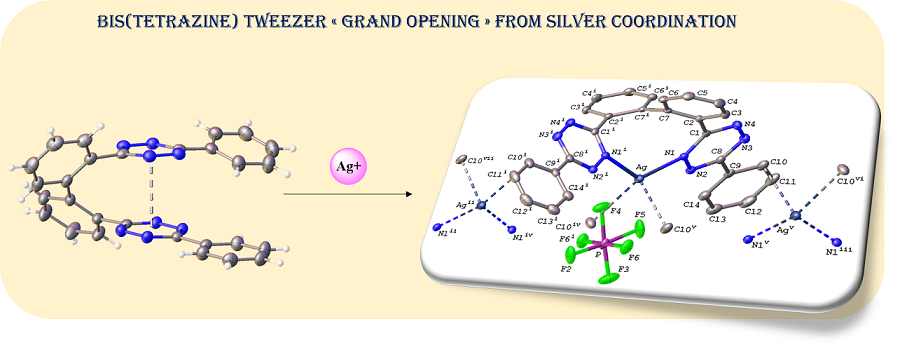
The carbon-carbon cross-coupling of phenyl s-tetrazine (Tz) units at their ortho-phenyl positions allows to form constrained bis(tetrazines) with original tweezer structures. In these compounds, the face-to-face positioning of the central tetrazine cores is endorsed by pi-staking of the electron-poor nitrogen-containing heteroaromatic moieties. The resulting tetra-aromatic structure can be used as a weak coordinating ligand with cationic silver. This coordination generates a set of bis(tetrazine)‐silver(I) coordination complexes tolerating a large variety of counter anions of various geometries, namely, PF6–, BF4–, SbF6–, ClO4–, NTf2–, OTf2–. These compounds were characterized in the solid-state by single crystal XRD and reflectance spectra, and in solution by 1H NMR, mass spectrometry, electroanalysis and UV-visible absorption spectroscopy. The X-ray diffraction (XRD) structure of complexes {[Ag(3)][PF6]}∞ (4) and {[Ag(3)][SbF6]∞ (6), where 3 is 3,3'-[(1,1'-biphenyl)-2,2'-diyl]-6,6'-bis[phenyl]-1,2,4,5-tetrazine, revealed the formation of 1D polymeric chains, characterized by an evolution to a large opening of the original tweezer and a coordination of silver(I) via two chelating nitrogen atom and some C=C pi-interactions. Electrochemical and UV spectroscopic properties of the original tweezer and of the corresponding silver complex are reported and compared. 1H NMR titrations with AgNTf2 allowed to determine the stoichiometry, and apparent stability of two solution species, namely [Ag(3)]+ and [Ag(3)2]2+, that formed in CDCl3/CD3OD 2:1 v/v mixtures.