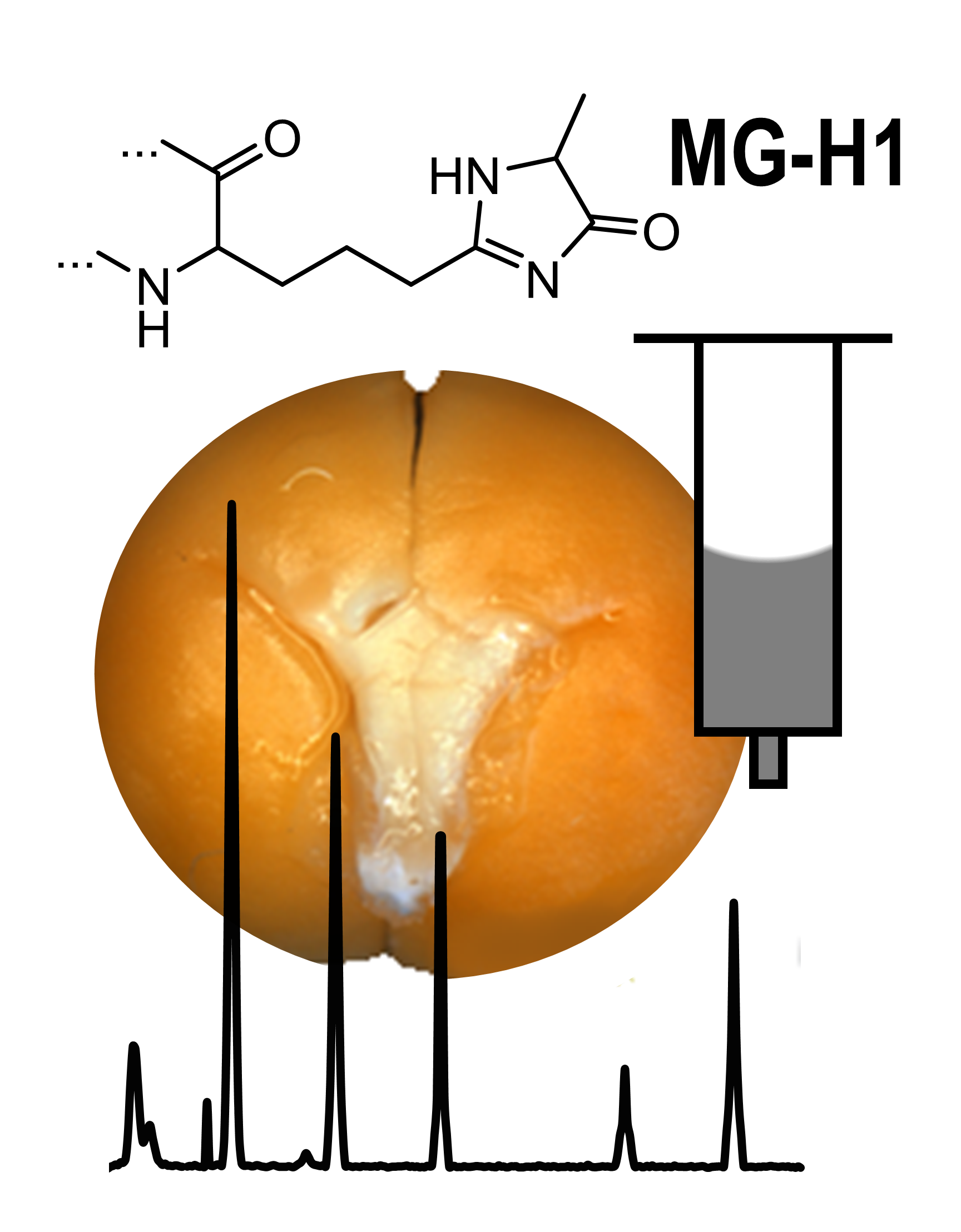
Seeds represent the major source of food protein, impacting on both human nutrition and animal feeding. Therefore, seed quality needs to be appropriately addressed in the context of viability and food safety. Indeed, long-term and inappropriate storage of seeds might result in enhancement of protein glycation, which might affect their quality and longevity. Glycation of seed proteins can be probed by exhaustive acid hydrolysis and quantification of the glycation adduct Nɛ-(carboxymethyl)lysine (CML) by liquid chromatography-mass spectrometry (LC-MS). This approach, however, does not allow analysis of thermally and chemically labile glycation adducts, like glyoxal-, methylglyoxal- and 3-deoxyglucosone-derived hydroimidazolones. Although enzymatic hydrolysis might be a good solution in this context, it requires aqueous conditions, which cannot ensure reconstitution of seed protein isolates. Because of this, the complete profiles of seed AGEs are not characterized so far. Therefore, here we propose the approach, giving access to quantitative solubilization of seed proteins in presence of sodium dodecyl sulfate (SDS) and their quantitative enzymatic hydrolysis prior to removal of SDS by reversed phase solid phase extraction (RP-SPE). Using MG-H1 as a case example, we demonstrate the applicability of this method for reliable and sensitive LC-MS-based quantification of chemically labile AGEs and its compatibility with bioassays.