present patterns and past trends of Caribbean mangroves. A) Present worldwide mangrove distribution (green patches) (Spalding et al., 2010) and the two main biogeographical regions (AEP, Atlantic-East Pacific; IWP, Indo-West Pacific). The location of the Caribbean region is indicated by a red box. B) The Caribbean region, as considered in this study (GA, Greater Antilles; LA, Lesser Antilles). C) The main genera of mangrove-forming trees (Rull, 2022a, b). D) Paleogene and Neogene evolution of Caribbean mangroves (Rull, 2022a, c, 2023a) in relation to paleographic (Iturralde-Vinent, 2006), paleoclimatic (Westerhold et al., 2020) and paleoeustatic (Miller et al., 2020) shifts. Chronology: Quat, Quaternary; Pli, Pliocene; E, Early, M. Middle; L, Late. Paleogeography: PI, Panama Isthmus. Paleoclimates: EECO, Early Eocene Climatic Optimum; MECO, Middle Eocene Climatic Optimum; EOT, Eocene‒Oligocene Transition; OMT, Oligocene/Miocene Transition; MCO, Miocene Climatic Optimum; Iceh, Icehouse; NQ, Neogene-Quaternary. Polar Ice Caps (IC): NH, Northern Hemisphere. Richness: NQ, Neogene-Quaternary. E) Holocene paleoclimatic (Haug et al., 2002, Lea et al., 2003), paleoeustatic (Khan et al., 2017) and cultural trends and events (Rull, 2022b). SST, Sea Surface Temperature; Ti, Titaniun concentration as a moisture proxy.
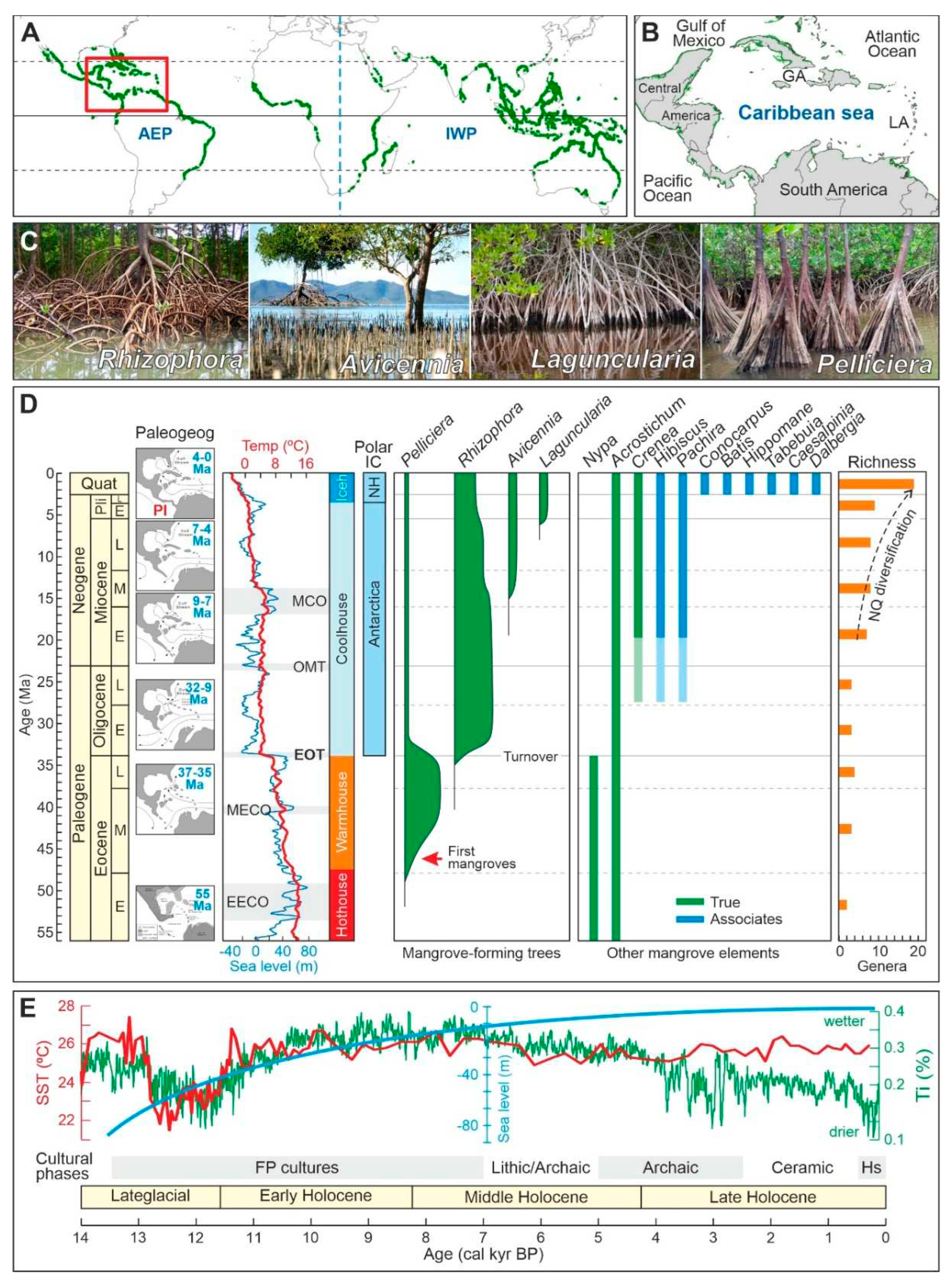
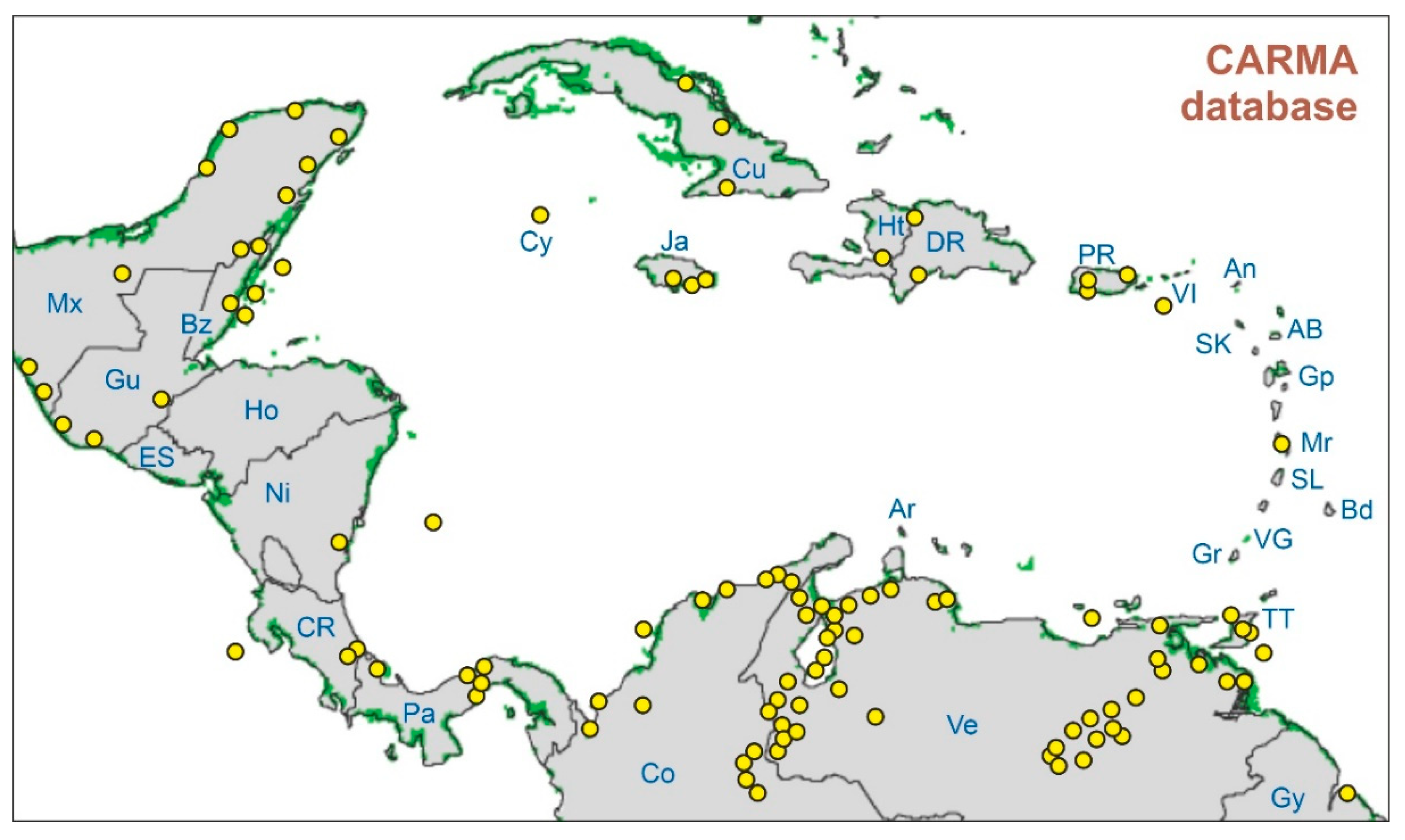
Figure 2.
Sketch-map indicating the location of the study sites compiled in the CARMA (CARibbean MAngroves) database (yellow dots). In a number of cases, a dot corresponds to more than one site (see the Supplementary Material for details and references). Present-day mangroves are indicated by green patches (Spalding et al. 2010). Country/island abbreviations as in
Table 2.
Figure 2.
Sketch-map indicating the location of the study sites compiled in the CARMA (CARibbean MAngroves) database (yellow dots). In a number of cases, a dot corresponds to more than one site (see the Supplementary Material for details and references). Present-day mangroves are indicated by green patches (Spalding et al. 2010). Country/island abbreviations as in
Table 2.
Figure 3.
Idealized transect showing the typical coastal zonation of Caribbean mangroves. The approximate ranges of the most important mangrove elements are indicated. After Rull (2022a).
Figure 3.
Idealized transect showing the typical coastal zonation of Caribbean mangroves. The approximate ranges of the most important mangrove elements are indicated. After Rull (2022a).
Table 1.
True (in bold) and associate Neotropical mangrove elements. Habitats: BC, beach communities; BM, back mangrove; BC, beach communities; CC, coastal communities; CS, coastal swamps; MF, mangrove fringe; RB, river banks; SM, Salt marshes; W, wetlands. Summarized from Tomlinson (2016).
Table 1.
True (in bold) and associate Neotropical mangrove elements. Habitats: BC, beach communities; BM, back mangrove; BC, beach communities; CC, coastal communities; CS, coastal swamps; MF, mangrove fringe; RB, river banks; SM, Salt marshes; W, wetlands. Summarized from Tomlinson (2016).
| Genus |
Family |
Mangrove species |
Life form |
| Acrostichum |
Pteridaceae |
A. aureum, A. danaefolium |
Fern |
| Amoora |
Meliaceae |
A. cucullata |
Tree |
| Amphitecna |
Bignoniaceae |
A. latifolia |
Tree |
| Anemopaegna |
Bignoniaceae |
A. chrysoleucum |
Vine |
| Avicennia |
Acanthaceae |
A. germinans, A. bicolor, A. shaueriana |
Tree |
| Batis |
Batidaceae |
B. maritima |
Shrub |
| Caesalpinia |
Fabaceae |
C. bonduc |
Vine |
| Conocarpus |
Combretaceae |
C. erectus |
Tree |
| Crenea |
Lythraceae |
C. patentinervis |
Herb |
| Dalbergia |
Fabaceae |
D. ecastophyllum, D. amerimnion |
Tree/Shrub |
| Hibiscus |
Malvaceae |
H. tiliaceum |
Tree |
| Hippomane |
Euphorbiaceae |
H. mancinella |
Tree |
| Laguncularia |
Combretaceae |
L. racemosa |
Tree |
| Mora |
Fabaceae |
M. oleifera |
Tree |
| Muellera |
Fabaceae |
M. moniliformis |
Tree |
| Pachira |
Bombacaceae |
P. aquatica |
Tree |
| Pavonia |
Malvaceae |
P. paludicola, P. rhizophorae |
Shrub |
| Pelliciera |
Tetrameristaceae |
P. rhizophorae |
Tree |
| Phryganocydia |
Bignoniaceae |
P. phellosperma |
Vine |
| Pluchea |
Asteraceae |
P. odorata |
Herb |
| Rhabdadenia |
Apocynaceae |
R. biflora |
Vine |
| Rhizophora |
Rhizophoraceae |
R. mangle, R. racemosa |
Tree |
| Rustia |
Rubiaceae |
R. occidentalis |
Tree/Shrub |
| Scaevola |
Goodeniaceae |
S. plumieri |
Shrub |
| Tabebuia |
Bignoniaceae |
T. palustris |
Tree |
| Thespesia |
Malvaceae |
T. populnea, T. populneiodes |
Tree |
| Tuberostylis |
Asteraceae |
T. axillaris, T. rhizophorae |
Shrub |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).