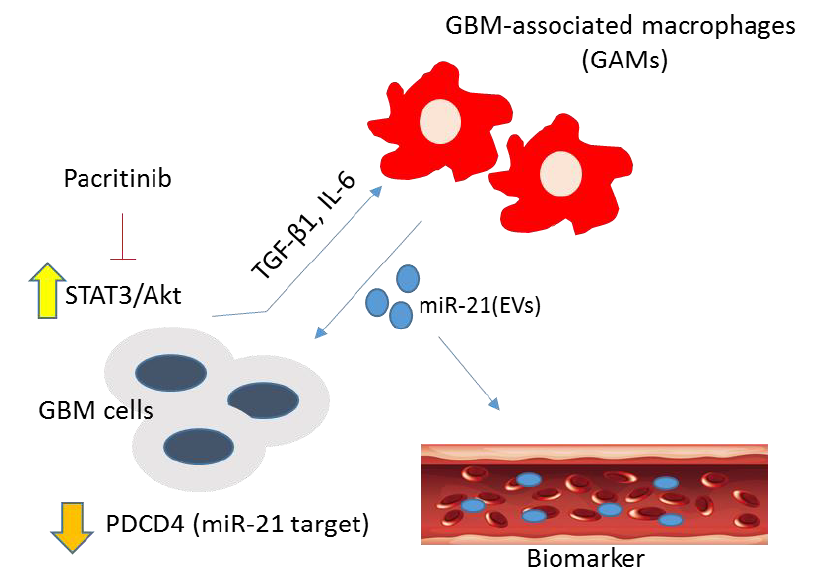
Background: Tumor microenvironment (TME) plays a crucial role in virtually every aspect of tumorigenesis of glioblastoma multiforme (GBM). The dysfunctional TME promotes drug resistance, disease recurrence and distant metastasis. Recent evidence indicates that exosomes released by stromal cells within TME may promote oncogenic phenotypes via transferring signaling molecules such as cytokines, proteins and microRNAs. Results: In this study, clinical GBM samples were collected and analyzed. We found that GBM-associated macrophages (GAMs) secreted exosomes which were enriched with oncomiR-21. Co-culture of GAMs (and GAM derived exosomes) and GBM cell lines resulted in the increased GBM cells’ resistance against temozolomide (TMZ) by upregulating pro-survival gene, PDCD4 and stemness markers Sox2, STAT3, Nestin and miR-21-5p and increased M2 cytokines, IL-6 and TGF-β1 secreted by GBM cells, promoting the M2 polarization of GAMs. Subsequently, pacritinib treatment suppressed GBM tumorigenesis and stemness; more importantly, pacritinib-treated GBM cells showed markedly reduced ability to secret M2 cytokines and reduced miR-21 enriched exosomes secreted by GAMs. Pacritinib-mediated effects were accompanied by a reduction of oncomiR miR-21-5p, by which tumor suppressor PDCD4 was targeted. We subsequently established a patient-derived xenograft models where mice bore patient GBM and GAMs. The treatment of pacritinib, and the combination of pacritinib/TMZ appeared to significantly reduce tumorigenesis of GBM/GAM PDX mice, overcome TMZ-resistance, and M2 polarization of GAMs. Conclusion: In summation, we showed that potential of pacritinib alone or in combination with TMZ for suppressing GBM tumorigenesis via modulating STAT3/miR-21/PDCD4 signaling. Further investigations are warranted for adopting pacritinib for the treatment of TMZ-resistant GBM in the clinical settings.