Submitted:
02 January 2023
Posted:
06 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
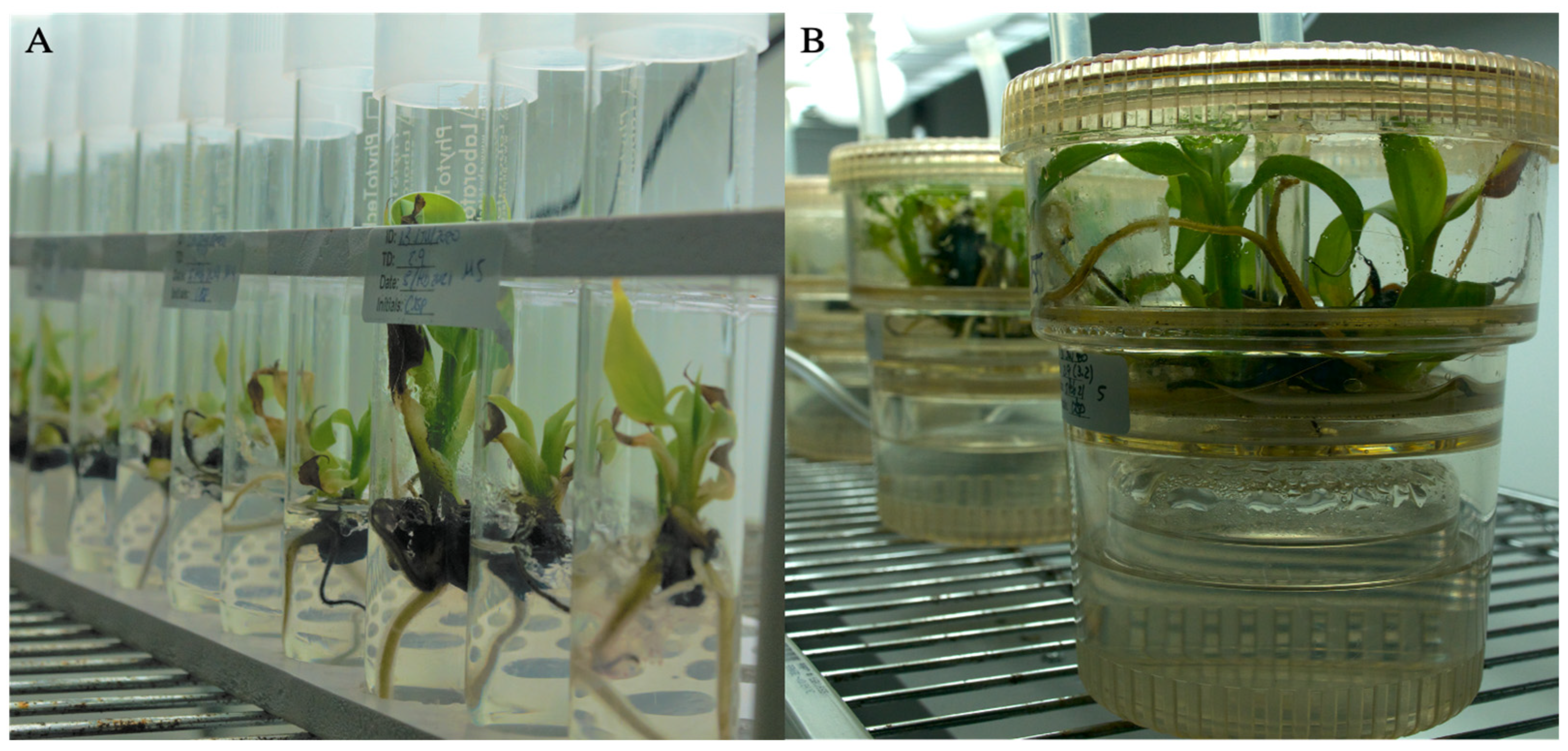
2.2. Temporary Immersions Bioreactor (TIB) and Micropropagation Assays
2.3. Growth Comparison in TIB vs Conventional Micropropagation
2.4. RNA Isolation and cDNA Preparation:
2.5. Oligonucleotide Design
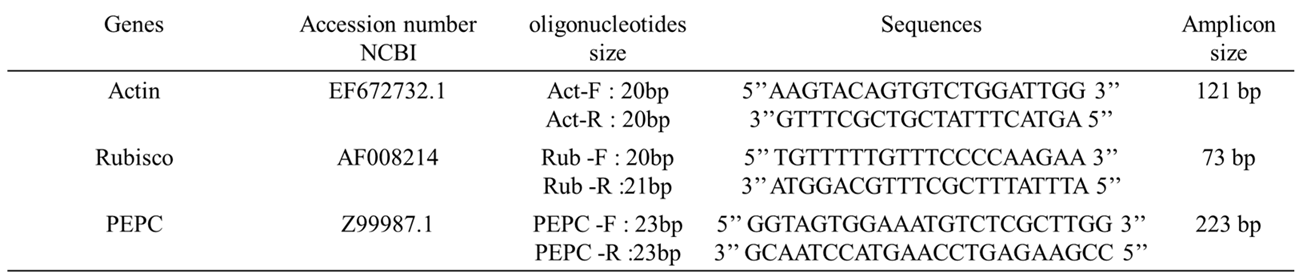
2.6. Quantitative Real Time- Polymerase Chain Reaction (qRT-PCR)
2.7. Photosynthetic Activity Measurement
2.8. ICP-OES Qualitative Analysis
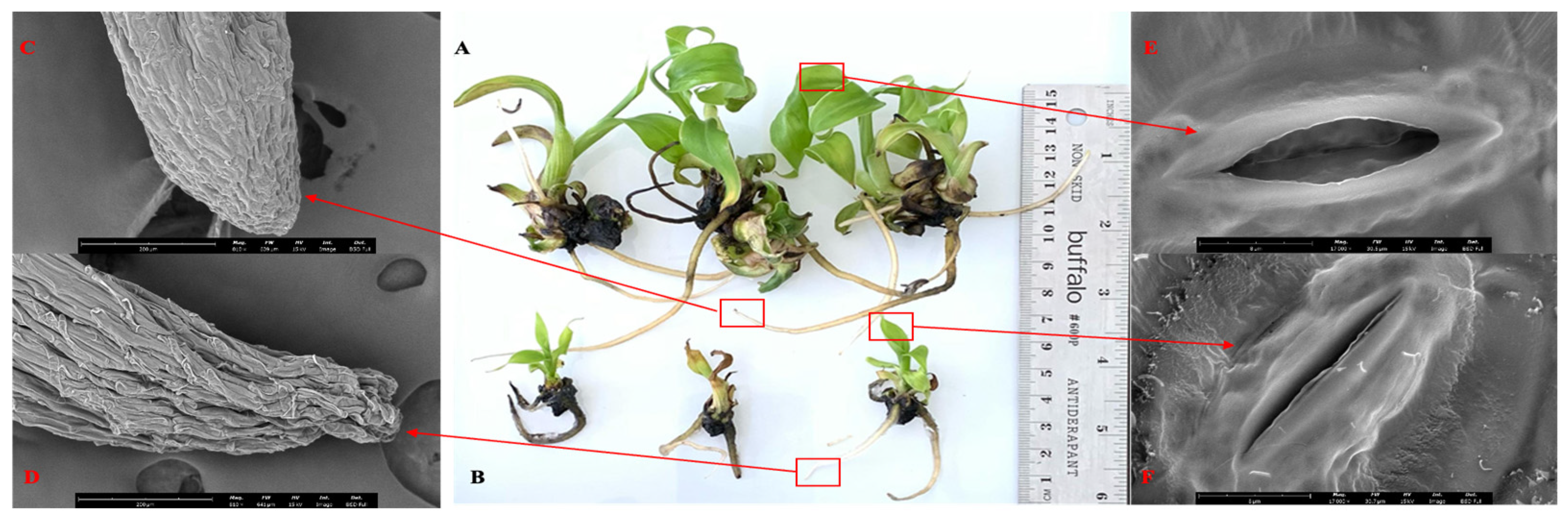
2.9. Scanning Electron Microscopy
3. Results
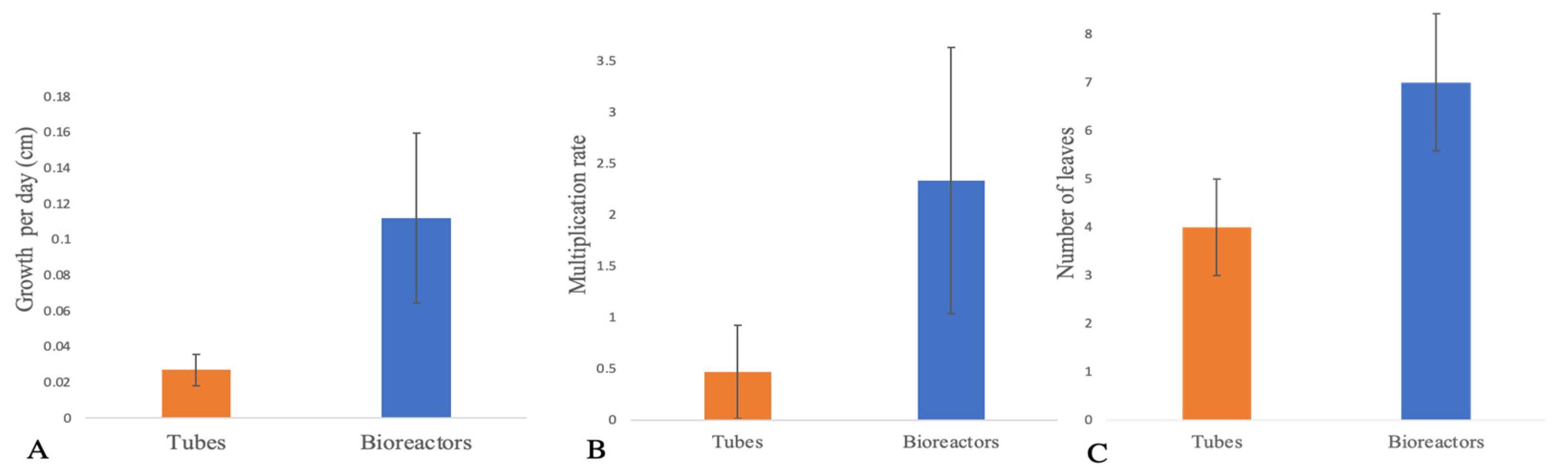
3.1. Comparison of Growth Parameters in TIB vs Conventional Micropropagation
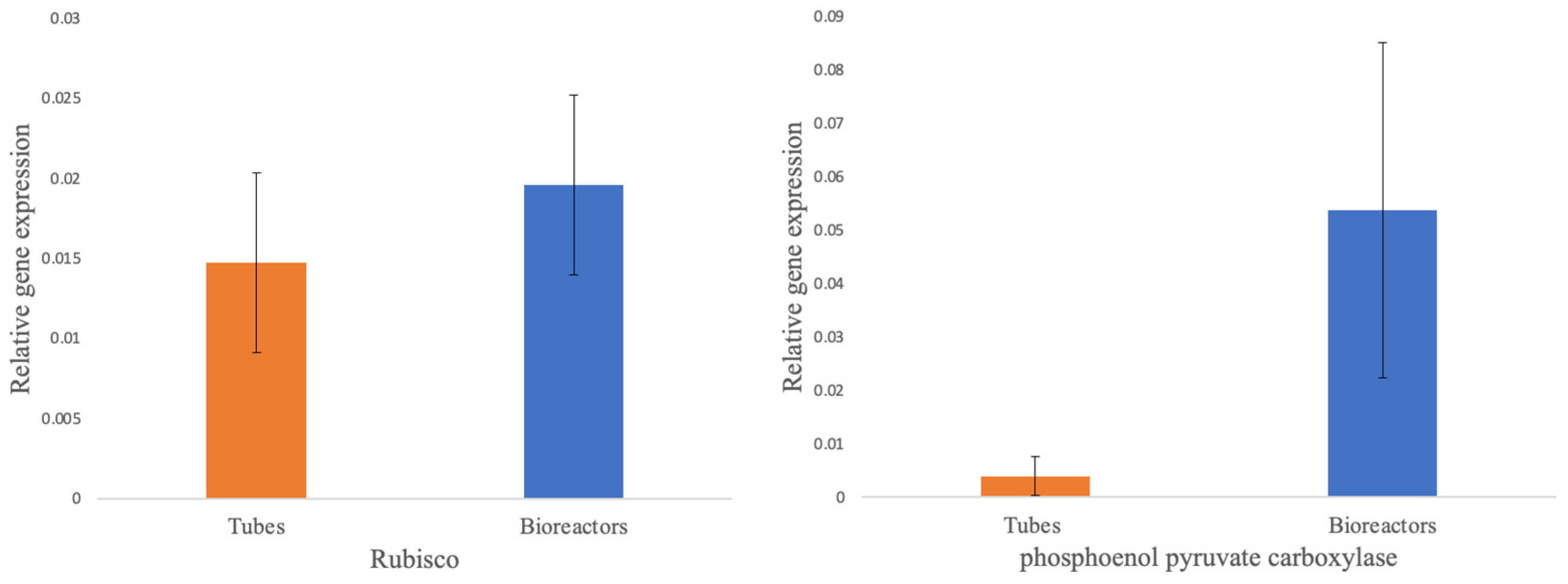
3.3. Gene Expression
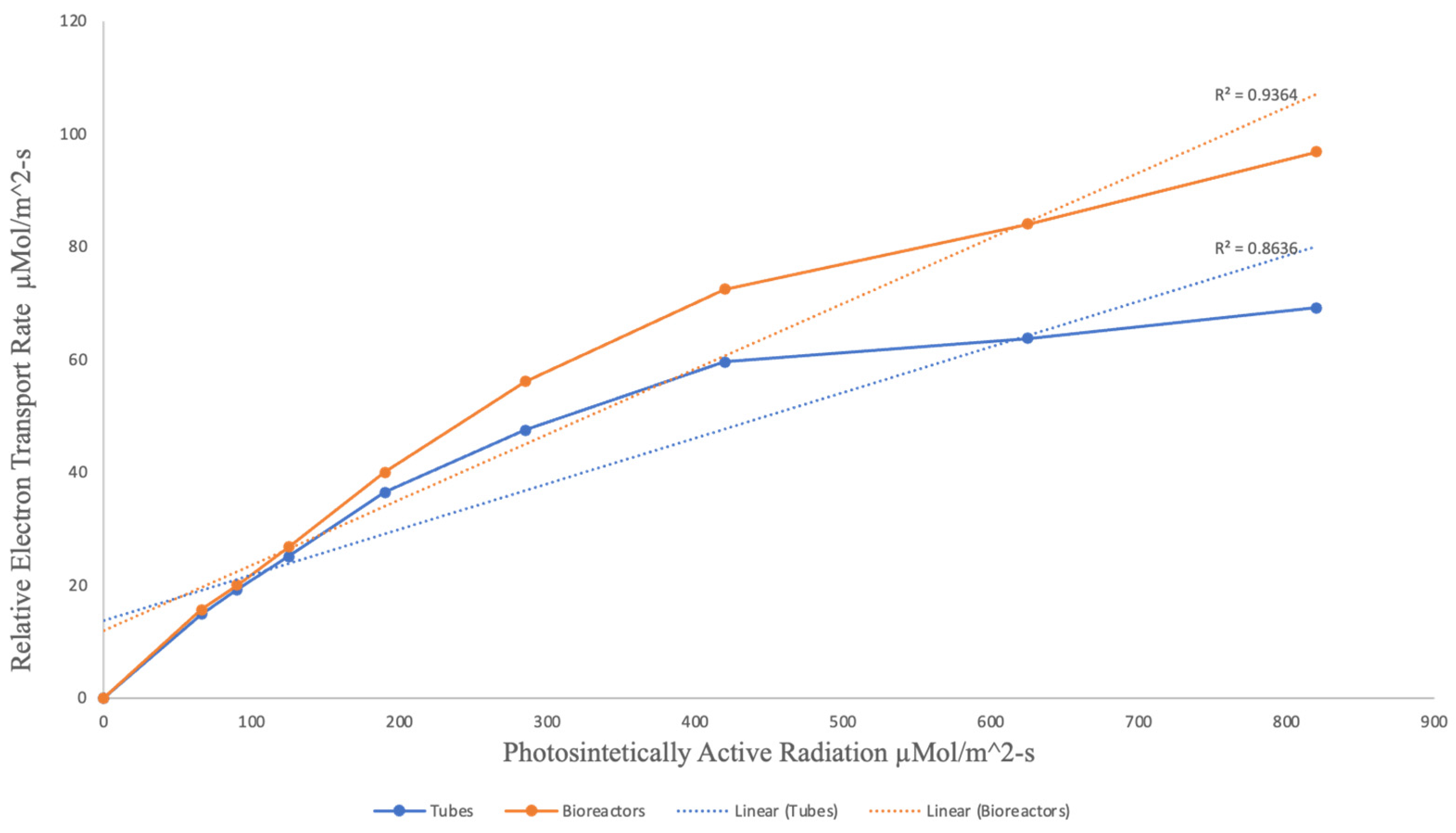
3.4. Photosynthetic Activity
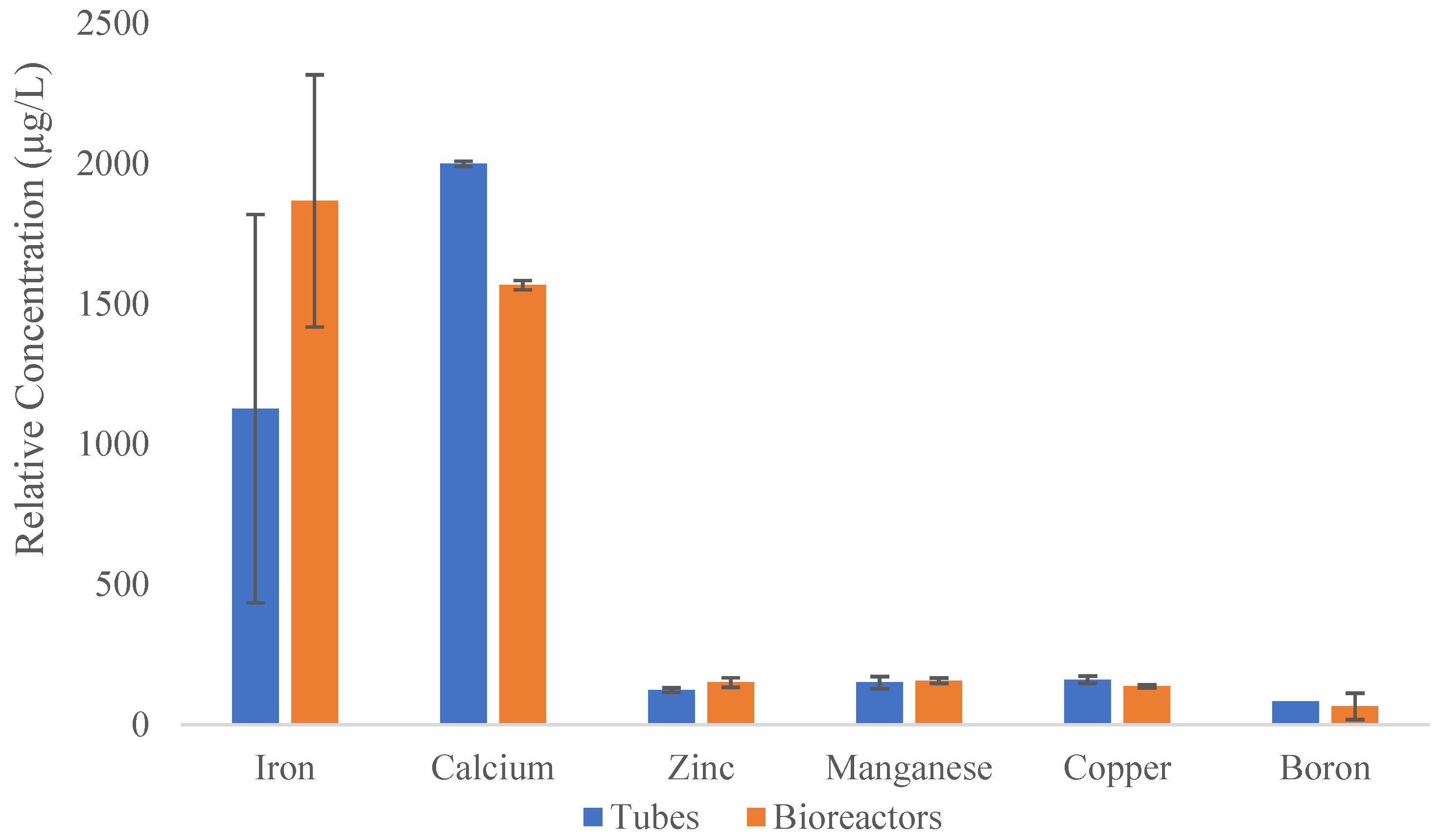
3.5. Digestion and ICP-OES Qualitative Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Jing, G.; Wang, H.; Duan, X.; Qu, H.; Jiang, Y. The combined effects of Phenylurea and gibberellins on quality maintenance and shelf life extension of banana fruit during storage. Science Horticulture 2014, 167, 36–42. [Google Scholar] [CrossRef]
- Nelson, S., Ploetz, R.C, & Kepler, A.K. (2006). Musa Species (bananas and plantains) Species Profiles for Pacific Island Agroforestry. Permanent Agriculture Resource. https://agroforestry.org/images/pdfs/Musa-banana-plantain.pdf.
- Kumar, N.; Reddy, M. In vitro Plant Propagation: A Review. Journal of Forest Science 2011, 27, 61–72. [Google Scholar] [CrossRef]
- Rodríguez Cruz, L., & Niles, M. (2018). Hurricane Maria’s Impacts on Puerto Rican Farmers: Experience, Challenges, and Perceptions. University of Vermont: Burlington.
- Smith, M., & Meade, B. (2019, June 3). Global Food Security.USDA. https://www.ers.usda.gov/amber-waves/2019/june/who-are-the-world-s-food-insecure-identifying-the-risk-factors-of-food-insecurity-around-the-world/.
- Bellido, M. S. (September 10, 2014). Food Security in Puerto Rico: Vulnerable Food Supply. American Society for Nutrition. https://nutrition.org/food-security-puerto-rico-vulnerable-food-supply/.
- Roels, S.; Escalona, M.; Cejas, I.; Noceda, C.; Rodriguez, R.; Canal, M.; Debergh, P. Optimization of plantain (Musa AAB) micropropagation by temporary immersion system. Plant Cell, Tissue and Organ Culture 2005, 82, 57–66. [Google Scholar] [CrossRef]
- Nhut, D. T., Trinh Don, N., Hong Vu, N., Quoc Thien, N., Thi Thu Thuy, D., Duy, N., & A. Teixeira da Silva, J. (2006). Advanced Technology in Micropropagation of Some Important Plants. Global Science Book.
- Escalona, M.; Lorenzo, J.C.; Gonza´ lez, B.; Daquinta, M.; Gonza´ lez, J.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Reports 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Florez, S.; Curtis, M.S.; Shaw, S.E.; Hamaker, N.K.; Larsen, J.S.; Curtis, W.R. A Temporary Immersion Plant Propagation Bioreactor with Decoupled Gas and Liquid Flows for Enhanced Control of Gas Phase. American Institute of Chemical Engineers 2016, 32, 337–345. [Google Scholar] [CrossRef]
- Albarrán, J.; Bertrand, B.; Lartaud, M.; Etienne, H. Cycle characteristics in a temporary immersion bioreactor affect regeneration, morphology, water, and mineral status of coffee (Coffea arabica) somatic embryos. Plant Cell, Tissue and Organ Culture 2005, 81, 27–36. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Engineering in Life Sciences 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Paek, K.; Chakrabarty, D.; Hahn, E. Application of bioreactor systems for large-scale production of horticultural and medicinal plants. Plant Cell, Tissue and Organ Culture 2005, 81, 287–300. [Google Scholar] [CrossRef]
- Alvard, D.; Cote, F.; Teisson, C. Comparison of methods of liquid medium culture for banana micropropagation. Plant Cell, Tissue and Organ Culture 1993, 32, 55–60. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion in plant micropropagation. Plant Cell Tissue and Organ Culture 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Tumer, N.; Clark, W.; Tabor, G.; Hironaka, C.; Fraley, R.; Shah, D. The genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase are expressed differentially in petunia leaves. Nucleic Acids Research 1986, 14, 3325–3342. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Bautsoens, N.; Boer, B.; Rie, J.; Gallé, A. A Conserved Sequence from Heat-Adapted Species Improves Rubisco Activase Thermostability in Wheat1. Plant Physiology 2019, 181, 43–54. [Google Scholar] [CrossRef]
- Marcus, Y., Altman-Gueta, H., Snir, A., Wolff, Y., & Gurevitz, M. Does Rubisco Limit the Rate of Photosynthesis? Photosynthesis. Energy from the Sun: 14th International Congress on Photosynthesis 2008, 863-866. Springer. [CrossRef]
- Suzuki, Y.; Makino, A. Availability of Rubisco small subunit up-regulates the transcript levels of large subunit for stoichiometric assembly of its holoenzyme in rice. Plant Physiology 2012, 160, 533–540. [Google Scholar] [CrossRef]
- Kanai, R., & Edward, G. (1999). The biochemistry of C4 photosynthesis. In R Monson, ed, C4 Plant Biology. Academic Press.
- Cousins, A.; Baroli, I.; Badger, M.; Ivakov, A.; Lea, P.; Leegood, R.; Caemmerer, S. The Role of Phosphoenolpyruvate Carboxylase during C4 Photosynthetic Isotope Exchange and Stomatal Conductance. Plant Physiology 2007, 145, 1006–1017. [Google Scholar] [CrossRef]
- Aragón, C.; Escalona, M.; Capote, I.; Pina, D.; Cejas, I.; Rodriguez, R.; Canal, M.; Sandoval, J.; Roels, S.; Debergh, P.; Gonzalez - Olmedo, J. Photosynthesis and carbon metabolism in plantain (Musa AAB) growing in temporary immersion bioreactor (TIB) and ex vitro acclimatization. In Vitro Cellular & Developmental Biology - Plant 2005, 41, 550–554. [Google Scholar] [CrossRef]
- Aragón, C.; Sanchez, C.; Gonzalez - Olmedo, J.; Escalona, M.; Carvalho, L.; Amancio, S. Comparison of plantain plantlets propagated in temporary immersion bioreactors and gelled medium during in vitro growth and acclimatization. Biologia Plantarum 2014, 1, 29–38. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and Iron Homeostasis in Plants: The Challenges of Oxidative Stress. Antioxidants & Redox Signaling 2013, 19. [Google Scholar] [CrossRef]
- Yruela, I. Transition metals in plant photosynthesis. Metallomics. 5 2013, 1090–1109. [Google Scholar] [CrossRef]
- Merchant, S., Wise, R., & Hoober, J. Trace metal utilization in chloroplasts. The Structure and Function of Plastids 2006, 199– 218. Springer. [CrossRef]
- Raven, J.; Evans, M.; Korb, R. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynthesis Research 1999, 60, 111–149. [Google Scholar] [CrossRef]
- Valderrama-Cháirez, M.L.; Cruz-Hernández, A.; Paredes-López, O. Isolation of functional RNA from cactus fruit. Plant Mol. Biol. Rep. 2002, 20, 279–286. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Peraza-Echeverría, L.; Islas-Flores, I.; Canto-Canché, B.; Grijalva, R. Isolation of retro-transcribed RNA from in vitro Mycosphaerella fijiensis-infected banana leaves. Genetics and molecular research: GMR. 2010, 9, 1460–1468. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A novel temporary immersion bioreactor system for large-scale multiplication of banana (Rasthali AAB-Silk). Scientific Reports 2021, 11, 20371. [Google Scholar] [CrossRef]
- Yang, L.; Zambrano, Y.; Hu, C.-J.; Carmona, E. Sugarcane metabolites produced in CO2-rich temporary immersion bioreactors (TIBs) induce tomato (Solanum lycopersicum)resistance against bacterial wilt (Ralstonia solanacearum) In Vitro Cellular and Developmental Biology. Plant 2010, 46, 558–568. [Google Scholar] [CrossRef]
- Aragón, C.; Escalona, M.; Rodriguez, R.; Cañal, M. Effect of sucrose, light, and carbon dioxide on plantain micropropagation in temporary immersion bioreactors. In Vitro Cellular & Developmental Biology - Plant 2009, 46, 89–94. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.; Francia, F.; Lemaire, S. Redox regulation of the Calvin–Benson cycle: something old, something new. Frontiers in Plant Science 2013, 4, 1–21. [Google Scholar] [CrossRef]
- Matthijs, D., Gielis, J., & Debergh, P. (1995). Automation and Environmental Control in Plant Tissue Culture. Kluwer Academic Publishers. [CrossRef]
- Maes, K.; Debergh, P. Volatiles emitted from in vitro grown tomato shoots during abiotic and biotic stress. Plant Cell, Tissue and Organ Culture. 2003, 75, 73–78. [Google Scholar] [CrossRef]
- Buddendorf-Joosten, J.; Woltering, E. Components of the gaseous environment and their effect on plant growth and development in-vitro. Plant Growth Regulation. 1994, 15, 1–16. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Escudero, V.; Saéz, Á.; Tejada-Jiménez, M. Transition Metal Transport in Plants and Associated Endosymbionts: Arbuscular Mycorrhizal Fungi and Rhizobia. Frontiers in Plants Science 2016, 7, 1088. [Google Scholar] [CrossRef]
- Gonzalez, E. (2005). Mass propagation of tropical crops in temporary immersion systems. Springer. [CrossRef]
- Seyed Ali, G., & Hassan, E. (2020). The Importance of Boron in Plant Nutrition. In Metalloids in Plants. John Wiley & Sons, Inc. [CrossRef]
- Santiago, A.; Höller, S.; Meier, B.; Peite, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Frontiers in Plant Science 2020, 11, 1–23. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: a central regulator of plant growth and development. The Plant cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





