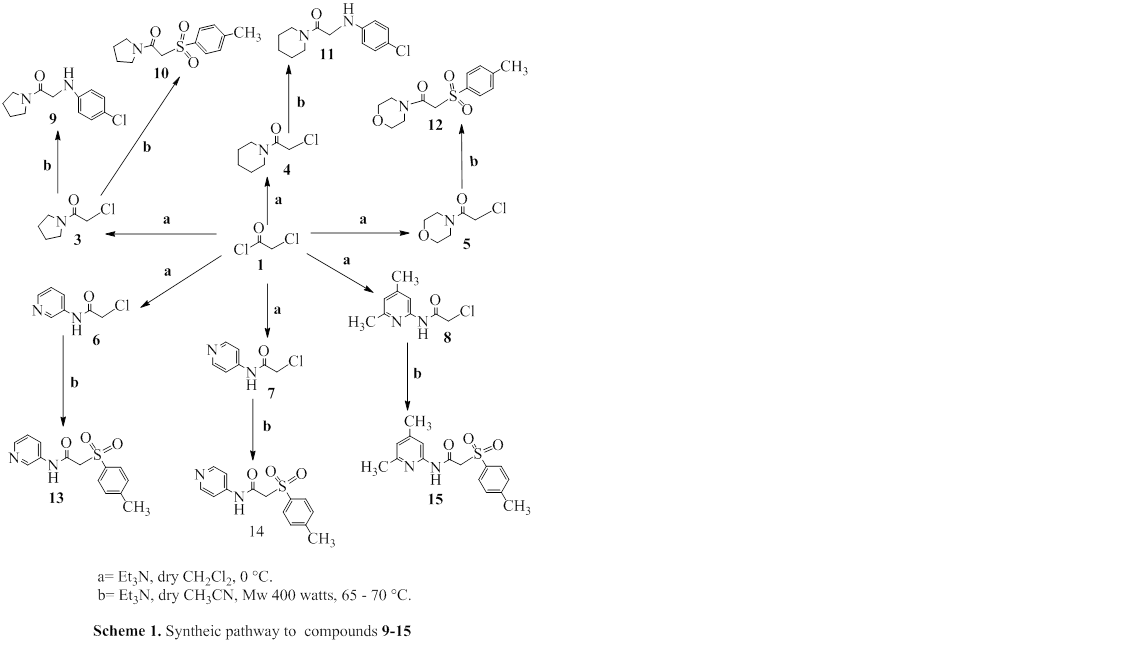
A sequence of new acetamide derivatives 9-15 of primary, secondary amine, and para-toluene sulphinate sodium salt have been synthesized under microwave irradiation and assessed in vitro for their antibacterial activity against one Gram-positive and two Gram-negative bacterial species such as S. pyogenes, E. coli, and P.mirabilis using the Mueller-Hinton Agar diffusion (well diffusion) method. The synthesized compounds with significant differences in inhibition diameters and MICs were compared with those of amoxicillin, ampicillin, cephalothin, azithromycin and doxycycline. All of the evaluated acetamide derivatives were used with varying inhibition concentrations of 6.25, 12.5, 37.5, 62.5, 87.5, 112.5 and 125 µg/ml. The results show that the most important antibacterial properties exercised by the synthetic compounds 9 and 11 bearing para-chlorophenyl moiety incorporated into the 2-position moiety of acetamide 2. The molecular structures of the new compounds were determined using FT-IR, 1H-NMR techniques.