Submitted:
18 January 2023
Posted:
19 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
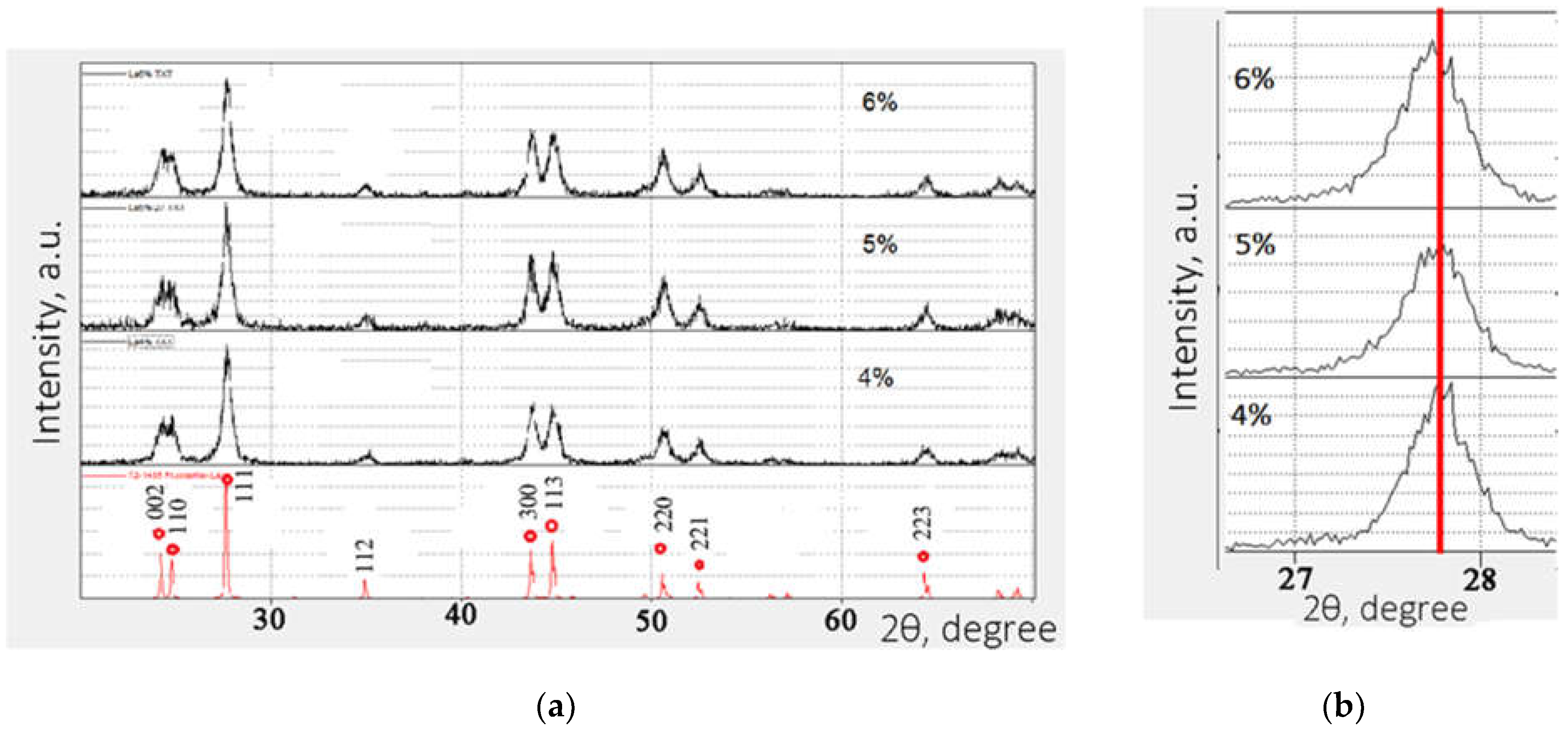
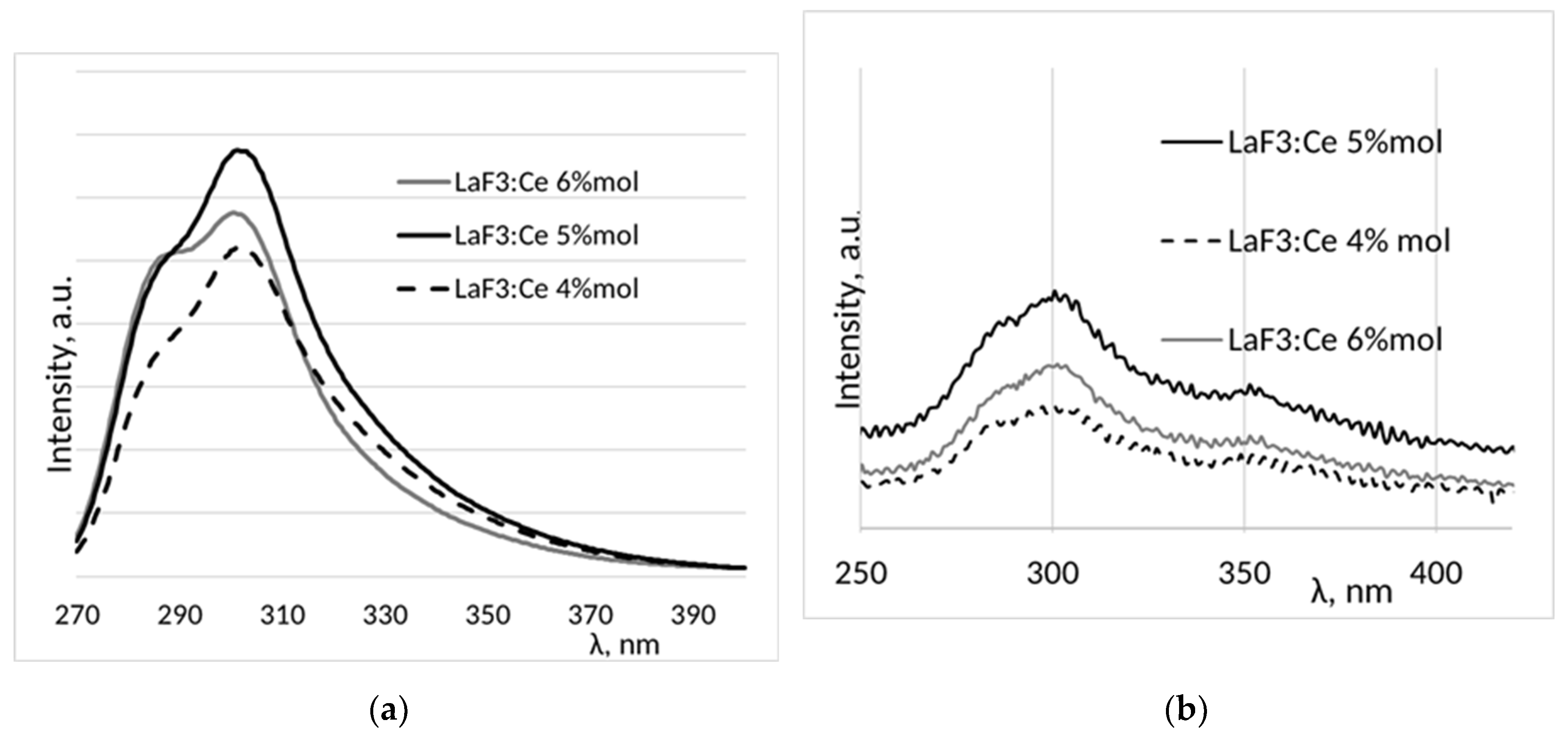
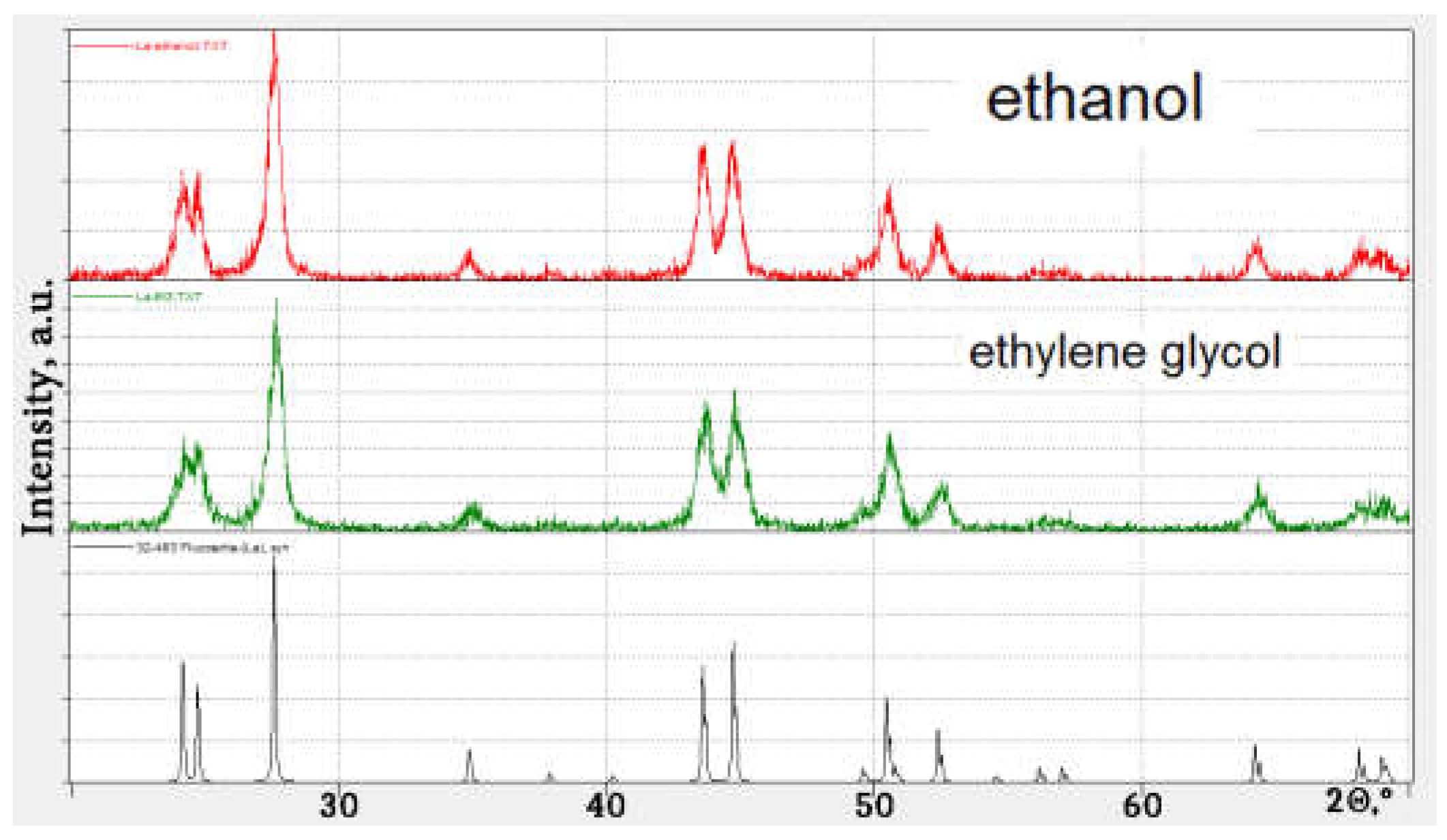
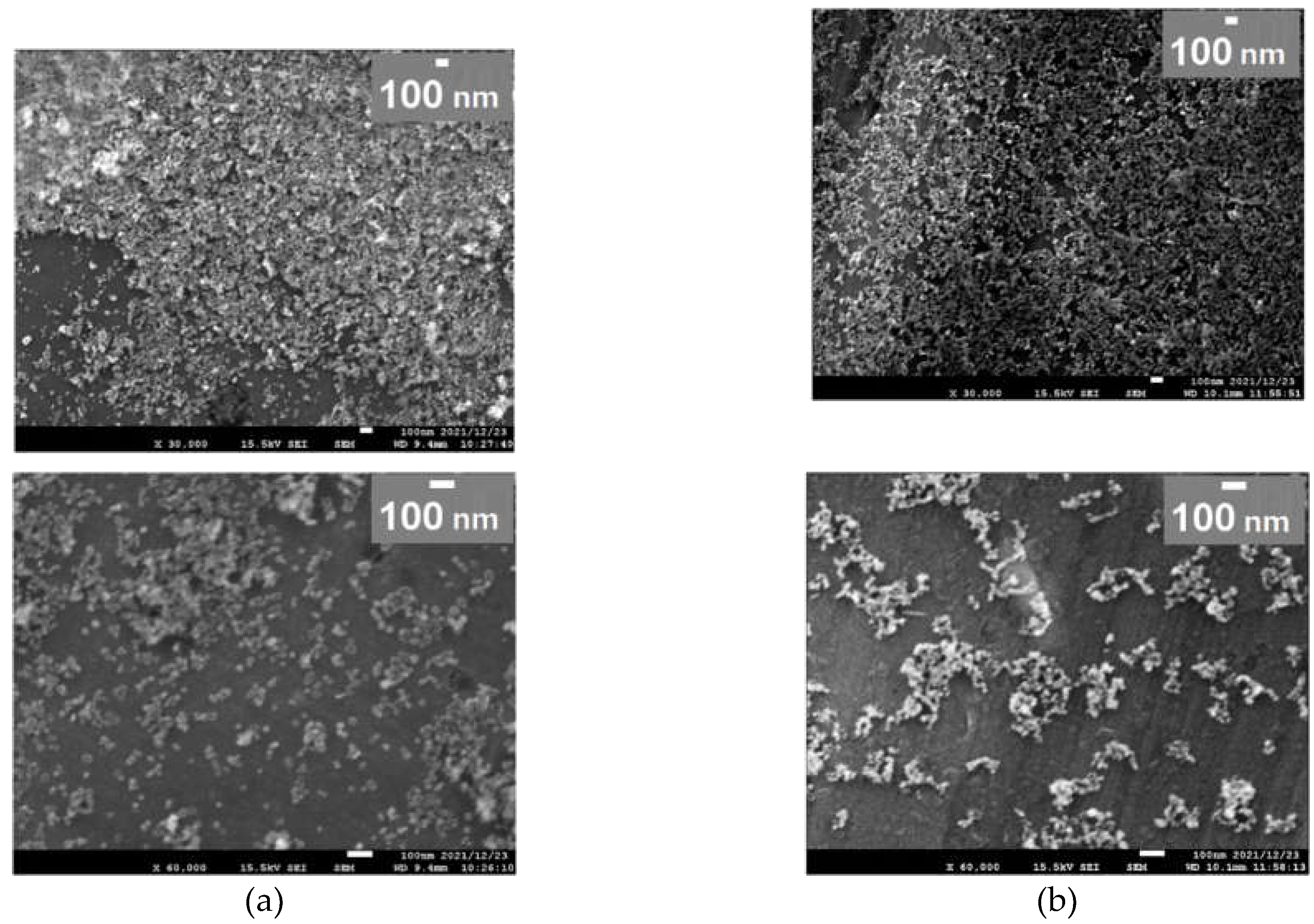
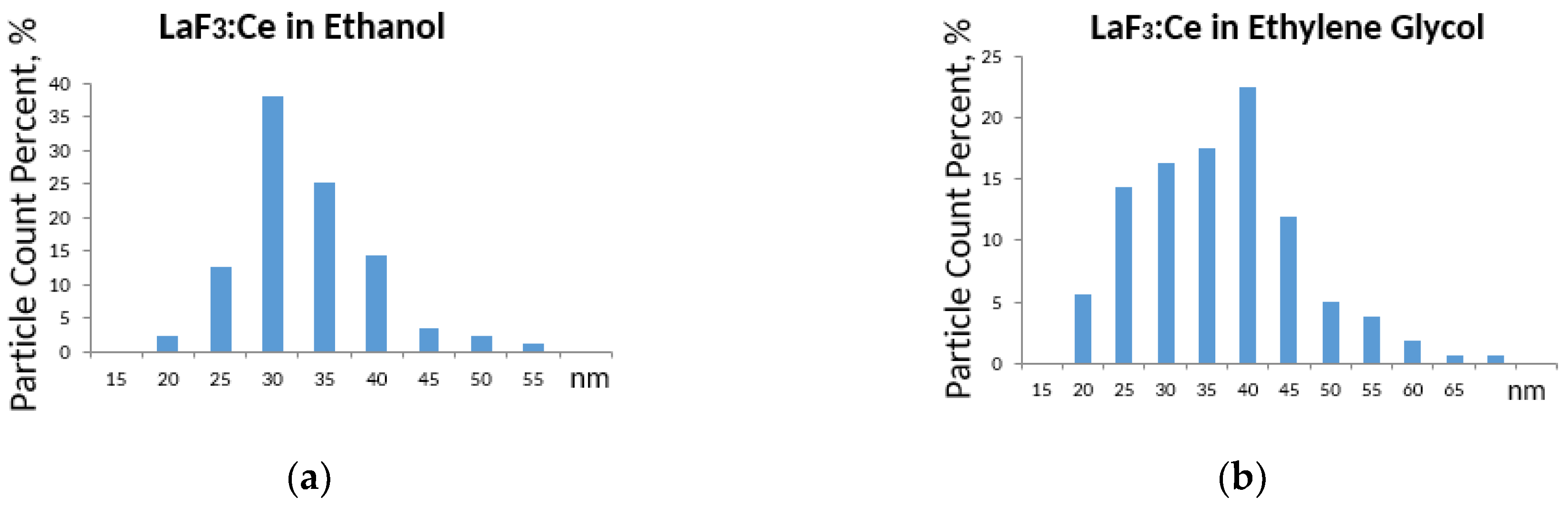
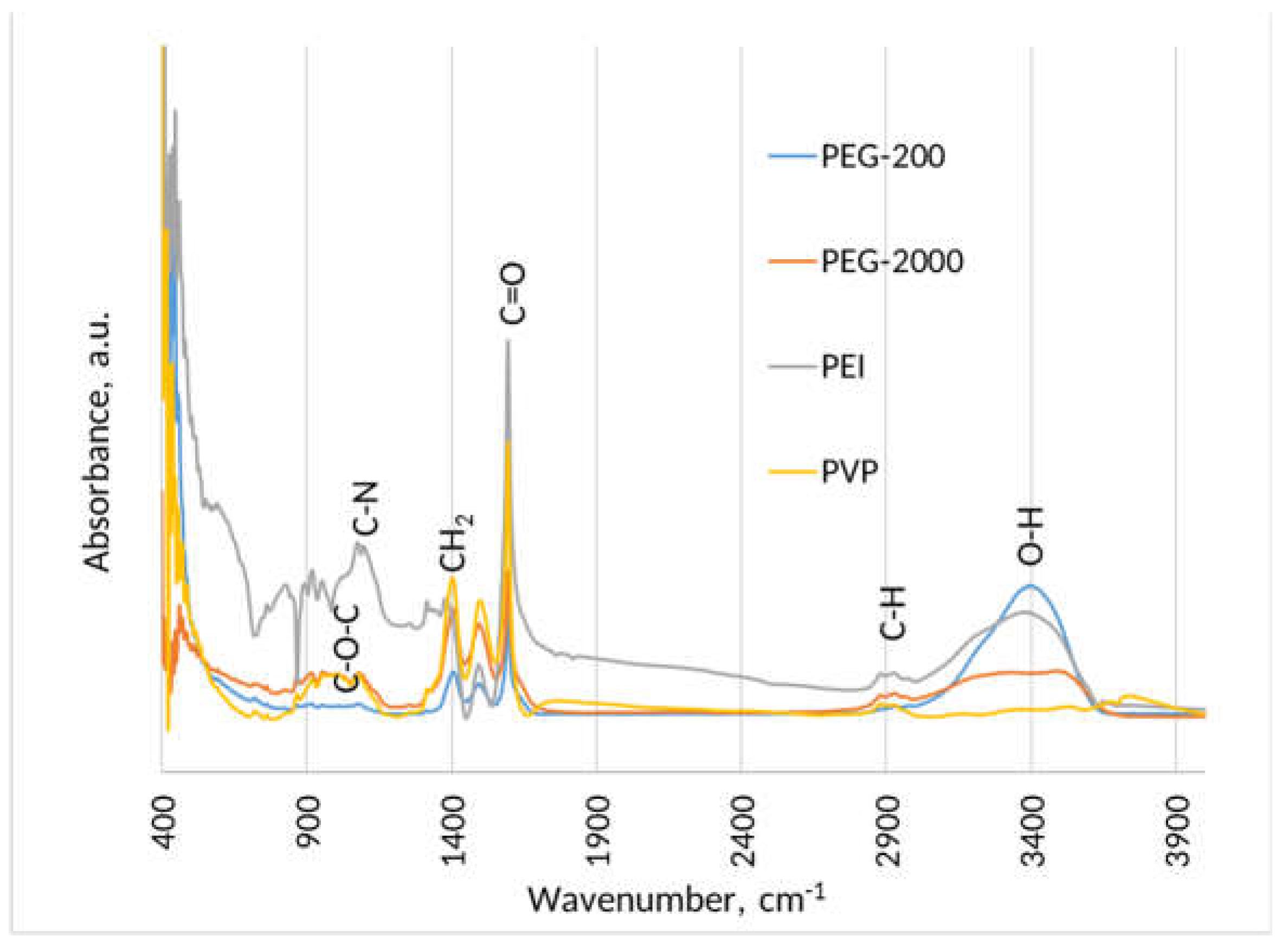
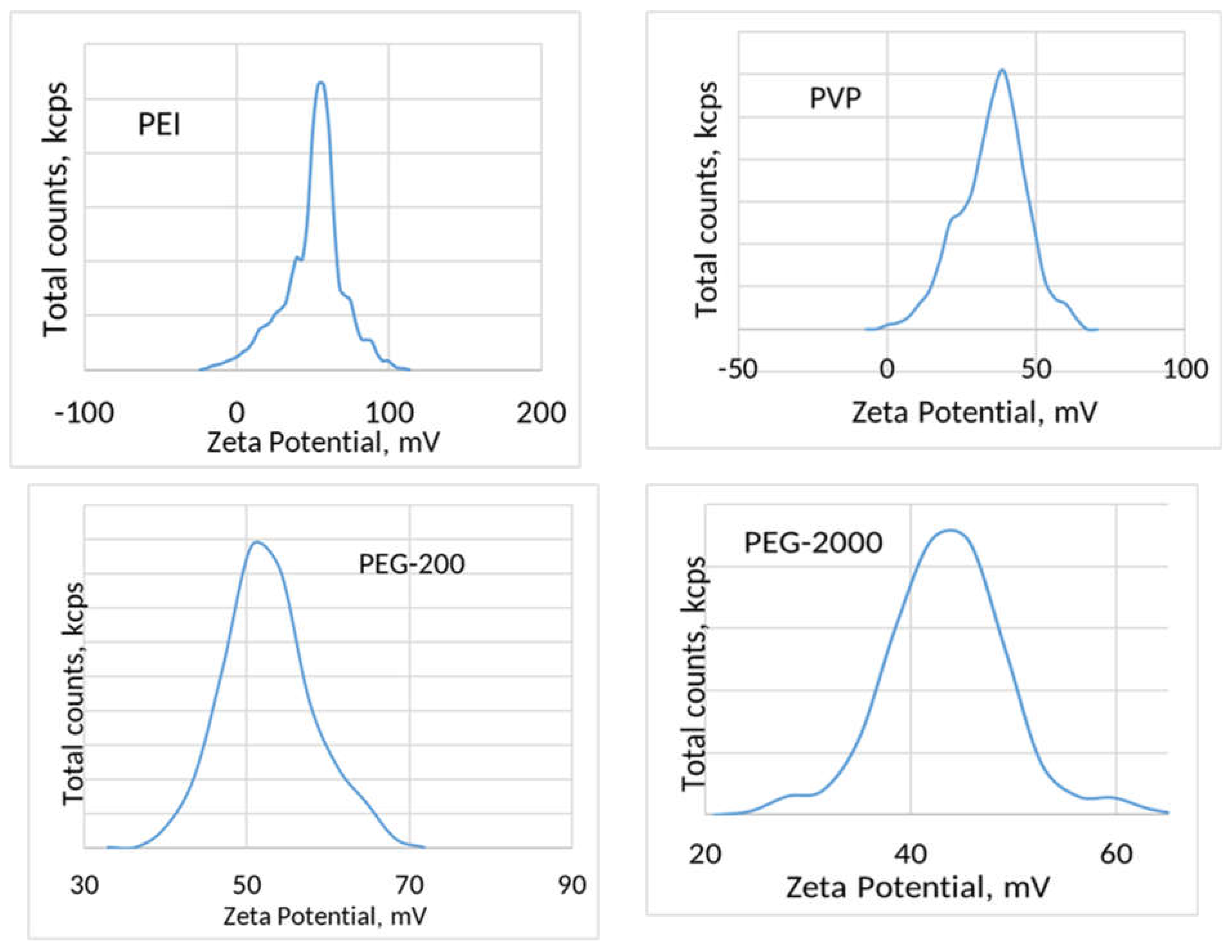
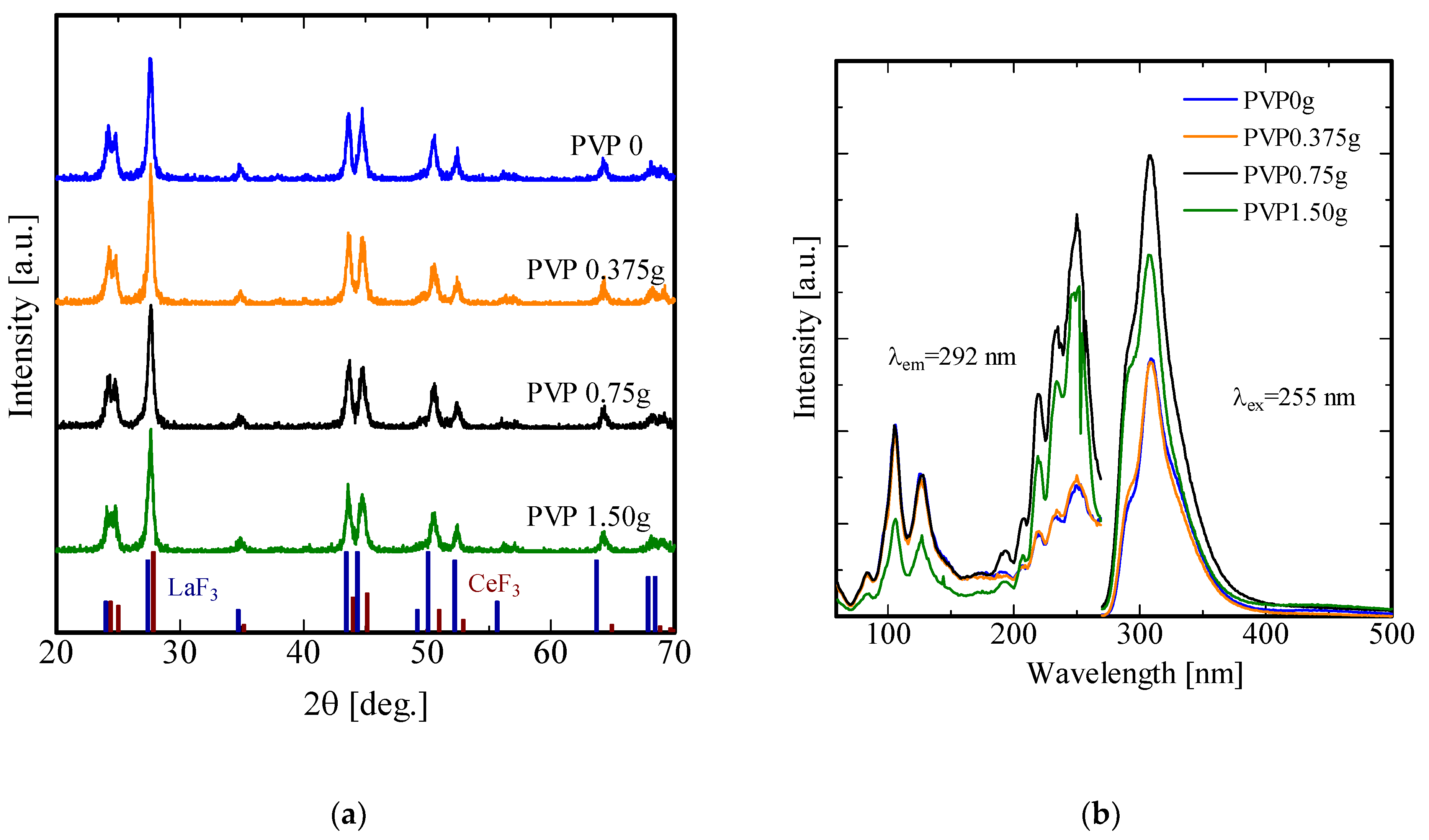
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yan, R.X.; Li, Y.D. Down/Up Conversion in Ln3+-Doped YF3 Nanocrystals. Adv. Funct. Mater. 2005, 15, 763. [Google Scholar] [CrossRef]
- Tan, M.C.; Al-Baroudi, L.; Riman, R.E. Surfactant effects on efficiency enhancement of infrared-to-visible upconversion emissions of NaYF4:Yb-Er. Appl. Mater. Interfaces 2011, 3, 3910–5. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chan, H.L.W.; Li, H.L.; Hao, J.H. Highly efficient low-voltage cathodoluminescence of LaF3:Ln3+ (Ln=Eu3+,Ce3+,Tb3+) spherical particles. Appl. Phys. Lett. 2008, 94, 141106. [Google Scholar] [CrossRef]
- Jia, P.Y.; Lin, J.; Yu, M. Sol–gel deposition and luminescence properties of LiYF4:Tb3+ thin films. Journal of Luminescence 2007, 122–123, 134–136. [Google Scholar] [CrossRef]
- Tuyet, D.T.; Quan, V.T.H.; Bondzior, B.; Dereń, P.J.; Velpula, R.T.; Nguyen, H.P.T.; Tuyen, L.A.; Hung, N.Q.; Nguyen, H.-D. Deep red fluoride dots-in-nanoparticles for high color quality micro white light-emitting diodes. Opt. Express 2020, 28, 26189–26199. [Google Scholar] [CrossRef]
- Kemnitz, E.; Mahn, S.; Krahl, T. Nano metal fluorides: small particles with great properties. ChemTexts 2020, 6, 19. [Google Scholar] [CrossRef]
- Quan, Z.; Yang, D.; Yang, P.; Zhang, X.; Lian, H.; Liu, X.; Lin, J. Uniform Colloidal Alkaline Earth Metal Fluoride Nanocrystals: Nonhydrolytic Synthesis and Luminescence Properties. Inorg. Chem. 2008, 47, 20–9509. [Google Scholar] [CrossRef]
- Heer, S.K.; Kцmpe, *!!! REPLACE !!!*; H.-U., Gьdel; Haase, M. Highly Efficient Multicolour Upconversion Emission in Transparent Colloids of Lanthanide-Doped NaYF4 Nanocrystals. Adv. Mater. 2004, 16, 2102. [Google Scholar] [CrossRef]
- Schietinger, S.; Menezes, L.S.; Lauritzen, B.; Benson, O. Observation of Size Dependence in Multicolor Upconversion in Single Yb3+, Er3+ Codoped NaYF4 Nanocrystals. Nano Lett. 2009, 9, 2477–2481. [Google Scholar] [CrossRef]
- Yi, G.S.; Lu, H.C.; Zhao, S.Y.; Ge, Y.; Yang, W.J.; Chen, D.P.; Guo, L.H. Synthesis, Characterization, and Biological Application of Size-Controlled Nanocrystalline NaYF4:Yb,Er Infrared-to-Visible Up-Conversion Phosphors. Nano Lett. 2004, 4, 2191–2196. [Google Scholar] [CrossRef]
- Nam, S.H.; Bae, Y.M.; Park, Y.I.; Kim, J.H.; Kim, H.M.; Choi, J.S.; Lee, K.T.; Hyeon, T.; Suh, Y.D. Long-term real-time tracking of lanthanide ion doped upconverting nanoparticles in living cells. Angew Chem Int Ed Engl. 2011, 50, 6093–7. [Google Scholar] [CrossRef]
- Zhou, S.F.; Jiang, N.; Miura, K.; Tanabe, S.; Shimizu, M.; Sakakura, M.; Shimotsuma, Y.; Nishi, M.; Qiu, J.R.; Hirao, K. Simultaneous Tailoring of Phase Evolution and Dopant Distribution in the Glassy Phase for Controllable Luminescence. J. Am. Chem. Soc. 2010, 132, 17945–17952. [Google Scholar] [CrossRef]
- Wang, Z.L.; Quan, Z.W.; Jia, P.Y.; Lin, C.K.; Luo, Y.; Chen, Y.; Fang, J.; Zhou, W.; O’Connor, C.J.; Lin, J. Cerium (III) Fluoride Thin Films by XPS. Chem. Mater. 2006, 18, 2030. [Google Scholar] [CrossRef]
- Sivakumar, S.; van Veggel, F.C.J.M.; Raudsepp, M. Bright White Light through Up-Conversion of a Single NIR Source from Sol−Gel-Derived Thin Film Made with Ln3+-Doped LaF3 Nanoparticles. J. Am. Chem. Soc 2005, 127, 12464–12465. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-M.; Chen, W.; W, Z.-G. Luminescence Nanocrystals for Solar Cell Enhancement. Journal of Nanoscience and Nanotechnology 2010, 10, 1418–1429. [Google Scholar] [CrossRef]
- Liu, Y.F.; Chen, W.; Wang, S.P.; Joly, A.G. Investigation of water-soluble x-ray luminescence nanoparticles for photodynamic activation. Appl. Phys. Lett. 2008, 92, 043901. [Google Scholar] [CrossRef]
- Chen, W. Nanoparticle Self-Lighting Photodynamic Therapy for Cancer Treatment. Journal of Biomedical Nanotechnology 2008, 4, 369–376. [Google Scholar] [CrossRef]
- Wang, F.; Han, Y.; Lim, C.S.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature. 2010, 463, 1061–5. [Google Scholar] [CrossRef] [PubMed]
- Moses, W.W.; Derenzo, S.E.; Weber, M.J.; Ray-Chaudhuri, A.K.; Cerrina, F. Scintillation mechanisms in cerium fluoride. Journal of Luminescence 1994, 59, 89–100. [Google Scholar] [CrossRef]
- Yao, M.; Joly, A.G.; Chen, W. Formation and Luminescence Phenomena of LaF3:Ce3+ Nanoparticles and Lanthanide−Organic Compounds in Dimethyl Sulfoxide. J.Phys. Chem. C 2010 2010, 114, 2–826. [Google Scholar] [CrossRef]
- Birowosuto, M.D.; Dorenbos, P.; van Eijk, C.W.E.; Krmer, K.W.; Gudel, H.U. Scintillation properties and anomalous Ce3+ emission of Cs2NaREBr6:Ce3+ (RE = La,Y,Lu). J. Phys. : Condens. Matter 2006, 18, 6133. [Google Scholar] [CrossRef]
- Petousis, I.; Mrdjenovich, D.; Ballouz, E. High-throughput screening of inorganic compounds for the discovery of novel dielectric and optical materials. Sci Data 2017, 4, 160134. [Google Scholar] [CrossRef]
- Tabatabaee, F.; Sabbagh Alvani, A.A.; Sameie, H. Ce3+-doped LaF3 nanoparticles: Wet-chemical synthesis and photo-physical characteristics “optical properties of LaF3:Ce nanomaterials. Met. Mater. Int. 2014, 20, 169–176. [Google Scholar] [CrossRef]
- Canning, A.; Chaudhry, A.; Boutchko, R.; Jensen, N.G. First-principles study of luminescence in Ce-doped inorganic scintillators. Phys. Rev. 2011, 83, 125115. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Spray-drying of solid lipid nanoparticles (SLNTM). European Journal of Pharmaceutics and Biopharmaceutics 1998, 46, 145–151. [Google Scholar] [CrossRef]
- Shah, S.; McRae, A.F.; Marioni, R.E.; Harris, S.E.; Gibson, J.; Henders, A.K.; Redmond, P.; Cox, S.R.; Pattie, A.; Corley, J.; Murphy, L.; Martin, N.G.; Montgomery, G.W.; Starr, J.M.; Wray, N.R.; Deary, I.J.; Visscher, P.M. Genetic and environmental exposures constrain epigenetic drift over the human life course. Genome Res. 2014, 24, 1725–33. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.-R.; Wan, H.-T. Discussion about Several Potential Drawbacks of PEGylated Therapeutic Proteins. Biological and Pharmaceutical Bulletin, 2014, 37, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Sudheendra, L.; Das, G.K.; Li, C.; Stark, D.; Cena, J.; Cherry, S.; Kennedy, I.M. NaGdF4:Eu3+ Nanoparticles for Enhanced X-ray Excited Optical Imaging. Chem. Mater. 2014, 26, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Vinila, V.S.; Isac, J. Design, Fabrication, and Characterization of Multifunctional Nanomaterials; Elsevier, 2022. [Google Scholar]
- Guo, H.; Zhang, T.; Qia, Y.M.; Zhao, L.H.; Li, Z.Q. Ionic Liquid-Based Approach to Monodisperse Luminescent LaF3:Ce,Tb Nanodiskettes: Synthesis, Structural and Photoluminescent Properties. Journal of Nanoscience and Nanotechnology 2010, 10, 1913–1919. [Google Scholar] [CrossRef]
- Nakashima, K.; Ueno, S.; Wada, S. Solvothermal synthesis of KNbO3 nanocubes using various organic solvents. Journal of the Ceramic Society of Japan 2014, 122, 547–551. [Google Scholar] [CrossRef]
- Dorokhina, A.M. Study of the properties of Ce3+-doped fluoride nanophosphors: phase composition, morphology, luminescence. J. Phys.: Conf. Ser. 2021, 2056, 012048. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Wang, X.; Lin, P.H.; Yao, Q.; Chen, C. Chlorotyrosine promotes human aortic smooth muscle cell migration through increasing superoxide anion production and ERK1/2 activation. Atherosclerosis. 2008, 201, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.; Fechner, P.M.; Martin, A.L. , et al. Polyethylenimine-graftpoly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug Chem. 2002, 13, 845–854. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Chapter 3 - Lipid-Based Nanoparticles for Drug Delivery Systems, Editor(s): Shyam S. Mohapatra, Shivendu Ranjan, Nandita Dasgupta, Raghvendra Kumar Mishra, Sabu Thomas, In Micro and Nano Technologies, Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier; pp. 47–76.
- Joseph, E.; Singhvi, G. Chapter 4 - Multifunctional nanocrystals for cancer therapy: a potential nanocarrier, Editor(s): Alexandru Mihai Grumezescu, Nanomaterials for Drug Delivery and Therapy; William Andrew Publishing, 2019; pp. 91–116. [Google Scholar]
- Chen, Y.-P.; Luo, D.-L.; Xu, Q.-Y.; Yang, S.-L.; Tang, T.; Wang, X.-Y. 无机材料学报Structure and Fluorescence Quenching of Li2O-MgO-Al2O3-SiO2 Glasses Doped with Trivalent Cerium. 2014, 29, 967–971. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




