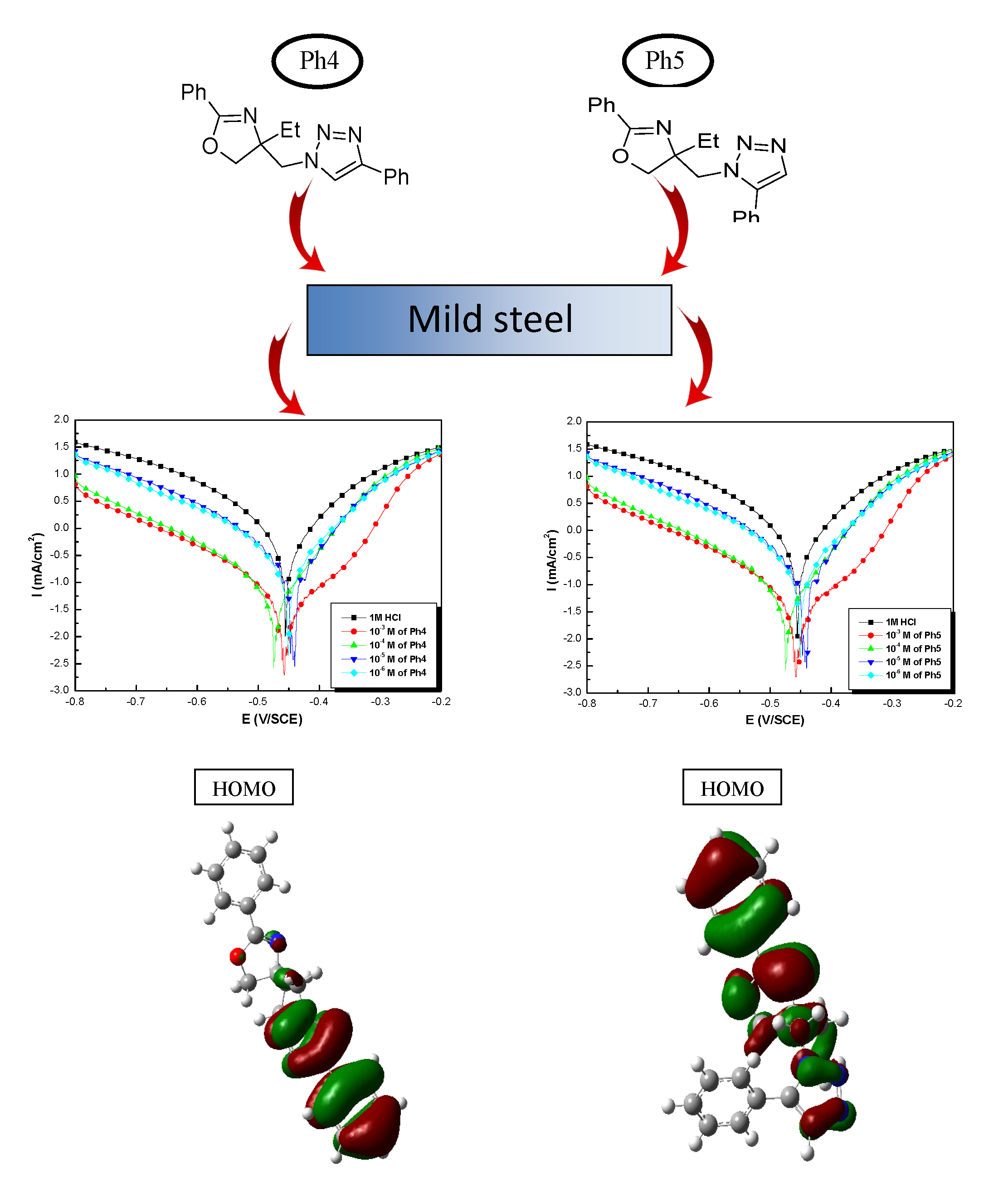
1-[(4-ethyl-2-phenyl-4,5-dihydro-1,3-oxazol-4-yl)methyl]-4-phenyl-1H-1,2,3-triazole (Ph4) and1-[(4-ethyl-2-phenyl-4,5-dihydro-1,3-oxazol-4-yl)methyl]-5-phenyl-1H-1,2,3-triazole (Ph5) are new isomers of the triazole derivative family, were synthesized and tested on the corrosion of mild steel in molar hydrochloric acid molar media using weight loss, electrochemical polarization and impedance spectroscopy. Then the experimental results were confirmed by quantum chemical calculations using DFT at B3LYP /6-31G (d,p). The compound Ph4 is the best inhibitor and its inhibitory efficiency increased with increasing concentration and reaching 95% at 10−3 M. Polarization curves studies show that both compounds tested are mixed-type inhibitors. Nyquist curves presented a single capacitive loop, their diameter increases progressively with both inhibitors concentration. The change of the substitution phenyl from position 5 to position 4 in the triazole ring increases the inhibitory effect of the triazole compounds. The effect of temperature on the corrosion behavior of iron indicates that the inhibitory efficiency of the two inhibitors decreases with increasing temperature in the range of 308 to 338K. DFT study is in good correlationwith the experimental results.