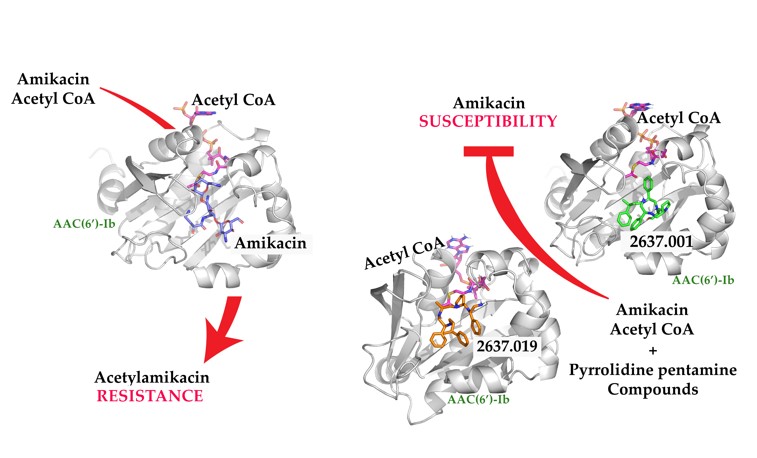
The aminoglycoside 6′-N-acetyltransferase type Ib [AAC(6′)-Ib] is a common cause of resistance to amikacin and other aminoglycosides in Gram-negatives. Utilization of mixture-based combinatorial libraries and application of the positional scanning strategy identified an inhibitor of AAC(6′)-Ib. This inhibitor’s chemical structure consists of a pyrrolidine pentamine scaffold substituted at four locations (R1, R3, R4, and R5). The substituents are two S-phenyl (R1 and R4), an S-hydroxymethyl (R3), and a 3-phenylbutyl (R5) groups. Another location, R2, does not have a substitution, but it is named because its stereochemistry was modified in some compounds utilized in this study. Structure-activity relationship (SAR) analysis using derivatives with different functionalities, modified stereochemistry, and truncations were carried out by assessing the effect of the addition of each compound at 8 µM to 16 µg/ml amikacin-containing media and performing checkerboard assays varying the concentrations of the inhibitor analogs and the antibiotic. The results showed that: 1) the aromatic functionalities at R1 and R4 are essential, but the stereochemistry is essential only at R4, 2) the stereochemical conformation at R2 is critical, 3) the hydroxyl moiety at R3 as well as stereoconformation are required for full inhibitory activity, 4) the phenyl functionality at R5 is not essential and can be replaced by aliphatic groups, 5) the location of the phenyl group on the butyl carbon chain at R5 is not essential, 6) the length of the aliphatic chain at R5 is not critical, 7) all truncations of the scaffold resulted in inactive compounds. Molecular docking revealed that all compounds preferentially bind to the kanamycin C binding cavity, and binding affinity correlates with the experimental data for most of the compounds evaluated. The SAR results in this study will serve as the basis for the design of new analogs in an effort to improve their ability to induce phenotypic conversion to susceptibility in amikacin-resistant pathogens.