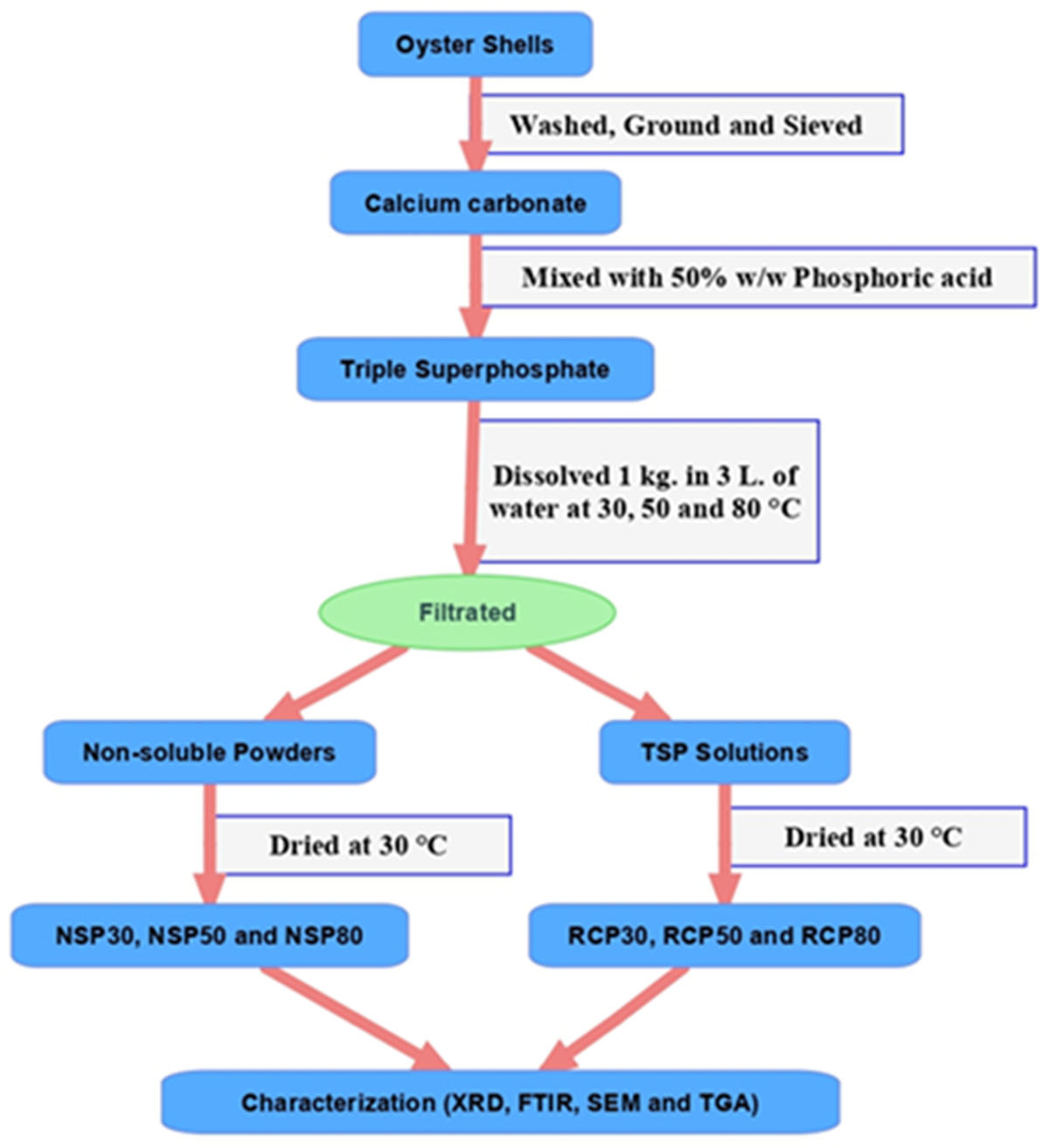
Calcium dihydrogen phosphate monohydrate [Ca(H2PO4)2·H2O] (a fertilizer) was successfully synthesized by the recrystallization process by using a prepared triple superphosphate (TSP) that derived from oyster shell waste as starting material. This bio-green, eco-friendly process to produce an important fertilizer can promote a sustainable society. The shell-waste-derived TSP was dissolved in distilled water and kept at 30, 50, and 80 °C. Non-soluble powder and TSP solution were obtained. The TSP solution fraction were then dried and the recrystallized products (RCP30, RCP50, and RCP80) were obtained and confirmed as Ca(H2PO4)2·H2O. Whereas the non-soluble products (NSP30, NSP50, and NSP80) were observed as calcium hydrogen phosphate dihydrate (CaHPO4·2H2O). The recrystallized yields of RCP30, RCP50, and RCP80 were found to be 51.0%, 49.6%, and 46.3%, whereas the soluble percentages were 98.72%, 99.16%, and 96.63%, respectively. RCP30 shows different morphological plate sizes, while RCP50 and RCP80 present the coagulate crystal plates. X-ray diffractograms confirm the formation of both the NSP and RCP. The infrared adsorption spectra confirmed the vibrational characteristics of HPO42‒, H2PO4‒ and H2O existed in CaHPO4·2H2O and Ca(H2PO4)2·H2O. Three thermal dehydration steps of Ca(H2PO4)2·H2O (physisorbed water, polycondensation, and re-polycondensation) were observed. Ca(H2PO4)2 and CaH2P2O7 are the thermodecomposed products from the first and second steps, whereas the final product is CaP2O6.