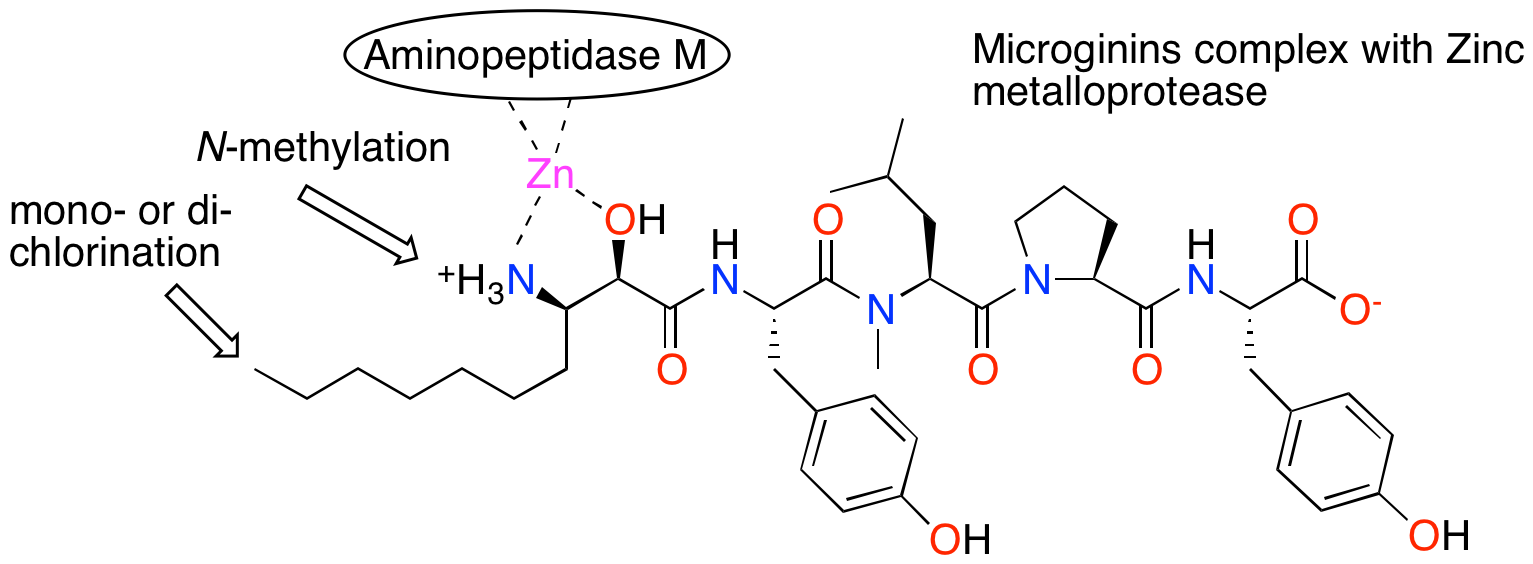
During blooms, cyanobacteria produce diverse modified peptides. Among these are the microginins, which inhibit zinc-containing metalloproteases. Ten microginins, microginins KR767 (1), KR801(2), KR835 (3), KR785 (4), KR604 (5), KR638 (6), KR781 (7), KR815 (8), FR3 (9), and FR4 (10) were isolated from the extract of a bloom material of Microcystis sp. (IL-405) collected from the Kishon Reservoir, Israel in the fall of 2009. The structures of the pure compounds were elucidated using 1D and 2D NMR techniques and high-resolution mass spectrometry. The absolute configuration of the chiral centers of the amino acids were determined by Marfey’s and advance Marfey’s methods and by comparison of 1H and 13C NMR chemical shifts of the Ahda derivatives with those of known microginins. These microginins differ in sequence and absolute configuration of the chiral centers of the Ahda moieties and by N-methylation of Ahda amine group and extent of chlorination of Ahda terminal methyl group. The compounds were evaluated for inhibition of the zinc metalloprotease aminopeptidase M and exhibited low- to sub-nanomolar IC50 values.