1. Introduction
The in vitro culture (IVC) of embryos is a traditional technique widely used in the in vitro production (IVP) of animal embryos [
1]. IVC has great potential for applications in artificial assisted reproduction technology (ART) and understanding the biological basis [
2,
3,
4]. In the past few decades, although some progress has been made in the field of IVP, the efficiency and quality of IVP are not as good as in vivo production because embryos are affected by the external environment and metabolic characteristics during growth and development [
5,
6,
7]. Oxidative stress and the resulting excess reactive oxygen species (ROS) are examples of in vivo characteristics that affect embryonic development [
8]. Excessive accumulation of ROS can result in various damages to embryo and oocyte, including mitochondrial dysfunction[
9], lipid peroxidation[
10], apoptosis[
10] and DNA damage [
11]. Therefore, it is helpful to improve the in vitro oocyte maturation and subsequent embryo development of animals by continuously studying compounds that exert antioxidant activity by reducing ROS levels [
12].
Among common livestock, pig embryos contain more lipid droplets and will transfer in the early stage of development, which is more susceptible to ROS and oxidative stress [
13,
14]. Therefore, porcine oocytes are often used as a model for antioxidant research during embryonic culture in vitro. Adding antioxidants at the early stage of porcine embryo development can reduce oxidative stress and improve the developmental capacity. Multifarious antioxidants, such as melatonin [
15,
16], laminarin [
17], imperatorin [
18], oroxin A [
19], and wedelolactone [
20], have been reported to have antioxidant activity and can improve the early development ability of porcine embryos. However, the current IVC environment does not greatly imitate the environment in vivo. Therefore, it is indispensable to further elucidate the mechanisms of antioxidant damage and continue to explore effective antioxidants for oocyte culture to improve embryonic development in vitro [
8].
Flavonoids are polyphenolic compounds, which are abundant in nature and food, and have functions related to diet and disease prevention [
21]. Chrysoeriol (CHE), a flavonoid compound also known as 5,7- dihydroxy-2-(4-hydroxy-3-methoxyphenyl) chromen-4-one, is mainly distributed among plants, such as the genuses
Perilla frutescens [
22],
Phyllanthus[
23], and
Salvia verticillata [
24] and the leaves of Digitalis purpurea (foxglove) [
25]. According to previous reports, CHE has various physiological and pharmacological effects, including anti-inflammatory [
25,
26], antioxidant[
24,
27], anti-tumor [
28,
29], and cardiovascular and liver protective effects [
30,
31,
32]. Therefore, CHE is a potent antioxidant, but the effect of CHE on reproductive system and oocytes has not been studied and reported. Therefore, we hypothesized that adding CHE in the IVC stage can improve the quality of oocyte development of porcine embryos by decreasing oxidative stress.
2. Materials and Methods
2.1. Animals and chemicals
All reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated. All manipulations were performed on a heated stage set as 37.5 °C. Porcine ovaries used in this study were obtained from a slaughterhouse (Jiangmen, China).
2.2. Oocyte collection and in vitro maturation (IVM)
Obtained the porcine ovaries from the local slaughterhouse, then store them in a thermos bottle filled with sterile saline solution supplemented with 100 µg/mL penicillin G, keep the ovaries at ~37 °C, and send them to the laboratory within two to three hours. Next, the cumulus–oocyte complexes (COCs) were extracted from ovarian three to eight mm antral follicles by using a 10 mL syringe and an 18-gauge needle. Approximately 100 oocytes were placed in each well of four-well embryo culture plates (Nunc, Roskilde, Denmark) containing 500 µL of mineral oil-covered IVM medium (M199 medium with 10% porcine follicular fluid, 0.91 mM sodium pyruvate, 10 ng/mL of epidermal growth factor, 1µg/mL insulin, 10 IU/mL of follicle-stimulating hormone, and 10 IU/mL of luteinizing hormone). The COCs were cultured at 38.5 °C in an atmosphere of 5% CO2 for 44 to 46 hours.
2.3. Parthenogenetic activation and embryo IVC
After 45 hours of IVM culture, COCs were transferred to 400 µL of 0.1% hyaluronidase for pipetting about 30 times, then oocytes and granulosa cells would be separated. The oocytes were selected and put into the activation solution (300 mM mannitol containing 0.5 mM HEPES, 0.05 mM CaCl2•2H2O, 0.1 mM MgSO4•7H2O, and 0.01% polyvinyl alcohol), and parthenogenetic activation using two direct-current (DC) pulses of 120 V for 60 µs. Next, parthenogenetic activated oocytes were transferred to IVC medium containing 7.5mg/mL cytochalasin B for at 38.5 °C in an atmosphere of 5% CO2 for three hours. Thereby inhibiting the discharge of the second polar body. Oocytes were washed four to five times with IVC after three hours of cytochalasin B treatment, and about 40 to 50 oocytes were placed in each well of the four-well plate with 50 µL IVC medium with or without (0 µM, control group) CHE (TargetMol, USA) that was finally dissolved with IVC at concentrations of 0, 0.1, 1, and 3 µM, and then cultured at 38.5 °C in an atmosphere of 5% CO2 for seven days.
2.4. Total cell number count and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay
Following the manufacturer’s instructions, apoptosis was analyzed using the TUNEL detection kit (Roche Diagnostics, IN, USA). In short, blastocysts formed on the Day 7 were collected and washed four times with phosphate-buffered saline (PBS) with 0.1% polyvinyl alcohol (PBS-PVA). The washed blastocysts were fixed in PBS-PVA containing 3.7% paraformaldehyde for 30 minutes, and then containing 0.1% Triton X-100 permeabilized for 30 minutes, all at room temperature. After permeabilized, the blastocysts were washed in 96-well plate for three to four times, and fixed in blocking buffer (1% BSA in PBS-PVA) for one hour. Next, the blastocysts were incubated with fluorescein-conjugated dUTP and the terminal deoxynucleotidyl transferase enzyme (Roche) in the dark at 37.5 ℃ for one hour. Subsequently, the blastocysts were incubated with 10 µg/mL Hoechst 33342 in the dark at 37.5 ℃ for seven minutes to mark the nuclei. Lastly, inverted fluorescence microscope (Ti2eU; Nikon, Tokyo, Japan) and ImageJ software (NIH, Bethesda, MD, USA) were used to analyze the fluorescence intensities, the numbers of total and apoptotic nuclei. We compared the level of apoptosis by calculating the percentage of apoptotic nuclei in the total number of cells in blastocysts.
2.5. 5-Ethynyl-2′-deoxyuridine (EdU) assay
Following the manufacturer’s instructions, cell proliferation was analyzed using the BeyoClick EdU-555 Cell Proliferation Assay Kit (Beyotime, Shanghai, China). In short, on the Day 6 of oocyte cultured, 10% EdU was added to IVC culture medium with or without CHE, then incubated in the dark at 38.5 ℃ in an atmosphere of 5% CO2 for 10 hours. After incubated, the blastocysts were washed four times with PBS-PVA, fixed in PBS-PVA containing 3.7% paraformaldehyde for 30 minutes, and then containing 0.1% Triton X-100 permeabilized for 30 minutes, all at room temperature After permeabilized, the blastocysts were washed four times, then mixed with 5% BeyoClick Additive Solution and incubated in the dark at 38.5 ℃ in an atmosphere of 5% CO2 for 15 hours. Finally, the blastocysts were incubated with 10 µg/mL Hoechst 33342 in the dark at 37.5 ℃ for seven minutes to mark the nuclei. Using inverted fluorescence microscope (Ti2eU; Nikon, Tokyo, Japan) and ImageJ software (NIH, Bethesda, MD, USA) to count the number of EdU-positive cells and total cells. We compared the level of cell proliferation by calculating the percentage of EdU-positive cells in the total number of cells in blastocysts.
2.6. Intracellular ROS and GSH level assay
We detected ROS and GSH levels using oocytes that had reached the stage of 4-cell development. The oocytes were incubated in PBS-PVA which containing 10 µM 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA, Beyotime, Shanghai, China) and 10 µM 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (CMF2HC, Beyotime, Shanghai, China) in the dark at 37.5℃ for 30 minutes. Next, washing the oocytes four times in PBS-PVA, then placed into 3 µL of droplets of PBS-PVA. Finally, the pictures were photographed using an inverted fluorescence microscope (Ti2eU; Nikon, Tokyo, Japan) and the fluorescence intensity was analyzed using ImageJ software (NIH, Bethesda, MD, USA).
2.7. Mitochondrial membrane potential (MMP, ∆Ψ) assay
The oocytes in the 4-cell stage are also used to detect mitochondrial membrane potential (MMP) that were incubated in PBS-PVA containing 10 µg/mL 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzi-midazolylcarbocyanineiodide iodide (JC-1, Beyotime, Shanghai, China) in the dark at 38.5℃ in an atmosphere of 5% CO2 for 17 hours.After washing the oocytes four times with PBS-PVA, the stained oocytes were put into a 3 µL droplet of PBS-PVA. Using inverted fluorescence microscope (Ti2eU; Nikon, Tokyo, Japan) to detect the red/green fluorescence signals and analyze the fluorescence intensities with ImageJ software. The average mitochondrial membrane potential (MMP) of oocytes were calculated as the ratios of red fluorescence intensity (J-aggregates) to green fluorescence intensity (J-monomers).
2.8. Immunofluorescence staining
The Day 7 blastocysts were collected and were washed four times with PBS-PVA. The washed blastocysts were fixed in PBS-PVA containing 3.7% paraformaldehyde for 30 minutes, and then containing 0.1% Triton X-100 permeabilized for 30 minutes, all at room temperature. Next, the blastocysts were blocked with blocking buffer (1% BSA in PBS-PVA) for one hour. After being blocked, the blastocysts were incubated with a primary LC3B antibody (1:200; Abcam, Cambridge, MA, USA; #ab48394) overnight in the dark at 4°C. The blastocysts were washed four times the next day with PBS-PVA then incubated with a secondary antibody (1:500, Cell Signaling; #8889, for LC3B staining) for one hour at 37.5℃. After incubated, the oocytes were washed five times with PBS-PVA and incubated with 10 µg/mL Hoechst 33342 for 10 minutes. Finally, the oocytes were washed five times with PBS-PVA and detected by inverted fluorescence microscope (Ti2eU; Nikon, Tokyo, Japan). The level of autophagy in the oocytes was measured by the number of LC3B positive dots.
2.9. Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR)
Blastocysts on the Day 7 with similar size and quality were selected from the with or without CHE group, and the extraction of total mRNA was following the manufacturer's instructions using the Dynabeads™ mRNA DIRECT™ Purification Kit (Invitrogen). The first strand cDNA was obtained by reverse transcription using reverse transcription kit (TIANGEN, Beijing, China).
According to the instructions, KAPA SYBR FAST Qpcr Master Mix (2X) Kit (Kapa Biosystems, USA) was used to prepare qRT-PCR systems, each system was 20 µL, which contained 1 µL of cDNA template and 1 µL each of forward and reverse primers.
The qRT-PCR process included initial denaturation for 30 minutes at 93°C, then, 40 cycles were carried out, and the conditions of each cycle were 3 seconds at 95℃, 30 seconds at 60℃ and 20 seconds at 72℃, and a final extension for 30 seconds at 37°C. The gene expression of two groups were analyzed by the LightCycler96, and the 2
−ΔΔCt method with GAPDH was the internal standard based. The annealing temperature of all reactions is 60 C. All primers used are showed in
Table 1.
2.10. Statistical analysis
The number of oocytes/blastocysts (n) used in each group and the number of repeated independent experiments (R) are shown in the figure note. Using the Student's t-test and a one-way ANOVA (Tukey-Kramer) to compare two and more means. The statistical analyze was using SPSS version 22.0 software (IBM Corp, Chicago, IL, USA) and results are expressed as the mean ± standard deviation (SD). ALL results were considered significant by *P < 0.05, **P < 0.01, and ***P < 0.001.
3. Results
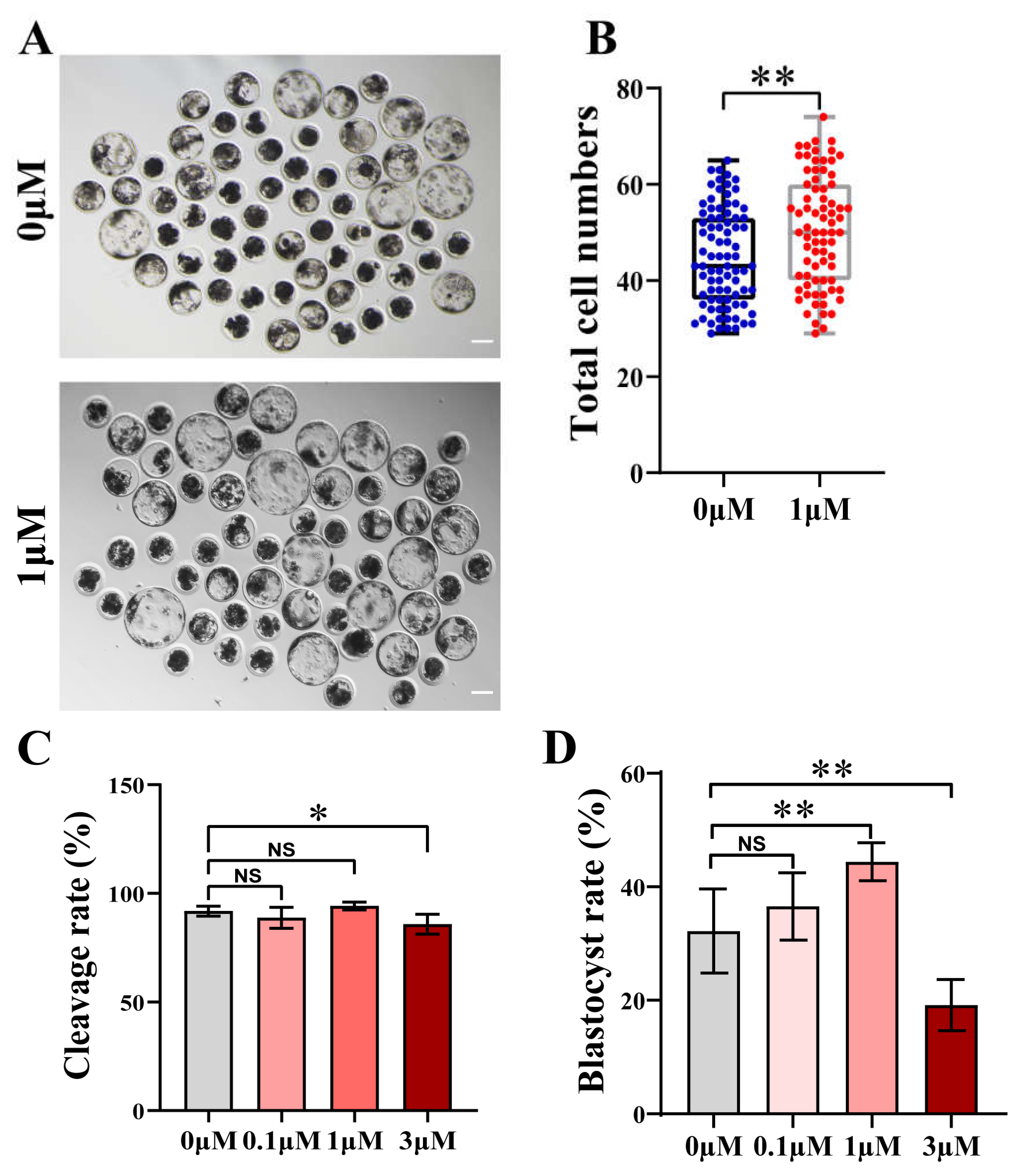
3.1. CHE enhanced the early developmental rates of porcine embryos
In a preliminary experiment, we found that no blastocysts formation was observed at CHE concentrations of 5 μM or 10 μM into IVC medium (data not shown). Then, four concentrations (0, 0.1, 1, and 3 μM) of CHE were added into IVC medium for formal experiments. We found that the development of the cells treated with 1μM was better than that of the other treatment groups (
Figure 1A). The cleavage rate of oocytes in the CHE-treated group was decreased at 3 μM, but other concentrations were not significant difference (
Figure 1C).
In terms of blastocyst formation rate, a significant increase was observed in the 1 μM CHE treatment group, and 1 μM was determined to be the optimal treatment concentration for subsequent experiments (35.15 ± 4.52% vs. 28.39 ± 7.64%, 44.53 ± 4.87%, and 20.22 ± 3.99%; P < 0.05, P < 0.01, P < 0.001) (
Figure 1D).
In addition, the total cell number of blastocysts in the CHE treated group (50.20 ± 11.35%) was obviously higher than that in the control group (44.96 ± 10.11%, P < 0.01) (
Figure 1B).
3.2. CHE improved cell proliferation level and reduced apoptosis of blastocysts
To explore why the development rate of oocytes can be improved after 1 μM CHE treatment in IVC stage, we first detected cell proliferation and apoptosis in blastocysts. The proportion of EdU-positive to total nuclei in the CHE treated group (0.39 ± 0.12) was higher compared with the control group (0.30 ± 0.10, P < 0.001) (
Figure 2C,D). We also measured the level of apoptosis in blastocysts with TUNEL assay, results showed the proportion of apoptotic nuclei to total nuclei in the CHE treated group (0.07 ± 0.04) was decreased compared with the control group (0.14 ± 0.05, P < 0.001) (
Figure 2A,B). As expected, qRT-PCR results showed a significant increase in mRNA expression levels of genes associated with pluripotency and cell proliferation in the CHE treated group, including octamer-binding transcription factor 4 (
OCT4), SRY-box transcription factor 2 (
SOX2) and Nanog homeobox (
NANOG). Among the genes related to apoptosis, the mRNA expression levels of anti-apoptotic gene B-cell lymphoma 2 (
BCL2) was significantly increased, while BCL2-Associated X (
BAX) and caspase 3 (
CASP3) were decreased in the CHE treated group (
Figure 2E,F).
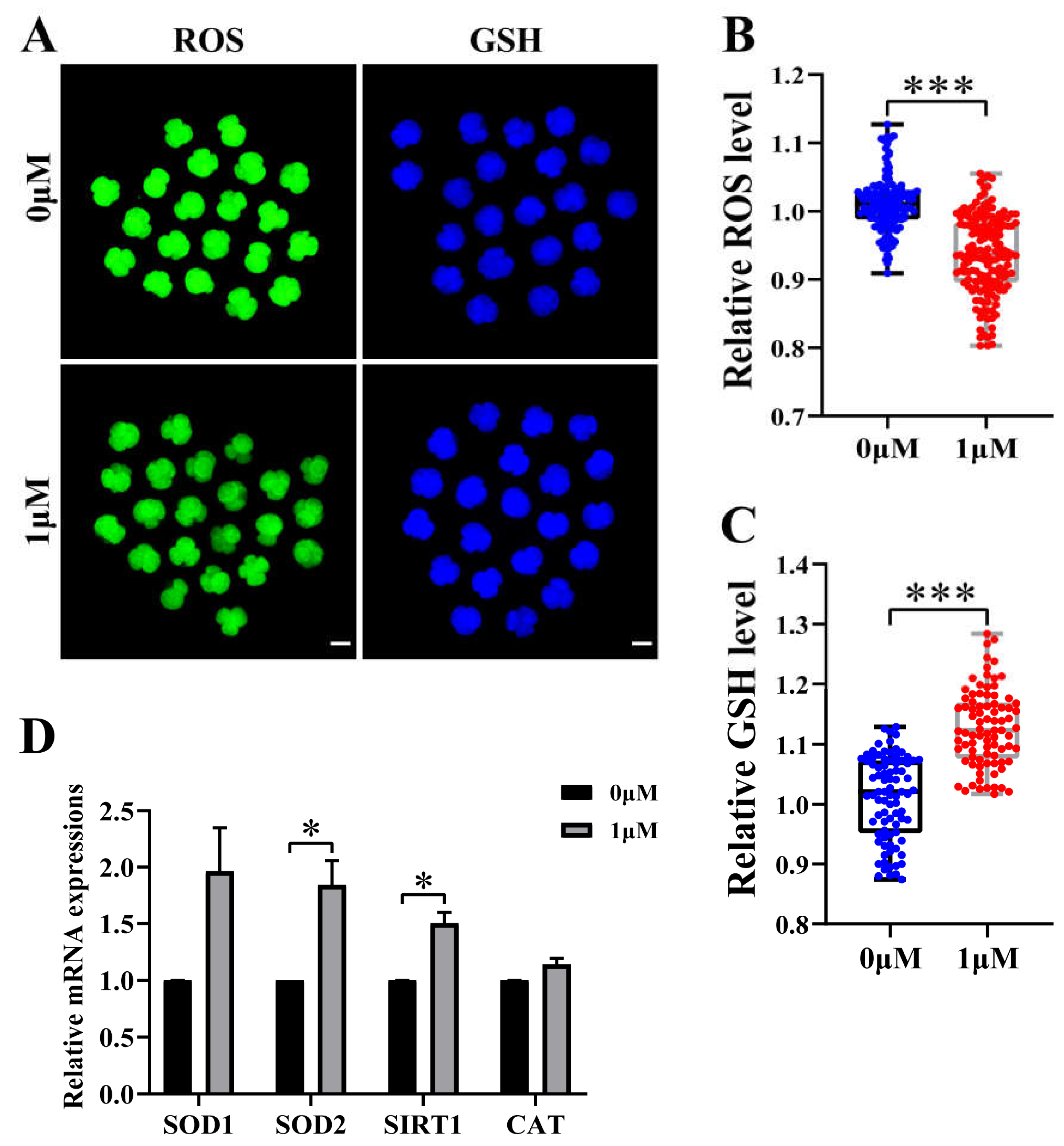
3.3. CHE improved the antioxidant capacity of early-stage embryos
We chose to test reactive oxygen species (ROS) and glutathione (GSH) to explore whether CHE increased antioxidant capacity during early embryonic development. Therefore, we detected the expression levels of ROS with 2′,7′-dichlorodihydrofluorescein diacetate (H
2DCFDA) and GSH with 4-chloromethyl-6,8-difluoro-7-hydroxycoumarin (CMF
2HC) at the 4-cell stage of porcine embryos (
Figure 3A), respectively. The staining results showed that the relative level of ROS in the CHE-treated group was much lower than in the control group (P < 0.001) (
Figure 3B). At the same time, the relative level of GSH in the CHE-treated group was significantly higher than in the control group (P < 0.001) (
Figure 3C). As expected, qRT-PCR results showed a significant increase in mRNA expression levels of genes associated with oxidative stress in the CHE treated group, including superoxide dismutase type 1 and 2 (
SOD1 and
SOD2), sirtuin 1 (
SIRT1), and catalase (
CAT) (
Figure 3D).
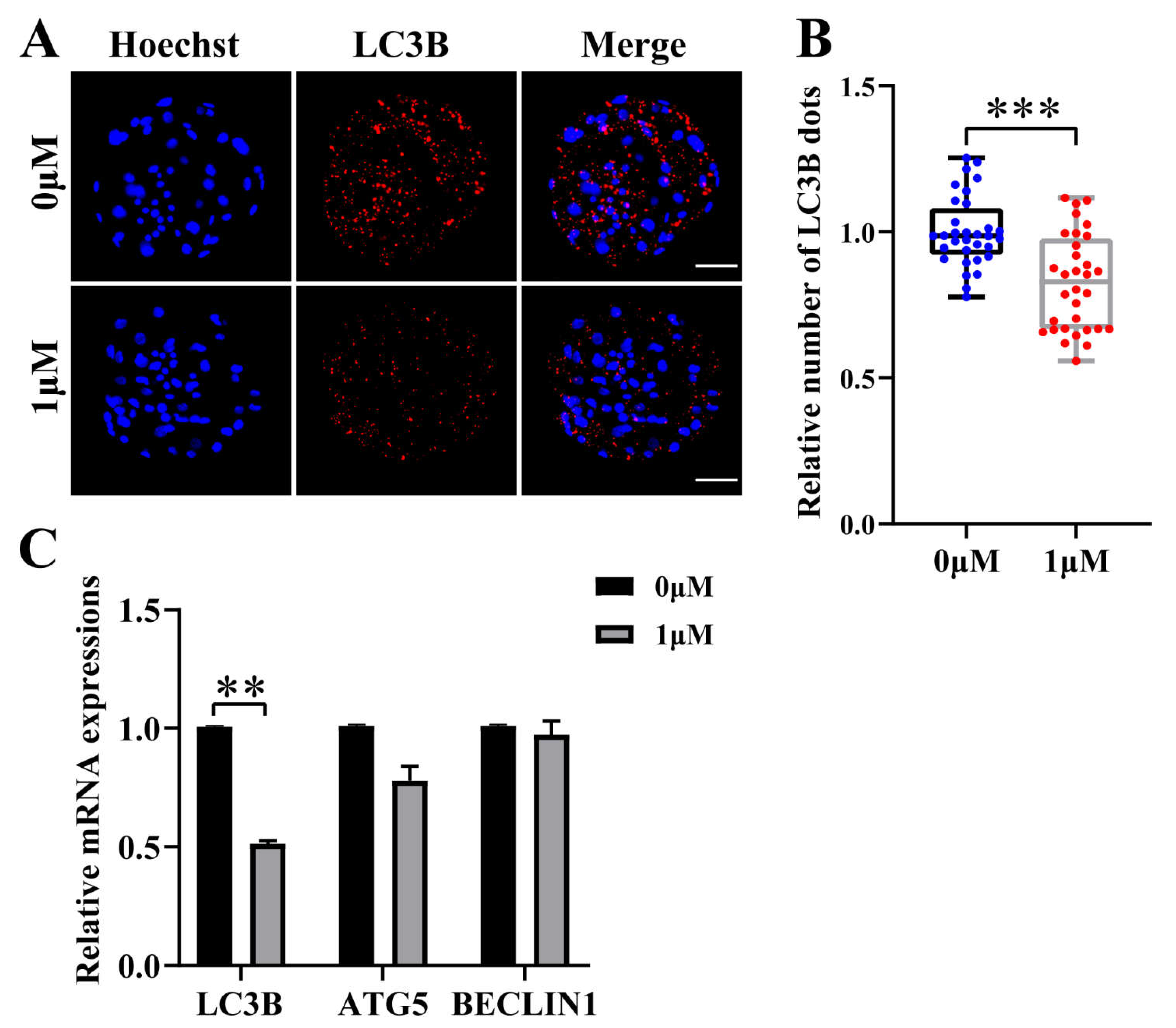
3.4. CHE reduces autophagy levels during porcine embryo development
To evaluate the effect on the level of autophagy in the embryo after the addition of CHE, blastocysts were stained and the number of LC3B-positive spots was counted. (
Figure 4A), which are closely related to the level of autophagy and are widely used to monitor autophagic activity. The results showed that the number of LC3B positive dots in CHE treatment group was significantly lower than that in control group. (P < 0.001) (
Figure 4B). As expected, qRT-PCR results showed that decrease in mRNA expression levels of genes associated with autophagy in the CHE treated group, including microtubule associated protein 1 light chain 3 beta (
LC3B), autophagy-related gene 5 (
ATG5) and
BECLIN1 (
Figure 4C).
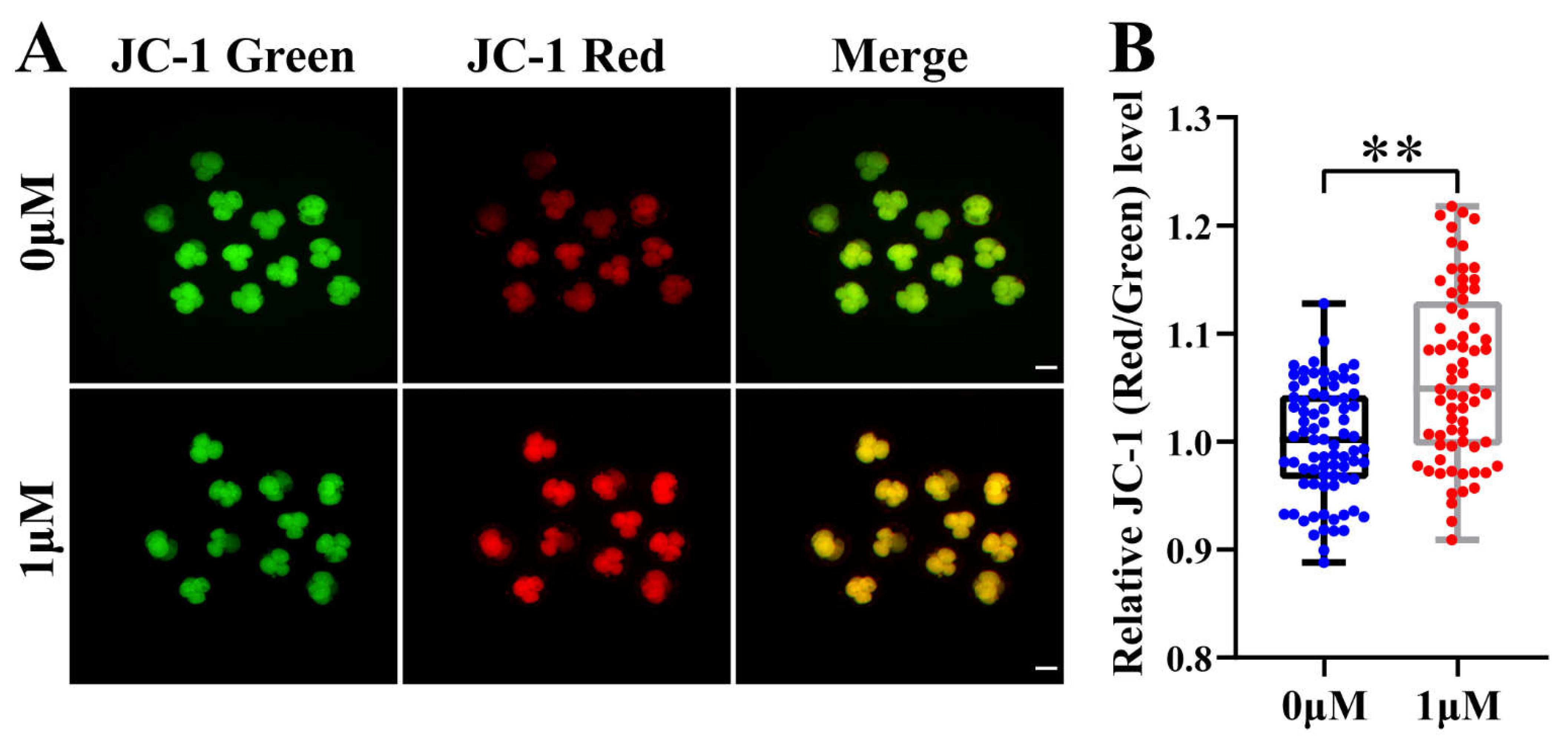
3.5. CHE improved the mitochondrial function of early-stage porcine embryos
Mitochondrial membrane potential (MMP,
ΔΨm) is often used to measure mitochondrial function and is also an important factor affecting embryonic development. We have already shown that CHE supplementation increases cell proliferation and improves embryonic development. Therefore, to further explore other potential mechanisms of CHE, we assay the MMP at the 4-cell stage of oocytes. In this experiment, JC-1 stain was selected to detect MMP, and the evaluation index was the ratio of fluorescence intensity of JC-1 in different colors (red/green). Representative images showed that MMP was increased in the CHE treatment group than in the control group (P < 0.01) (
Figure 5A,B). According to the results, CHE can up-regulate MMP to improve mitochondrial function and prevent mitochondrial damage and defects.
4. Discussion
The mature quality and subsequent developmental potential of embryos cultured in vitro are not as potential as in vivo embryos due to the inability to achieve a perfect IVC medium and the difference of cell external environment [
33,
34]. In addition, embryos are susceptible to ROS-induced oxidative stress during IVC culture [
35], and oxidative stress can lead to stunted embryonic development and various early damage.
Recently, research on antioxidants in food and compounds has become increasingly widespread. The previous research on the chemical chrysoeriol (CHE) mainly focused on its extraction from plants and some antioxidant activity, but its effect on the reproductive system has not been reported. Thus, we speculate that CHE can improve embryo development due to its antioxidant effects.
In this experiment, parthenogenetic activation was used to simulate the in vivo fertilization process, and the oocytes were used to simulate the development process of zygote in vitro. Parthenogenetically activated oocytes form cavities with inner cell masses and fluid when cultured to six to seven days and contain trophoblasts, called blastocysts [
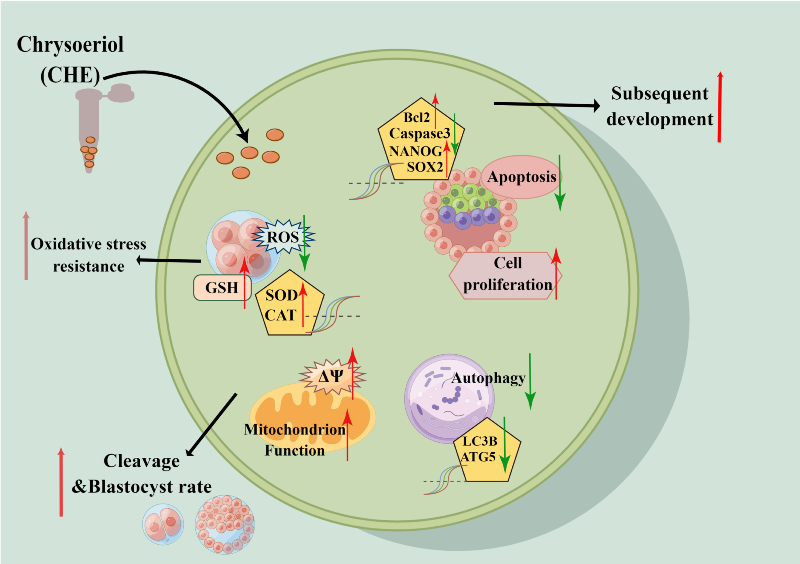
36]. Blastocysts formed on the Day-7 were selected for testing of various indicators to evaluate embryonic development capacity. The rate of blastocyst formation and the total number of cells in blastocysts were obviously increased when 1 μM CHE was added to IVC medium. In addition, CHE treatment can also improve cell proliferation and reduce the level of autophagy. All these results have shown that CHE can improve the early embryonic development ability of porcine.
Porcine embryos also produce reactive oxygen species (ROS), and ROS include several types, including hydroxyl radical (OH), radical (O2
−), non-radical (H2O2) and organic peroxides [
37]. To investigate possible mechanisms by which CHE enhances early embryonic development with antioxidant capacity, we detected ROS and GSH levels and MMP in the 4-cell stage of embryos. In our study, the level of ROS in the CHE-treated group was significantly decreased compared with the control group. Also, CHE reduced GSH levels and inhibited cellular endogenous ROS levels, which is consistent with the effect of supplementing other antioxidants.
Mitochondria can provide energy and perform other functions, playing an important role in the early stages of embryonic development. [
38]. So, mitochondrial dysfunction does not only impede the embryonic development, but also leads to apoptosis, autophagy, and even complete death [
39]. MMP was detected after the addition of CHE, and the increase of MMP was observed, indicating that CHE had the function of protecting mitochondria. Combined with the trend of blastocyst rate, total cell number, and ROS and GSH levels, these results suggest that CHE can promote embryonic development and stabilize or even activate mitochondrial function by reducing ROS.
Autophagy is necessary during early embryonic development, and defects can lead to developmental stagnation. Autophagy is also associated with the response to oxidative stress, redox signaling, cell death, and survival [
40]. Our results suggest that the addition of CHE to IVC medium reduced the autophagy levels, and this alteration was associated with changes in ROS levels and mitochondrial function in embryos.
Lastly, we detected the mRNA expression of the oxidative stress-related genes, SOD1, SOD2, SIRT1, and CAT, the pluripotency-related genes NANOG, OCT4, and SOX2, found them to be significantly upregulated upon after CHE supplementation. These results indicate that addition of CHE improves embryo developmental competence and the ability of embryos to resist oxidative stress. On the contrary, the apoptosis-related genes including CASP3, BAX, autophagy-related genes including LC3B, ATG5, and BECLIN1, were significant decreased. Specially, the anti-apoptosis gene BCL2 was significantly upregulated. The results of gene expression have shown that CHE can reduce autophagy and apoptosis levels in porcine embryos.
Taken together, we evaluated the effect of CHE on enhancing the antioxidant capacity of porcine oocytes. Whether CHE can also improve the embryo development of pigs and other animals in other ways, as well as its effect on other pathways, still needs further study.
5. Conclusion
In summary, we determined that at the optimal concentration, chrysoeriol (CHE) could improve the early development of porcine embryos by increasing GSH levels and decreasing ROS level. Furthermore, supplementation of CHE could also reduce the proportion of apoptosis, enhance mitochondrial function, and participate in regulating autophagy levels. These conclusions contribute to the improvement of in vitro culture and in vitro production of embryos.
Acknowledgments & Author Contributions
Conceptualization, Chao-Rui Wang; Data curation, Sheng-Yan He; Formal analysis, Rong-Ping Liu and Xin-Qin Wang; Funding acquisition, Namhyung Kim; Investigation, Sheng-Yan He and Jing Wang; Methodology, Chao-Rui Wang and He-Wei Ji; Project administration, Ying-Hua Li; Resources, Chao-Rui Wang and Namhyung Kim; Software, Chao-Rui Wang and Chu-Man Huang; Supervision, Ying-Hua Li; Validation, Yong-Nan Xu, Ying-Hua Li and Namhyung Kim; Visualization, Ying-Hua Li; Writing – original draft, Chao-Rui Wang and He-Wei Ji; Writing – review & editing, Chao-Rui Wang and Yong-Nan Xu.
Competing Interests
The authors declare no conflict of interest.
Funding
This research was funded by the Department of Education of Guangdong Province (Grant No. 2021ZDZX2046); Start-up Fund for Scientific Research of High-level Talents of Wuyi University (Grant NO. 2021AL022).
References
- el Hajj, N.; Haaf, T. Epigenetic disturbances in in vitro cultured gametes and embryos: implications for human assisted reproduction. Fertil. Steril. 2013, 99, 632–641. [Google Scholar] [CrossRef]
- Hammadeh, M.E.; Fischer-Hammadeh, C.; Ali, K.R. Assisted hatching in assisted reproduction: a state of the art. J. Assist. Reprod. Genet. 2010, 28, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Z.; Lin, E.; He, P.; Ru, G. Oxidative damage-induced hyperactive ribosome biogenesis participates in tumorigenesis of offspring by cross-interacting with the Wnt and TGF-β1 pathways in IVF embryos. Exp. Mol. Med. 2021, 53, 1792–1806. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Takayama, S. Application of microfluidic technologies to human assisted reproduction. Mol. Hum. Reprod. 2017, 23, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.R.; Redel, B.K.; Kerns, K.C.; Spate, L.D.; Prather, R.S. Challenges and Considerations during In Vitro Production of Porcine Embryos. Cells 2021, 10, 2770. [Google Scholar] [CrossRef] [PubMed]
- de Souza-Fabjan, J.M.G.; Panneau, B.; Duffard, N.; Locatelli, Y.; de Figueiredo, J.R.; Freitas, V.J.d.F.; Mermillod, P. In vitro production of small ruminant embryos: Late improvements and further research. Theriogenology 2014, 81, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Funahashi, H.; Yoshioka, K.; Kikuchi, K. Up date of in vitro production of porcine embryos. Front. Biosci. 2006, 11, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- W. Sun, H. W. Sun, H. Liu, H. Zhu, M. Gao, S. Xu, Eucalyptol antagonized the apoptosis and immune dysfunction of grass carp hepatocytes induced by tetrabromobisphenol A by regulating ROS/ASK1/JNK pathway, Environ Toxicol (2023).
- Sudharshan, S.J.; Narayanan, A.K.; Princilly, J.; Dyavaiah, M.; Nagegowda, D.A. Betulinic acid mitigates oxidative stress-mediated apoptosis and enhances longevity in the yeast Saccharomyces cerevisiae model. Free. Radic. Res. 2022, 56, 699–712. [Google Scholar] [CrossRef]
- Ding, Z.-M.; Jiao, X.-F.; Wu, D.; Zhang, J.-Y.; Chen, F.; Wang, Y.-S.; Huang, C.-J.; Zhang, S.-X.; Li, X.; Huo, L.-J. Bisphenol AF negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chem. Interactions 2017, 278, 222–229. [Google Scholar] [CrossRef]
- Khazaei, M.; Aghaz, F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. 11, 70. [CrossRef]
- Kikuchi, K.; Ekwall, H.; Tienthai, P.; Kawai, Y.; Noguchi, J.; Kaneko, H.; Rodriguez-Martinez, H. Morphological features of lipid droplet transition during porcine oocyte fertilisation and early embryonic development to blastocyst in vivo and in vitro. Zygote 2002, 10, 355–366. [Google Scholar] [CrossRef]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. . 2000, 118, 163–70. [Google Scholar]
- Liang, S.; Jin, Y.-X.; Yuan, B.; Zhang, J.-B.; Kim, N.-H. Melatonin enhances the developmental competence of porcine somatic cell nuclear transfer embryos by preventing DNA damage induced by oxidative stress. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Osorio, N.; Kim, I.J.; Wang, H.; Kaya, A.; Memili, E. Melatonin increases cleavage rate of porcine preimplantation embryos in vitro. J. Pineal Res. 2007, 43, 283–288. [Google Scholar] [CrossRef]
- Jiang, H.; Liang, S.; Yao, X.-R.; Jin, Y.-X.; Shen, X.-H.; Yuan, B.; Zhang, J.-B.; Kim, N.-H. Laminarin improves developmental competence of porcine early stage embryos by inhibiting oxidative stress. Theriogenology 2018, 115, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, J.-B.; Peng, Y.-X.; Liu, J.-B.; Han, D.-X.; Wang, Y.; Zhang, Z.; Yuan, B.; Gao, Y.; Chen, C.-Z.; et al. Imperatorin improves in vitro porcine embryo development by reducing oxidative stress and autophagy. Theriogenology 2019, 146, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, X.; Wang, J.; Dan, L.; Li, Y.; Jiang, H.; Xu, Y.; Kim, N. Oroxin A reduces oxidative stress, apoptosis, and autophagy and improves the developmental competence of porcine embryos in vitro. Reprod. Domest. Anim. 2022, 57, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- X.Q. Wang, R.P. X.Q. Wang, R.P. Liu, J. Wang, D. Luo, Y.H. Li, H. Jiang, Y.N. Xu, N.H. Kim, Wedelolactone facilitates the early development of parthenogenetically activated porcine embryos by reducing oxidative stress and inhibiting autophagy, PeerJ 10 (2022) e13766.
- Hamsalakshmi; Alex, A. M.; Marappa, M.A.; Joghee, S.; Chidambaram, S.B. Therapeutic benefits of flavonoids against neuroinflammation: a systematic review. Inflammopharmacology 2022, 30, 111–136. [Google Scholar] [CrossRef]
- Kim, M.H.; Kwon, S.Y.; Woo, S.-Y.; Seo, W.D.; Kim, D.Y. Antioxidative Effects of Chrysoeriol via Activation of the Nrf2 Signaling Pathway and Modulation of Mitochondrial Function. Molecules 2021, 26, 313. [Google Scholar] [CrossRef]
- Limboonreung, T.; Tuchinda, P.; Chongthammakun, S. Chrysoeriol mediates mitochondrial protection via PI3K/Akt pathway in MPP+ treated SH-SY5Y cells. Neurosci. Lett. 2019, 714, 134545. [Google Scholar] [CrossRef]
- Nickavar, B.; Rezaee, J.; Nickavar, A. Effect-Directed Analysis for the Antioxidant Compound in Salvia verticillata. Iran J Pharm Res 2016, 15, 241–6. [Google Scholar] [PubMed]
- Choi, D.-Y.; Lee, J.Y.; Kim, M.-R.; Woo, E.-R.; Kim, Y.G.; Kang, K.W. Chrysoeriol potently inhibits the induction of nitric oxide synthase by blocking AP-1 activation. J. Biomed. Sci. 2005, 12, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ikejima, T.; Li, L.; Wu, R.; Yuan, X.; Zhao, J.; Wang, Y.; Peng, S. Impairment of Mitochondrial Biogenesis and Dynamics Involved in Isoniazid-Induced Apoptosis of HepG2 Cells Was Alleviated by p38 MAPK Pathway. Front. Pharmacol. 2017, 8, 753. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Priyadarsini, K.; Kumar, M.; Unnikrishnan, M.; Mohan, H. Effect of O -glycosilation on the antioxidant activity and free radical reactions of a plant flavonoid, chrysoeriol. Bioorganic Med. Chem. 2003, 11, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Min, D.Y.; Jung, E.; Ahn, S.S.; Lee, Y.H.; Lim, Y.; Shin, S.Y. Chrysoeriol Prevents TNFα-Induced CYP19 Gene Expression via EGR-1 Downregulation in MCF7 Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Min, Q.; Palida, A.; Zhang, Y.; Tang, R.; Chen, L.; Li, H. 8-Chrysoeriol, as a potential BCL-2 inhibitor triggers apoptosis of SW1990 pancreatic cancer cells. Bioorganic Chem. 2018, 77, 478–484. [Google Scholar] [CrossRef]
- S.M.S. Abo-Qotb, A.M.M. S.M.S. Abo-Qotb, A.M.M. Hassanein, S.Y. Desoukey, A.S. Wanas, H.M. Tawfik, M.A.A. Orabi, In vivo anti-inflammatory and hepatoprotective activities of Orobanche crenata (Forssk.) aerial parts in relation to its phytomolecules, Nat Prod Res 36(4) (2022) 1067-1072.
- Cha, B.-Y.; Shi, W.L.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.-T. An Inhibitory Effect of Chrysoeriol on Platelet-Derived Growth Factor (PDGF)-Induced Proliferation and PDGF Receptor Signaling in Human Aortic Smooth Muscle Cells. J. Pharmacol. Sci. 2009, 110, 105–110. [Google Scholar] [CrossRef]
- Liu, Z.; Song, X.-D.; Xin, Y.; Wang, X.-J.; Yu, H.; Bai, Y.-Y.; Liu, J.-H.; Zhang, C.-N.; Hui, R.-T. Protective effect of chrysoeriol against doxorubicin-induced cardiotoxicity in vitro. . 2009, 122, 2652–2656. [Google Scholar] [PubMed]
- Niu, Y.; Sun, N.; Li, C.; Lei, Y.; Huang, Z.; Wu, J.; Si, C.; Dai, X.; Liu, C.; Wei, J.; et al. Dissecting primate early post-implantation development using long-term in vitro embryo culture. Science 2019, 366, eaaw5754. [Google Scholar] [CrossRef]
- Tam, P.P.L. Modeling the early development of a primate embryo. Science 2019, 366, 798–799. [Google Scholar] [CrossRef]
- Choi, J.; Park, S.; Lee, E.; Kim, J.; Lee, J.; Park, S.; Kim, H.; Hossein, M.S.; Jeong, Y.; Kim, S.; et al. Anti-apoptotic effect of melatonin on preimplantation development of porcine parthenogenetic embryos. Mol. Reprod. Dev. 2008, 75, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Azpiroz, F.; Lopes, S.M.C.d.S. Engineered models of the human embryo. Nat. Biotechnol. 2021, 39, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: an update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.J. Mitochondria in early development: linking the microenvironment, metabolism and the epigenome. Reproduction 2019, 157, R159–R179. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 2011, 441, 523–540. [Google Scholar] [CrossRef]
- J. Navarro-Yepes, M. J. Navarro-Yepes, M. Burns, A. Anandhan, O. Khalimonchuk, L.M. del Razo, B. Quintanilla-Vega, A. Pappa, M.I. Panayiotidis, R. Franco, Oxidative stress, redox signaling, and autophagy: cell death versus survival, Antioxid Redox Signal 21(1) (2014) 66-85.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).