Submitted:
01 January 2023
Posted:
09 January 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
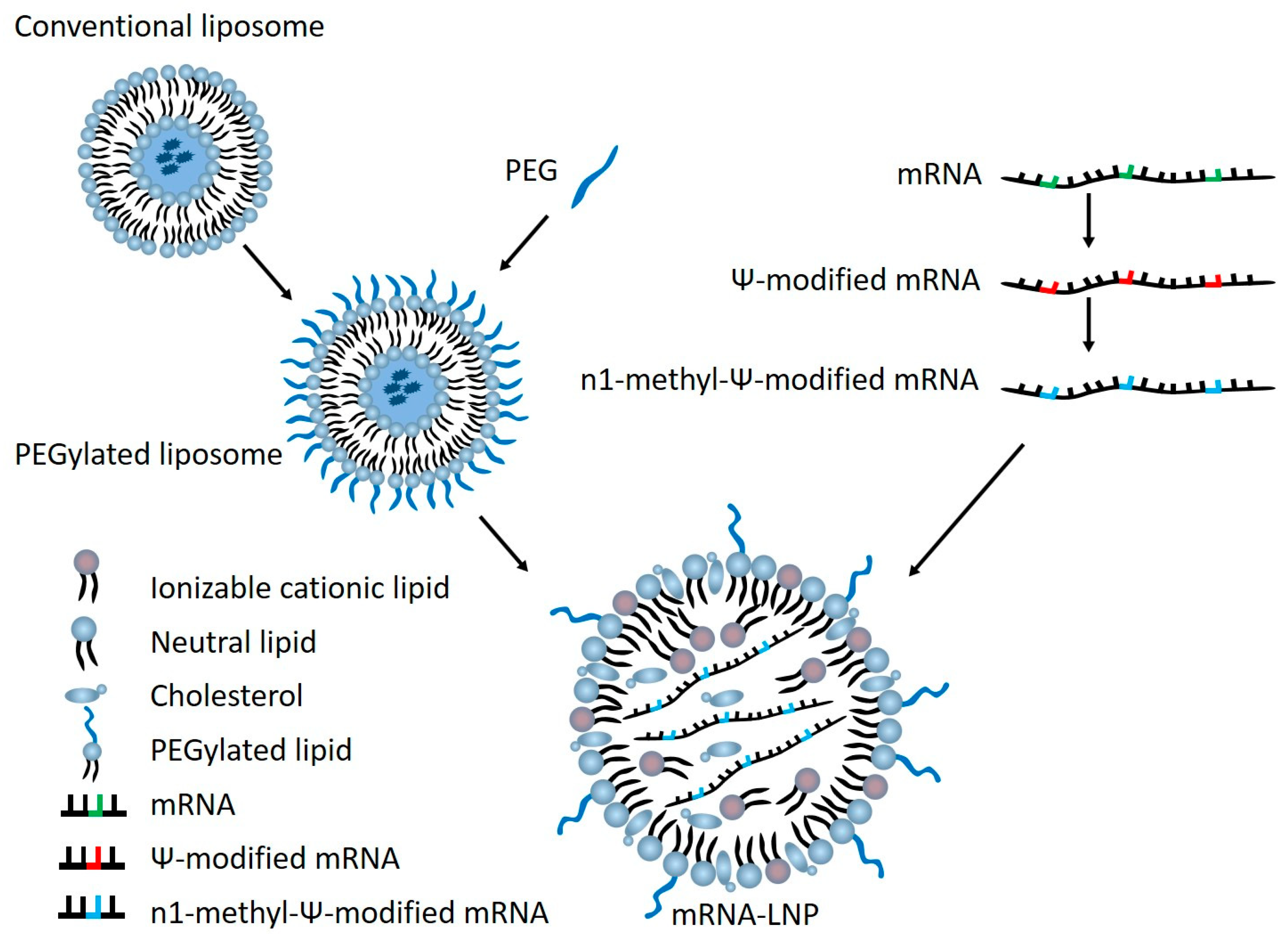
2. mRNA Vaccine Elements and Potential for Harm
2.1. Harms Due to Lipid Nanoparticle (LNP)
2.2. Harms Due to Exogenous RNA
2.3. Harms Due to In Vitro Transcribed (IVT) RNA
2.4. Harms of RNA Vaccination
2.5. Harms of Coronavirus Vaccination
2.6. Harms of RNA Vaccination with SARS-CoV-2 Spike (S) Antigen
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagaich, U.; Sadhna, D. Drug Recall: An Incubus for Pharmaceutical Companies and Most Serious Drug Recall of History. Int J Pharm Investig 2015, 5, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gagne, J.J.; Choudhry, N.K. The Epidemiology of Drug Recalls in the United States. Arch Intern Med 2012, 172, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.J.; Sokolov, E.; Franklin, J.M.; Kesselheim, A.S. Comparison of Rates of Safety Issues and Reporting of Trial Outcomes for Medical Devices Approved in the European Union and United States: Cohort Study. BMJ 2016, 353, i3323. [Google Scholar] [CrossRef]
- Vajapey, S.P.; Li, M. Medical Device Recalls in Orthopedics: Recent Trends and Areas for Improvement. J Arthroplasty 2020, 35, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Bekele, T.; Gunn, A.; Chapman, N.; Chowdhary, V.; Corrigan, K.; Dahora, L.; Martinez, S.; Permar, S.; Persson, J.; et al. Developing New Health Technologies for Neglected Diseases: A Pipeline Portfolio Review and Cost Model 2020.
- Dowden, H.; Munro, J. Trends in Clinical Success Rates and Therapeutic Focus. Nature Reviews Drug Discovery 2019, 18, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of Clinical Trial Success Rates and Related Parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef]
- Dahlin, E.; Nelson, G.M.; Haynes, M.; Sargeant, F. Success Rates for Product Development Strategies in New Drug Development. J Clin Pharm Ther 2016, 41, 198–202. [Google Scholar] [CrossRef]
- Hayes, A.W. The Precautionary Principle. Arh Hig Rada Toksikol 2005, 56, 161–166. [Google Scholar]
- Ball, P. The Lightning-Fast Quest for COVID Vaccines — and What It Means for Other Diseases. Nature 2020, 589, 16–18. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Ogata, A.F.; Cheng, C.-A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of MRNA-1273 Vaccine Recipients. Clinical Infectious Diseases 2022, 74, 715–718. [Google Scholar] [CrossRef]
- Deb, A.; Abdelmalek, J.; Iwuji, K.; Nugent, K. Acute Myocardial Injury Following COVID-19 Vaccination: A Case Report and Review of Current Evidence from Vaccine Adverse Events Reporting System Database. J Prim Care Community Health 2021, 12, 21501327211029230. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines — a New Era in Vaccinology. Nat Rev Drug Discov 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Pepini, T.; Pulichino, A.-M.; Carsillo, T.; Carlson, A.L.; Sari-Sarraf, F.; Ramsauer, K.; Debasitis, J.C.; Maruggi, G.; Otten, G.R.; Geall, A.J.; et al. Induction of an IFN-Mediated Antiviral Response by a Self-Amplifying RNA Vaccine: Implications for Vaccine Design. The Journal of Immunology 2017, 198, 4012–4024. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.K.; Jasny, E.; Yoon, H.; Horscroft, N.; Schanen, B.; Geter, T.; Fotin-Mleczek, M.; Petsch, B.; Wittman, V. Adjuvant Effects of a Sequence-Engineered MRNA Vaccine: Translational Profiling Demonstrates Similar Human and Murine Innate Response. Journal of Translational Medicine 2017, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Theofilopoulos, A.N.; Baccala, R.; Beutler, B.; Kono, D.H. TYPE I INTERFERONS (α/β) IN IMMUNITY AND AUTOIMMUNITY. Annual Review of Immunology 2005, 23, 307–335. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.-J.; Gilliet, M. Plasmacytoid Predendritic Cells Initiate Psoriasis through Interferon-α Production. Journal of Experimental Medicine 2005, 202, 135–143. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune Imprinting, Breadth of Variant Recognition, and Germinal Center Response in Human SARS-CoV-2 Infection and Vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination Prior to Development of Antibodies: A Novel Mechanism for Immune Activation by MRNA Vaccines. The Journal of Immunology 2021, 207, 2405–2410. [Google Scholar] [CrossRef]
- Welsh, K.J.; Baumblatt, J.; Chege, W.; Goud, R.; Nair, N. Thrombocytopenia Including Immune Thrombocytopenia after Receipt of MRNA COVID-19 Vaccines Reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2021, 39, 3329–3332. [Google Scholar] [CrossRef]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 MRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr Issues Mol Biol 2022, 44, 1115–1126. [Google Scholar] [CrossRef]
- Wang, X.; Ishida, T.; Kiwada, H. Anti-PEG IgM Elicited by Injection of Liposomes Is Involved in the Enhanced Blood Clearance of a Subsequent Dose of PEGylated Liposomes. Journal of Controlled Release 2007, 119, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Rare PEG Allergy Triggered Postvaccination Anaphylaxis. JAMA 2021, 325, 1931. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.; Khalib, K.; Conlon, N. PEG That Reaction: A Case Series of Allergy to Polyethylene Glycol. The Journal of Clinical Pharmacology 2021, 61, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Nasser, S.; Ewan, P. Polyethylene Glycol-Induced Systemic Allergic Reactions (Anaphylaxis). J Allergy Clin Immunol Pract 2021, 9, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.C.; Phillips, E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N Engl J Med 2020, NEJMra2035343. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Greenhawt, M.; Shaker, M.; Kosowan, L.; Singer, A.G. Primary Care Provider-Reported Prevalence of Vaccine and Polyethylene Glycol Allergy in Canada. Ann Allergy Asthma Immunol 2021, 127, 446–450.e1. [Google Scholar] [CrossRef] [PubMed]
- Ohgoda, O.; Robinson, I. Toxicological Evaluation of DSPC (1,2-Distearoyl-Sn-Glycero- 3-Phosphocholine). Fundamental Toxicological Sciences 2020, 7. [Google Scholar] [CrossRef]
- Doktorovová, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical Safety of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Current Evidence from in Vitro and in Vivo Evaluation. Eur J Pharm Biopharm 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front Immunol 2014, 5, 461. [Google Scholar] [CrossRef]
- Wienert, B.; Shin, J.; Zelin, E.; Pestal, K.; Corn, J.E. In Vitro–Transcribed Guide RNAs Trigger an Innate Immune Response via the RIG-I Pathway. PLOS Biology 2018, 16, e2005840. [Google Scholar] [CrossRef]
- Kannemeier, C.; Shibamiya, A.; Nakazawa, F.; Trusheim, H.; Ruppert, C.; Markart, P.; Song, Y.; Tzima, E.; Kennerknecht, E.; Niepmann, M.; et al. Extracellular RNA Constitutes a Natural Procoagulant Cofactor in Blood Coagulation. Proceedings of the National Academy of Sciences 2007, 104, 6388–6393. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Gerriets, T.; Wessels, C.; Walberer, M.; Kostin, S.; Stolz, E.; Zheleva, K.; Hocke, A.; Hippenstiel, S.; Preissner, K.T. Extracellular RNA Mediates Endothelial-Cell Permeability via Vascular Endothelial Growth Factor. Blood 2007, 110, 2457–2465. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Eyler, D.E.; Franco, M.K.; Batool, Z.; Wu, M.Z.; Dubuke, M.L.; Dobosz-Bartoszek, M.; Jones, J.D.; Polikanov, Y.S.; Roy, B.; Koutmou, K.S. Pseudouridinylation of MRNA Coding Sequences Alters Translation. Proc Natl Acad Sci U S A 2019, 116, 23068–23074. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Q.; Burgute, B.D.; Tzeng, S.-C.; Jing, C.; Jungers, C.; Zhang, J.; Yan, L.L.; Vierstra, R.D.; Djuranovic, S.; Evans, B.S.; et al. N1-Methylpseudouridine Found within COVID-19 MRNA Vaccines Produces Faithful Protein Products. Cell Rep 2022, 40, 111300. [Google Scholar] [CrossRef] [PubMed]
- Domazet-Lošo, T. MRNA Vaccines: Why Is the Biology of Retroposition Ignored? Genes (Basel) 2022, 13, 719. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, E.K.; Martinez, N.M.; Gilbert, W.V. Regulation and Function of RNA Pseudouridylation in Human Cells. Annu Rev Genet 2020, 54, 309–336. [Google Scholar] [CrossRef]
- Pang, H.; Ihara, M.; Kuchino, Y.; Nishimura, S.; Gupta, R.; Woese, C.R.; McCloskey, J.A. Structure of a Modified Nucleoside in Archaebacterial TRNA Which Replaces Ribosylthymine. 1-Methylpseudouridine. J Biol Chem 1982, 257, 3589–3592. [Google Scholar] [CrossRef]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N(1)-Methylpseudouridine-Incorporated MRNA Outperforms Pseudouridine-Incorporated MRNA by Providing Enhanced Protein Expression and Reduced Immunogenicity in Mammalian Cell Lines and Mice. J Control Release 2015, 217, 337–344. [Google Scholar] [CrossRef]
- Vlatkovic, I. Non-Immunotherapy Application of LNP-MRNA: Maximizing Efficacy and Safety. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic MRNA Delivery. Molecular Therapy 2019, 27, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yin, L.; Theisen, M.; Zhuo, J.; Siddiqui, S.; Levy, B.; Presnyak, V.; Frassetto, A.; Milton, J.; Salerno, T.; et al. Systemic MRNA Therapy for the Treatment of Fabry Disease: Preclinical Studies in Wild-Type Mice, Fabry Mouse Model, and Wild-Type Non-Human Primates. Am J Hum Genet 2019, 104, 625–637. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Schneller, J.L.; Frassetto, A.; Liang, S.; Zhu, X.; Park, J.-S.; Theisen, M.; Hong, S.-J.; Zhou, J.; Rajendran, R.; et al. Systemic Messenger RNA Therapy as a Treatment for Methylmalonic Acidemia. Cell Rep 2018, 24, 2520. [Google Scholar] [CrossRef]
- Jiang, L.; Berraondo, P.; Jericó, D.; Guey, L.T.; Sampedro, A.; Frassetto, A.; Benenato, K.E.; Burke, K.; Santamaría, E.; Alegre, M.; et al. Systemic Messenger RNA as an Etiological Treatment for Acute Intermittent Porphyria. Nat Med 2018, 24, 1899–1909. [Google Scholar] [CrossRef]
- Bartelds, G.M.; Krieckaert, C.L.M.; Nurmohamed, M.T.; van Schouwenburg, P.A.; Lems, W.F.; Twisk, J.W.R.; Dijkmans, B.A.C.; Aarden, L.; Wolbink, G.J. Development of Antidrug Antibodies against Adalimumab and Association with Disease Activity and Treatment Failure during Long-Term Follow-Up. JAMA 2011, 305, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Moots, R.J.; Xavier, R.M.; Mok, C.C.; Rahman, M.U.; Tsai, W.-C.; Al-Maini, M.H.; Pavelka, K.; Mahgoub, E.; Kotak, S.; Korth-Bradley, J.; et al. The Impact of Anti-Drug Antibodies on Drug Concentrations and Clinical Outcomes in Rheumatoid Arthritis Patients Treated with Adalimumab, Etanercept, or Infliximab: Results from a Multinational, Real-World Clinical Practice, Non-Interventional Study. PLOS ONE 2017, 12, e0175207. [Google Scholar] [CrossRef]
- Pratt, K.P. Anti-Drug Antibodies: Emerging Approaches to Predict, Reduce or Reverse Biotherapeutic Immunogenicity. Antibodies 2018, 7, 19. [Google Scholar] [CrossRef]
- Krishna, M.; Nadler, S.G. Immunogenicity to Biotherapeutics – The Role of Anti-Drug Immune Complexes. Front Immunol 2016, 7, 21. [Google Scholar] [CrossRef]
- Clarke, J.B. Mechanisms of Adverse Drug Reactions to Biologics. Handb Exp Pharmacol 2010, 453–474. [Google Scholar] [CrossRef]
- Pichler, W.J. Adverse Side-Effects to Biological Agents. Allergy 2006, 61, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Sathish, J.G.; Sethu, S.; Bielsky, M.-C.; de Haan, L.; French, N.S.; Govindappa, K.; Green, J.; Griffiths, C.E.M.; Holgate, S.; Jones, D.; et al. Challenges and Approaches for the Development of Safer Immunomodulatory Biologics. Nat Rev Drug Discov 2013, 12, 306–324. [Google Scholar] [CrossRef]
- Banugaria, S.G.; Prater, S.N.; Ng, Y.-K.; Kobori, J.A.; Finkel, R.S.; Ladda, R.L.; Chen, Y.-T.; Rosenberg, A.S.; Kishnani, P.S. The Impact of Antibodies on Clinical Outcomes in Diseases Treated with Therapeutic Protein: Lessons Learned from Infantile Pompe Disease. Genet Med 2011, 13, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat Rev Drug Discov 2021, 20, 817–838. [Google Scholar] [CrossRef]
- de Lusignan, S.; Damaso, S.; Ferreira, F.; Byford, R.; McGee, C.; Pathirannehelage, S.; Shende, V.; Yonova, I.; Schmidt, A.; Schuind, A.; et al. Brand-Specific Enhanced Safety Surveillance of GSK’s Fluarix Tetra Seasonal Influenza Vaccine in England: 2017/2018 Season. Hum Vaccin Immunother 2020, 16, 1762–1771. [Google Scholar] [CrossRef]
- Crescioli, S.; Correa, I.; Karagiannis, P.; Davies, A.M.; Sutton, B.J.; Nestle, F.O.; Karagiannis, S.N. IgG4 Characteristics and Functions in Cancer Immunity. Curr Allergy Asthma Rep 2016, 16, 7. [Google Scholar] [CrossRef]
- Schlaudecker, E.P.; McNeal, M.M.; Dodd, C.N.; Ranz, J.B.; Steinhoff, M.C. Pregnancy Modifies the Antibody Response to Trivalent Influenza Immunization. J Infect Dis 2012, 206, 1670–1673. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, H.; Peng, L.; Zhou, J.; Wang, M.; Li, J.; Liu, Z.; Zhang, W.; Zhao, Y.; Zeng, X.; et al. The Role of PD-1/PD-Ls in the Pathogenesis of IgG4-Related Disease. Rheumatology 2022, 61, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class Switch towards Non-Inflammatory, Spike-Specific IgG4 Antibodies after Repeated SARS-CoV-2 MRNA Vaccination. Sci Immunol 2022, eade2798. [Google Scholar] [CrossRef]
- Pfizer-BioNTech COVID-19 Vaccine, COMIRNATY® (Tozinameran). Available online: https://www.who.int/publications/m/item/comirnaty-covid-19-mrna-vaccine (accessed on 30 December 2022).
- Moderna MRNA-1273, COVID-19 Vaccine. Available online: https://www.who.int/publications/m/item/moderna-covid-19-vaccine-(mrna-1273) (accessed on 30 December 2022).
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R. Present Variants of Concern and Variants of Interest of Severe Acute Respiratory Syndrome Coronavirus 2: Their Significant Mutations in S-Glycoprotein, Infectivity, Re-Infectivity, Immune Escape and Vaccines Activity. Reviews in Medical Virology 2022, 32, e2270. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S. A Detailed Overview of Immune Escape, Antibody Escape, Partial Vaccine Escape of SARS-CoV-2 and Their Emerging Variants With Escape Mutations. Front Immunol 2022, 13, 801522. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Zheng, B.; Li, Y.; Poon, L.; Xie, Z.; Chan, K.; Li, P.; Tan, S.; Chang, Q.; Xie, J.; et al. Epidemiology and Cause of Severe Acute Respiratory Syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. The Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Gastañaduy, P.A. Update: Severe Respiratory Illness Associated with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) — Worldwide, 2012–2013. MMWR Morb Mortal Wkly Rep 2013, 62, 480–483. [Google Scholar]
- Li, Y.-D.; Chi, W.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.-C. Coronavirus Vaccine Development: From SARS and MERS to COVID-19. Journal of Biomedical Science 2020, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Weingartl, H.; Czub, M.; Czub, S.; Neufeld, J.; Marszal, P.; Gren, J.; Smith, G.; Jones, S.; Proulx, R.; Deschambault, Y.; et al. Immunization with Modified Vaccinia Virus Ankara-Based Recombinant Vaccine against Severe Acute Respiratory Syndrome Is Associated with Enhanced Hepatitis in Ferrets. J Virol 2004, 78, 12672–12676. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-T.; Sbrana, E.; Iwata-Yoshikawa, N.; Newman, P.C.; Garron, T.; Atmar, R.L.; Peters, C.J.; Couch, R.B. Immunization with SARS Coronavirus Vaccines Leads to Pulmonary Immunopathology on Challenge with the SARS Virus. PLoS One 2012, 7, e35421. [Google Scholar] [CrossRef]
- Bolles, M.; Deming, D.; Long, K.; Agnihothram, S.; Whitmore, A.; Ferris, M.; Funkhouser, W.; Gralinski, L.; Totura, A.; Heise, M.; et al. A Double-Inactivated Severe Acute Respiratory Syndrome Coronavirus Vaccine Provides Incomplete Protection in Mice and Induces Increased Eosinophilic Proinflammatory Pulmonary Response upon Challenge. J Virol 2011, 85, 12201–12215. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, Q.; Lin, Q.; Fang, J.; Wang, H.; Kwok, H.; Tang, H.; Nishiura, K.; Peng, J.; Tan, Z.; et al. Anti-Spike IgG Causes Severe Acute Lung Injury by Skewing Macrophage Responses during Acute SARS-CoV Infection. JCI Insight 2019, 4, 123158. [Google Scholar] [CrossRef]
- Doremalen, N. van; Haddock, E.; Feldmann, F.; Meade-White, K.; Bushmaker, T.; Fischer, R.J.; Okumura, A.; Hanley, P.W.; Saturday, G.; Edwards, N.J.; et al. A Single Dose of ChAdOx1 MERS Provides Broad Protective Immunity against a Variety of MERS-CoV Strains 2020, 2020.04.13.036293.
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-Dependent Enhancement and SARS-CoV-2 Vaccines and Therapies. Nat Microbiol 2020, 5, 1185–1191. [Google Scholar] [CrossRef]
- Wen, J.; Cheng, Y.; Ling, R.; Dai, Y.; Huang, B.; Huang, W.; Zhang, S.; Jiang, Y. Antibody-Dependent Enhancement of Coronavirus. Int J Infect Dis 2020, 100, 483–489. [Google Scholar] [CrossRef]
- Xu, L.; Ma, Z.; Li, Y.; Pang, Z.; Xiao, S. Antibody Dependent Enhancement: Unavoidable Problems in Vaccine Development. Adv Immunol 2021, 151, 99–133. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Zuno, G.A.; Matuz-Flores, M.G.; González-Estevez, G.; Nicoletti, F.; Turrubiates-Hernández, F.J.; Mangano, K.; Muñoz-Valle, J.F. A Review: Antibody-Dependent Enhancement in COVID-19: The Not so Friendly Side of Antibodies. Int J Immunopathol Pharmacol 2021, 35, 20587384211050200. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Smatti, M.K.; Ouhtit, A.; Cyprian, F.S.; Almaslamani, M.A.; Thani, A.A.; Yassine, H.M. Antibody-Dependent Enhancement (ADE) and the Role of Complement System in Disease Pathogenesis. Mol Immunol 2022, 152, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Ricke, D.O. Two Different Antibody-Dependent Enhancement (ADE) Risks for SARS-CoV-2 Antibodies. Front Immunol 2021, 12, 640093. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z.; et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J Virol 2020, 94, e02015–19. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Sasaki, T.; Koketsu, R.; Morita, R.; Yoshimura, Y.; Murakami, A.; Saito, Y.; Kusunoki, T.; Samune, Y.; Nakayama, E.E.; et al. Reevaluation of Antibody-Dependent Enhancement of Infection in Anti-SARS-CoV-2 Therapeutic Antibodies and MRNA-Vaccine Antisera Using FcR- and ACE2-Positive Cells. Sci Rep 2022, 12, 15612. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N Engl J Med 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- London, A.J.; Kimmelman, J. Against Pandemic Research Exceptionalism. Science 2020, 368, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I.R. Vaccination against Coronaviruses in Domestic Animals. Vaccine 2020, 38, 5123–5130. [Google Scholar] [CrossRef]
- Olsen, C.W.; Corapi, W.V.; Ngichabe, C.K.; Baines, J.D.; Scott, F.W. Monoclonal Antibodies to the Spike Protein of Feline Infectious Peritonitis Virus Mediate Antibody-Dependent Enhancement of Infection of Feline Macrophages. J Virol 1992, 66, 956–965. [Google Scholar] [CrossRef]
- Hohdatsu, T.; Yamada, M.; Tominaga, R.; Makino, K.; Kida, K.; Koyama, H. Antibody-Dependent Enhancement of Feline Infectious Peritonitis Virus Infection in Feline Alveolar Macrophages and Human Monocyte Cell Line U937 by Serum of Cats Experimentally or Naturally Infected with Feline Coronavirus. J Vet Med Sci 1998, 60, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Nakaguchi, M.; Doki, T.; Hohdatsu, T. Antibody-Dependent Enhancement of Serotype II Feline Enteric Coronavirus Infection in Primary Feline Monocytes. Arch Virol 2017, 162, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, Z.; Simpson, B.; Donald Reynolds, D.V.M. Antibody Dependent Enhancement of Infectious Bronchitis Virus in Poultry. UCARE Research Products 2022.
- H, T.; D, P.; Ra, G.; Vl, v.S.; Fw, v.G.; J, Z.; Ks, J. Infectious Bronchitis Virus Subpopulations in Vaccinated Chickens after Challenge. Avian diseases 2012, 56. [Google Scholar] [CrossRef]
- Brandão, P.E.; Berg, M.; Silva, S.O.S.; Taniwaki, S.A. Emergence of Avian Coronavirus Escape Mutants Under Suboptimal Antibody Titers. J Mol Evol 2022, 90, 176–181. [Google Scholar] [CrossRef] [PubMed]
- F, B.; Ss, A.; Mh, B.; H, M.; Ar, O. Progress and Challenges toward the Development of Vaccines against Avian Infectious Bronchitis. Journal of immunology research 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Eldemery, F.; Li, Y.; Yu, Q.; van Santen, V.L.; Toro, H. Infectious Bronchitis Virus S2 of 4/91 Expressed from Recombinant Virus Does Not Protect Against Ark-Type Challenge. Avian Dis 2017, 61, 397–401. [Google Scholar] [CrossRef]
- Ravikumar, R.; Chan, J.; Prabakaran, M. Vaccines against Major Poultry Viral Diseases: Strategies to Improve the Breadth and Protective Efficacy. Viruses 2022, 14, 1195. [Google Scholar] [CrossRef]
- Shao, G.; Chen, T.; Feng, K.; Zhao, Q.; Zhang, X.; Li, H.; Lin, W.; Xie, Q. Efficacy of Commercial Polyvalent Avian Infectious Bronchitis Vaccines against Chinese QX-like and TW-like Strain via Different Vaccination Strategies. Poult Sci 2020, 99, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Sjaak de Wit, J.J.; Cook, J.K.A.; van der Heijden, H.M.J.F. Infectious Bronchitis Virus Variants: A Review of the History, Current Situation and Control Measures. Avian Pathol 2011, 40, 223–235. [Google Scholar] [CrossRef]
- Cavanagh, D. Severe Acute Respiratory Syndrome Vaccine Development: Experiences of Vaccination against Avian Infectious Bronchitis Coronavirus. Avian Pathol 2003, 32, 567–582. [Google Scholar] [CrossRef]
- Legnardi, M.; Tucciarone, C.M.; Franzo, G.; Cecchinato, M. Infectious Bronchitis Virus Evolution, Diagnosis and Control. Vet Sci 2020, 7, E79. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.B.; Holladay, J.A.; Cave, J.S. A Neurologic Syndrome Associated with Use of a Canine Coronavirus-Parvovirus Vaccine in Dogs. The Compendium on continuing education for the practicing veterinarian (USA) 1986.
- Martin, M. Canine Coronavirus Enteritis and a Recent Outbreak Following Modified Live Virus Vaccination. Compendium on continuing education for the practicing veterinarian 1985, 7, 1012–1017. [Google Scholar]
- Pratelli, A.; Tinelli, A.; Decaro, N.; Martella, V.; Camero, M.; Tempesta, M.; Martini, M.; Carmichael, L.E.; Buonavoglia, C. Safety and Efficacy of a Modified-Live Canine Coronavirus Vaccine in Dogs. Vet Microbiol 2004, 99, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, A. High-cell-passage Canine Coronavirus Vaccine Providing Sterilising Immunity. J Small Anim Pract 2007, 48, 574–578. [Google Scholar] [CrossRef]
- Cho, K.-O.; Hasoksuz, M.; Nielsen, P.R.; Chang, K.-O.; Lathrop, S.; Saif, L.J. Cross-Protection Studies between Respiratory and Calf Diarrhea and Winter Dysentery Coronavirus Strains in Calves and RT-PCR and Nested PCR for Their Detection. Arch Virol 2001, 146, 2401–2419. [Google Scholar] [CrossRef]
- Heckert, R.A.; Saif, L.J.; Hoblet, K.H.; Agnes, A.G. A Longitudinal Study of Bovine Coronavirus Enteric and Respiratory Infections in Dairy Calves in Two Herds in Ohio. Vet Microbiol 1990, 22, 187–201. [Google Scholar] [CrossRef]
- Fulton, R.W.; d’Offay, J.M.; Landis, C.; Miles, D.G.; Smith, R.A.; Saliki, J.T.; Ridpath, J.F.; Confer, A.W.; Neill, J.D.; Eberle, R.; et al. Detection and Characterization of Viruses as Field and Vaccine Strains in Feedlot Cattle with Bovine Respiratory Disease. Vaccine 2016, 34, 3478–3492. [Google Scholar] [CrossRef]
- Hu, S.; Bruszewski, J.; Smalling, R.; Browne, J.K. Studies of TGEV Spike Protein Gp195 Expressed in E. Coli and by a TGE-Vaccinia Virus Recombinant. Adv Exp Med Biol 1985, 185, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Gómez, N.; Wigdorovitz, A.; Castañón, S.; Gil, F.; Ordá, R.; Borca, M.V.; Escribano, J.M. Oral Immunogenicity of the Plant Derived Spike Protein from Swine-Transmissible Gastroenteritis Coronavirus. Arch Virol 2000, 145, 1725–1732. [Google Scholar] [CrossRef]
- Lamphear, B.J.; Streatfield, S.J.; Jilka, J.M.; Brooks, C.A.; Barker, D.K.; Turner, D.D.; Delaney, D.E.; Garcia, M.; Wiggins, B.; Woodard, S.L.; et al. Delivery of Subunit Vaccines in Maize Seed. J Control Release 2002, 85, 169–180. [Google Scholar] [CrossRef]
- Gerdts, V.; Zakhartchouk, A. Vaccines for Porcine Epidemic Diarrhea Virus and Other Swine Coronaviruses. Vet Microbiol 2017, 206, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdallat, M.M.; Payne, D.C.; Alqasrawi, S.; Rha, B.; Tohme, R.A.; Abedi, G.R.; Al Nsour, M.; Iblan, I.; Jarour, N.; Farag, N.H.; et al. Hospital-Associated Outbreak of Middle East Respiratory Syndrome Coronavirus: A Serologic, Epidemiologic, and Clinical Description. Clinical Infectious Diseases 2014, 59, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.-K.; Kim, J.H. First Clinical Trial of a MERS Coronavirus DNA Vaccine. The Lancet Infectious Diseases 2019, 19, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Modjarrad, K.; Roberts, C.C.; Mills, K.T.; Castellano, A.R.; Paolino, K.; Muthumani, K.; Reuschel, E.L.; Robb, M.L.; Racine, T.; Oh, M.; et al. Safety and Immunogenicity of an Anti-Middle East Respiratory Syndrome Coronavirus DNA Vaccine: A Phase 1, Open-Label, Single-Arm, Dose-Escalation Trial. The Lancet Infectious Diseases 2019, 19, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-T.; Zhang, J.-S.; Su, N.; Xu, J.-G.; Wang, N.; Chen, J.-T.; Chen, X.; Liu, Y.-X.; Gao, H.; Jia, Y.-P.; et al. Safety and Immunogenicity from a Phase I Trial of Inactivated Severe Acute Respiratory Syndrome Coronavirus Vaccine. Antivir Ther 2007, 12, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Louder, M.K.; Holman, L.A.; Gordon, I.J.; Enama, M.E.; Larkin, B.D.; Andrews, C.A.; Vogel, L.; Koup, R.A.; Roederer, M.; et al. A SARS DNA Vaccine Induces Neutralizing Antibody and Cellular Immune Responses in Healthy Adults in a Phase I Clinical Trial. Vaccine 2008, 26, 6338–6343. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.; Dahlke, C.; Fathi, A.; Kupke, A.; Krähling, V.; Okba, N.M.A.; Halwe, S.; Rohde, C.; Eickmann, M.; Volz, A.; et al. Safety and Immunogenicity of a Modified Vaccinia Virus Ankara Vector Vaccine Candidate for Middle East Respiratory Syndrome: An Open-Label, Phase 1 Trial. The Lancet Infectious Diseases 2020, 20, 827–838. [Google Scholar] [CrossRef]
- Fathi, A.; Dahlke, C.; Krähling, V.; Kupke, A.; Okba, N.M.A.; Raadsen, M.P.; Heidepriem, J.; Müller, M.A.; Paris, G.; Lassen, S.; et al. Increased Neutralization and IgG Epitope Identification after MVA-MERS-S Booster Vaccination against Middle East Respiratory Syndrome. Nat Commun 2022, 13, 4182. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Bittaye, M.; Flaxman, A.; Lopez, F.R.; Bellamy, D.; Kupke, A.; Mair, C.; Makinson, R.; Sheridan, J.; Rohde, C.; et al. Safety and Immunogenicity of a Candidate Middle East Respiratory Syndrome Coronavirus Viral-Vectored Vaccine: A Dose-Escalation, Open-Label, Non-Randomised, Uncontrolled, Phase 1 Trial. The Lancet Infectious Diseases 2020, 20, 816–826. [Google Scholar] [CrossRef]
- Bosaeed, M.; Balkhy, H.H.; Almaziad, S.; Aljami, H.A.; Alhatmi, H.; Alanazi, H.; Alahmadi, M.; Jawhary, A.; Alenazi, M.W.; Almasoud, A.; et al. Safety and Immunogenicity of ChAdOx1 MERS Vaccine Candidate in Healthy Middle Eastern Adults (MERS002): An Open-Label, Non-Randomised, Dose-Escalation, Phase 1b Trial. Lancet Microbe 2022, 3, e11–e20. [Google Scholar] [CrossRef]
- Hsu, A.C.-Y.; Wang, G.; Reid, A.T.; Veerati, P.C.; Pathinayake, P.S.; Daly, K.; Mayall, J.R.; Hansbro, P.M.; Horvat, J.C.; Wang, F.; et al. SARS-CoV-2 Spike Protein Promotes Hyper-Inflammatory Response That Can Be Ameliorated by Spike-Antagonistic Peptide and FDA-Approved ER Stress and MAP Kinase Inhibitors in Vitro 2020, 2020.09.30.317818.
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 Spike Protein Induces Inflammation via TLR2-Dependent Activation of the NF-ΚB Pathway. Elife 2021, 10, e68563. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circulation Research 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Nyström, S.; Hammarström, P. Amyloidogenesis of SARS-CoV-2 Spike Protein. J Am Chem Soc 2022, 144, 8945–8950. [Google Scholar] [CrossRef] [PubMed]
- Muglia, J.J.; DiGiovanna, J.J. Phase 1 Clinical Trials. J Cutan Med Surg 1998, 2, 236–241. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The MRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Simberg, D. Pro-Inflammatory Concerns with Lipid Nanoparticles. Molecular Therapy 2022, 30, 2109–2110. [Google Scholar] [CrossRef]
- Kedmi, R.; Ben-Arie, N.; Peer, D. The Systemic Toxicity of Positively Charged Lipid Nanoparticles and the Role of Toll-like Receptor 4 in Immune Activation. Biomaterials 2010, 31, 6867–6875. [Google Scholar] [CrossRef]
| Platform | Vaccine | Group | Status | Severe adverse events | NCT ID | Study |
|---|---|---|---|---|---|---|
| SARS Vaccine Clinical Trials | ||||||
| Inactivated virus | Inactivated SARS-CoV vaccine (ISCV) | Sinovac | Phase I, completed | [0/24, 0%] | No NCT ID | [111] |
| DNA vaccine | VRC-SRSDNA015-00-VP | NIAID | Phase I, completed | [0/9, 0%] | NCT00099463 | [112] |
| MERS Vaccine Clinical Trials | ||||||
| DNA vaccine | GLS-5300 (INO-4700) | GeneOne Life Science/Inovio Pharmaceuticals/International Vaccine Institute | Phase I, completed | [0/75, 0%] *Infections in 36% of participants |
NCT02670187 | [110] |
| DNA vaccine | GLS-5300 (INO-4700) | GeneOne Life Science/Inovio Pharmaceuticals/ International Vaccine Institute |
Phase I/IIa, completed | No results available | NCT03721718 | |
| Viral vector vaccine | MVA-MERS-S | CTC North GmbH & Co. KG | Phase I, completed | [0/23,0%] | NCT03615911 | [113] |
| Viral vector vaccine | MVA-MERS-S_DF1 | CTC North GmbH & Co. KG | Phase Ib, not yet recruiting | No data | NCT04119440 | [114] |
| Viral vector vaccine | ChAdOx1 MERS | University of Oxford | Phase I, recruiting | [1/24, 4%] | NCT03399578 | [115] |
| Viral vector vaccine | ChAdOx1 MERS | King Abdullah International Medical Research Center/University of Oxford | Phase I, recruiting | [6/24, 25%] | NCT04170829 | [116] |
| Viral vector vaccine | BVRS-GamVac-Combi | Gamaleya Research Institute of Epidemiology and Microbiology/Acellena Contract Drug Research and Development | Phase I/II, recruiting | No data | NCT04128059 | |
| Viral vector vaccine | BVRS-GamVac | Gamaleya Research Institute of Epidemiology and Microbiology | Phase I/II, recruiting | No data | NCT04130594 | |
| 1 | According to a Canadian survey, the prevalence of vaccine allergy is 0.037%. and 0.01% of patients had an allergy to either PEG or common injectable medications containing PEG (CIMCP) [28]. |
| 2 | Event classification available at: https://rsc.niaid.nih.gov/sites/default/files/corrected-grading-table-v-2-1-with-all-changes-highlighted.pdf
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




