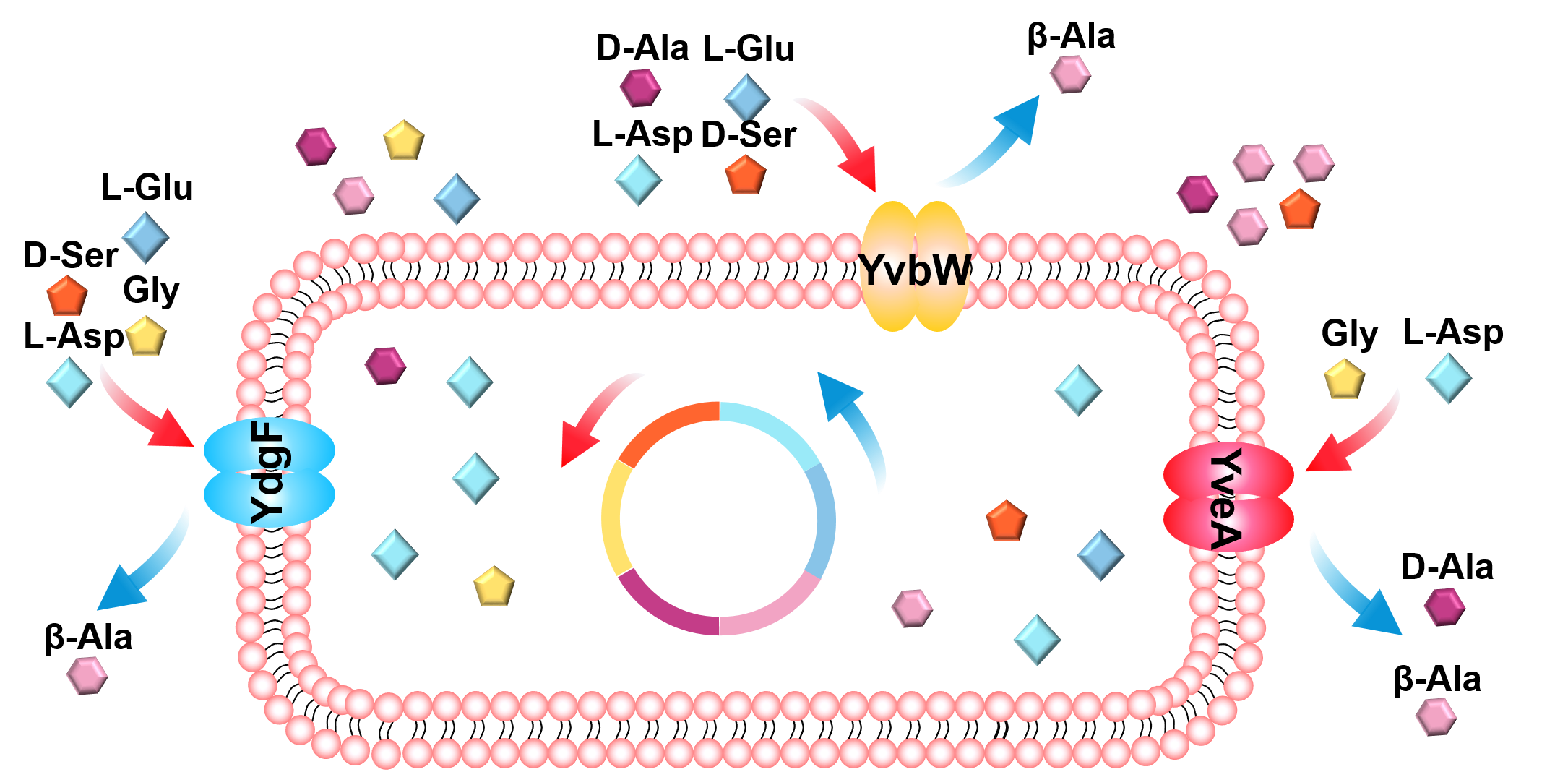
Amino acid efflux and influx transport systems play vital roles in industrial microorganisms’ cell growth and metabolism. However, although biochemically characterized, most amino acid transporters remain unknown at the molecular level in Bacillus licheniformis. This study focuses on the molecular and functional characterizations of three transporters, YdgF, YvbW, and YveA, mainly when catalyzing the cross-membrane flux of L-Aspartate. When growing in the minimal medium with L-Asp as the only carbon and nitrogen source, the growth of strains lacking proteins YdgF, YvbW, and YveA was significantly inhibited compared with wild-type strains, while supplementing the expression of the corresponding proteins in the single-gene knockout strains can alleviate the inhibition to some extent. Upon overexpression, the recombinant proteins mediate the accumulation of L-aspartate to varying degrees. Compared with wild-type strains, the single knockout strains of the three protein genes exhibited reduced absorption of L-aspartate. In addition, this paper focuses on the effects of these three proteins on the absorption of β-alanine, L-glutamate, D-serine, D-alanine, and glycine.