Submitted:
24 January 2026
Posted:
27 January 2026
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Eligibility Criteria
Searching and Screening
Data Collection Process
Planned Data Synthesis
Current Review Stage
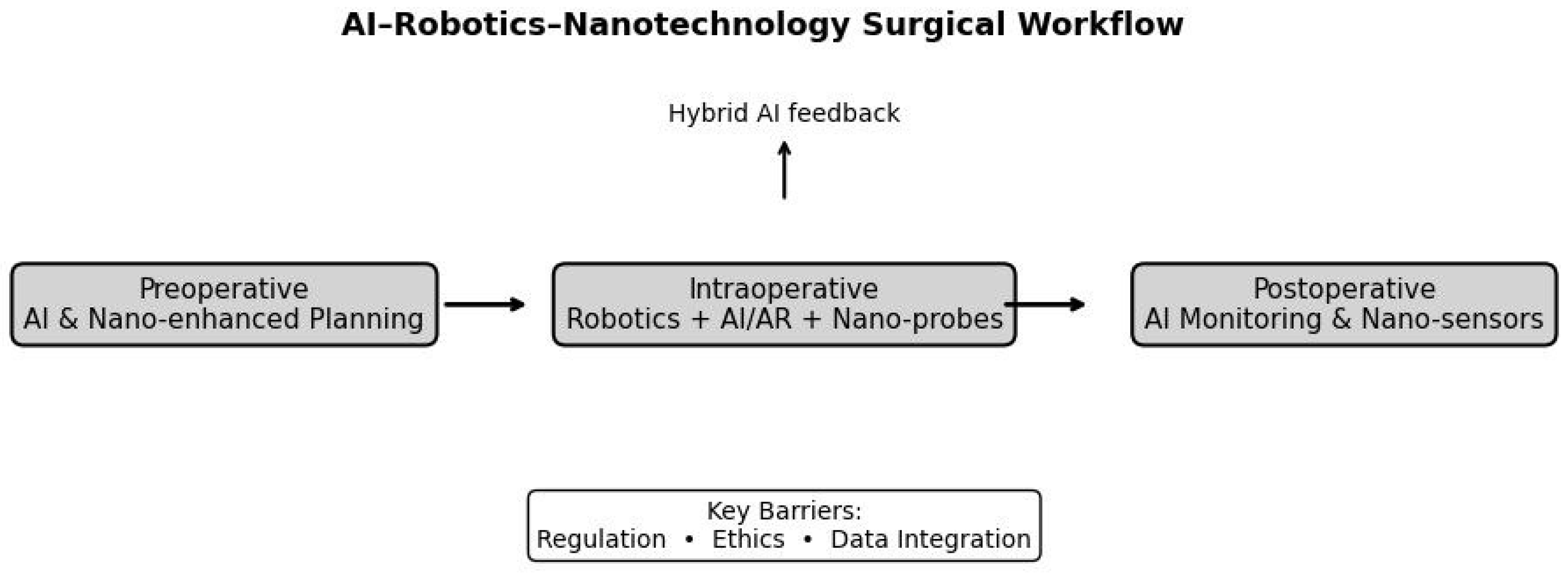
Conceptual Role in Preoperative Planning
Intraoperative Guidance and Execution
Postoperative Monitoring and Outcomes
Limitations, Safety Concerns, and Barriers to Adoption
| Nano Application | Evidence Level | Clinical Status |
|---|---|---|
| Fluorescence probes | Preclinical / early human pilots | Experimental |
| Drug-eluting particles | Preclinical | Not approved in US/EU |
| Targeted contrast agents | Animal models / phase I trials | Investigational |
| Nano-sensors for monitoring | Preclinical concepts | Not in clinical use |
Evidence for Hybrid Systems
| Aspect | Single-Technology (e.g., AI Alone, Robotics Alone, Nano Alone) | Hybrid Systems (e.g., AI-Robotics-Nano Integration) |
| Strengths | Simpler implementation; lower cost; focused on specific pain points (e.g., AI for prediction, robotics for dexterity). | Potential synergies for adaptive workflows; addresses multiple challenges simultaneously (e.g., real-time margin adjustment). |
| Weaknesses | Limited scope; may not handle complexity like deformation or heterogeneity. | Increased complexity; higher risk of compounded errors; regulatory hurdles. |
| Evidence Maturity | Higher for AI/robotics (trials); lower for nano (preclinical). | Mostly preclinical/pilot; lacks RCTs. |
| Clinical Value | High for routine cases; reduces cognitive load in isolated tasks. | May add value in complex resections; could degrade performance in routine ones due to latency/setup. |
| Adoption Barriers | Cost/learning curve for robotics; bias for AI. | Vendor lock-in; interoperability issues; equity gaps. |
| When to Prefer | Standard procedures where one tech suffices. | High-risk cases needing multi-layered support, pending validation. |
When Hybrid Systems May NOT Add Value
Future Roadmap
Conclusions
References
- Artificial Intelligence Applications in the Diagnosis, Treatment, and Prognosis of Hepatocellular Carcinoma - PMC - NIH. https://pmc.ncbi.nlm.nih.gov/articles/PMC12800664.
- Artificial intelligence in hepatocellular carcinoma screening: applications and challenges. https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2025.1713887/full.
- Machine learning integrating MRI and clinical features predicts early recurrence of hepatocellular carcinoma after resection | Scientific Reports - Nature. https://www.nature.com/articles/s41598-026-36261-3.
- Current status and prospects of artificial intelligence in liver cancer management. https://www.sciencedirect.com/science/article/pii/S295026162500038X.
- Study reveals AI strengths and limits in real-world liver cancer treatment - HAB Central. https://habcentral.habcommunity.com/article/study-reveals-ai-strengths-and-limits-in-real-world-liver-cancer-treatment?article_id=7066699&article_title=Study+reveals+AI+strengths+and+limits+in+real-world+liver+cancer+treatment.
- Can AI Guide the Decision to Transplant or Resect for Hepatocellular Carcinoma? | Gastroenterology and Hepatology | JAMA Network Open. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2839040.
- Artificial Intelligence in Hepatocellular Carcinoma: Applications and | IJGM - Dove Medical Press. https://www.dovepress.com/an-artificial-intelligence-pipeline-for-hepatocellular-carcinoma-from--peer-reviewed-fulltext-article-IJGM.
- Artificial intelligence in predicting recurrence after first-line treatment of liver cancer: a systematic review and meta-analysis | BMC Medical Imaging. https://link.springer.com/article/10.1186/s12880-024-01440-z.
- Robotic liver resection for HCC reduced length of hospital stay, ICU admissions - Healio. https://www.healio.com/news/gastroenterology/20221221/robotic-liver-resection-for-hcc-reduced-length-of-hospital-stay-icu-admissions.
- Emerging Role of MRI--Based Artificial Intelligence in Individualized Treatment Strategies for Hepatocellular Carcinoma: A Narrative Review - NIH. https://pmc.ncbi.nlm.nih.gov/articles/PMC12706715.
- Artificial Intelligence in Hepatopancreaticobiliary Surgery: A Systematic Review. https://journal.hep.com.cn/artis/EN/10.20517/ais.2022.20.
- The role of artificial intelligence and 3D printing in minimally invasive liver surgery. https://www.oaepublish.com/articles/2574-1225.2024.78.
- Artificial intelligence in the detection, characterisation and prediction of hepatocellular carcinoma: a narrative review - Translational Gastroenterology and Hepatology. https://tgh.amegroups.org/article/view/6429/html.
- Artificial Intelligence in Perioperative Planning and Management of Liver Resection | Request PDF - ResearchGate. https://www.researchgate.net/publication/377597661_Artificial_Intelligence_in_Perioperative_Planning_and_Management_of_Liver_Resection.
- Current state of artificial intelligence in liver transplantation - ScienceDirect.com. https://www.sciencedirect.com/science/article/pii/S2451959625000034.
- Machine learning integrating MRI and clinical features predicts early recurrence of hepatocellular carcinoma after resection - Nature. https://www.nature.com/articles/s41598-026-36261-3_reference.pdf.
- Role of artificial intelligence in the diagnosis and treatment of hepatocellular carcinoma. https://www.wjgnet.com/2644-3236/full/v3/i4/96.htm.
- Redefining precision medicine in hepatocellular carcinoma through omics, translational, and AI-based innovations. https://www.jprecisionmedicine.org/article/S3050-6328(25)00003-4/fulltext.
- Robotic versus laparoscopic hepatectomy: meta-analysis of... https://pmc.ncbi.nlm.nih.gov/articles/PMC11957917.
- An updated meta-analysis of the efficacy and safety of robot-assisted... https://journals.lww.com/md-journal/fulltext/2025/01030/an_updated_meta_analysis_of_the_efficacy_and.8.aspx.
- Robotic versus laparoscopic hepatectomy for liver malignancies... https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(24)00139-X/fulltext.
- Robotic Liver Resections: Current State-of-the-Art and Future... https://link.springer.com/article/10.1007/s40137-025-00469-5.
- Laparoscopic versus Robotic Hepatectomy: A Systematic Review... https://www.mdpi.com/2077-0383/11/19/5831.
- International experts consensus guidelines on robotic liver resection... https://pmc.ncbi.nlm.nih.gov/articles/PMC10494765.
- Robotic versus conventional laparoscopic liver resections. https://journals.sagepub.com/doi/10.1177/1457496920925637.
- Robotic-assisted laparoscopic liver resection in hepatocellular... https://journal.hep.com.cn/hepatomaresearch/EN/10.20517/2394-5079.2020.86.
- Robotic versus open resection for colorectal liver metastases in a... https://www.cell.com/heliyon/fulltext/S2405-8440(24)00831-4.
- Robotic versus laparoscopic liver resection: a systematic review and... https://journals.lww.com/international-journal-of-surgery/fulltext/2025/08000/robotic_versus_laparoscopic_liver_resection__a.46.aspx.
- Advanced nanotheranostic approaches for hepatocellular carcinoma management. https://www.sciencedirect.com/science/article/pii/S2949916X2500012X.
- Application of nanotechnology in the treatment of hepatocellular carcinoma - Frontiers. https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2024.1438819/full.
- Nanotechnology for the Diagnosis and Treatment of Liver Cancer - PMC - NIH. https://pmc.ncbi.nlm.nih.gov/articles/PMC11681781.
- Full article: Application of Nanotechnology in TACE Treatment of Liver Cancer. https://www.tandfonline.com/doi/full/10.2147/IJN.S527518.
- A Narrative Review on Functionalized Nanoparticles for the Treatment and Early Detection of Hepatocellular Carcinoma - MDPI. https://www.mdpi.com/2076-3417/15/14/7649.
- Convergence of nanotechnology and artificial intelligence in the fight against liver cancer: a comprehensive review | Discover Oncology | Springer Nature Link. https://link.springer.com/article/10.1007/s12672-025-01821-y.
- Recent advances in hepatocellular carcinoma-targeted nanoparticles - IOP Science. https://iopscience.iop.org/article/10.1088/1748-605X/ad46d3/pdf.
- Nanotechnology at the forefront of liver cancer diagnosis - ScienceDirect - DOI. [CrossRef]
- Advancements in nano drug delivery system for liver cancer therapy based on mitochondria-targeting - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0753332224014069.
- nanotechnology-driven therapeutics for liver cancer: clinical applications and pharmaceutical insights - ResearchGate. https://www.researchgate.net/publication/388876344_NANOTECHNOLOGY-DRIVEN_THERAPEUTICS_FOR_LIVER_CANCER_CLINICAL_APPLICATIONS_AND_PHARMACEUTICAL_INSIGHTS.
- Nanoparticles and their application in the diagnosis of hepatocellular carcinoma. https://www.researchgate.net/publication/385657291_Nanoparticles_and_their_application_in_the_diagnosis_of_hepatocellular_carcinoma.
- Engineering nanotheranostic strategies for liver cancer; Baishideng Publishing Group; https://www.wjgnet.com/1948-5204/full/v13/i10/1213.htm.
- The improving strategies and applications of nanotechnology-based drugs in hepatocellular carcinoma treatment - Frontiers. https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2023.1272850/full.
- Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma - Theranostics. https://www.thno.org/v11p5464.htm.
- Nanomedicine in Hepatocellular Carcinoma: A New Frontier in Targeted Cancer Treatment. https://www.mdpi.com/1999-4923/14/1/41.
- Engineered nanoparticles for imaging and targeted drug delivery in hepatocellular carcinoma | Experimental Hematology & Oncology - Springer Link. https://link.springer.com/article/10.1186/s40164-025-00658-z.
- Current advances in nanoparticle-based approaches for the hepatocellular carcinoma treatment - ScienceDirect. https://www.sciencedirect.com/science/article/abs/pii/S2210740124002298.
- Advances of Nanotechnology in the Diagnosis and Treatment of Hepatocellular Carcinoma. https://www.semanticscholar.org/paper/Advances-of-Nanotechnology-in-the-Diagnosis-and-of-Escutia-Guti%C3%A9rrez-Sandoval-Rodr%C3%ADguez/2774b8da70739a1ed08b337898cec1ab434a49af.
- (PDF) Recent advances in hepatocellular carcinoma-targeted nanoparticles - ResearchGate. https://www.researchgate.net/publication/380312181_Recent_advances_in_hepatocellular_carcinoma-targeted_nanoparticles.
- Liver Robotic Surgery: A Review of Current Use and Future Perspectives - MDPI. https://www.mdpi.com/2077-0383/14/19/7014.
- Robotic Liver Resections: Current State-of-the-Art and Future Perspectives - Springer Link. https://link.springer.com/article/10.1007/s40137-025-00469-5.
- Review Article Artificial Intelligence and liver: Opportunities and barriers - ScienceDirect.com. https://www.sciencedirect.com/science/article/pii/S1590865823009003.
- Liver Robotic Surgery: A Review of Current Use and Future Perspectives - ResearchGate. https://www.researchgate.net/publication/396188100_Liver_Robotic_Surgery_A_Review_of_Current_Use_and_Future_Perspectives.
- The current status and future directions of artificial intelligence in the prediction, diagnosis, and treatment of liver diseases - Sage Journals. https://journals.sagepub.com/doi/10.1177/20552076251325418.
- The rise of robotics and AI-assisted surgery in modern healthcare - PMC - PubMed Central. https://pmc.ncbi.nlm.nih.gov/articles/PMC12181090.
- Nano bio-robots: a new frontier in targeted therapeutic delivery. https://www.frontiersin.org/journals/robotics-and-ai/articles/10.3389/frobt.2025.1639445/full.
- The future of robotic liver surgery - Giulianotti. https://hbsn.amegroups.org/article/view/39794/html.
- A bright future for robotic surgery - Open MedScience. https://openmedscience.com/bright-future-for-robotic-surgeons.
- Robotic liver surgery: literature review and current evidence - OAE Publishing Inc. https://www.oaepublish.com/articles/2574-1225.2020.90.
- International experts consensus guidelines on robotic liver resection in 2023 - PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC10494765.
- Convergence of nanotechnology and artificial intelligence in the fight against liver cancer: a comprehensive review | Discover Oncology | Springer Nature Link. https://link.springer.com/article/10.1007/s12672-025-01821-y.
- Total robotic liver transplant: the final frontier of minimally invasive surgery - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S1600613524002405.
- Redefining precision medicine in hepatocellular carcinoma through omics, translational, and AI-based innovations. https://www.jprecisionmedicine.org/article/S3050-6328(25)00003-4/fulltext.
- AI-Integrated Micro/Nanorobots for Biomedical Applications: Recent Advances in Design, Fabrication, and Functions - MDPI. https://www.mdpi.com/2079-6374/15/12/793.
- The future of robotic surgery | Stanford University School of Engineering. https://engineering.stanford.edu/news/future-robotic-surgery.
- Medical Micro/Nanorobots in Precision Medicine - Soto - The Advanced Portfolio - Wiley; https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202002203.
- Advancements and Challenges in Robotic Liver Transplantation - AZoRobotics. https://www.azorobotics.com/News.aspx?newsID=15115.
- World’s First Remote Head & Neck Robotic Surgery | Yao Li | Borns Medical Robotics. https://www.youtube.com/watch?v=kEZjp93p7OI.
- Robotics as the natural evolution for minimally invasive surgery liver surgery: technological advances for patient and surgeon benefit - Digestive Medicine Research. https://dmr.amegroups.org/article/view/7827/html.
- Augmented and mixed reality in liver surgery: a comprehensive narrative review of novel clinical implications on cohort studies - PubMed Central. https://pmc.ncbi.nlm.nih.gov/articles/PMC12245099.
- Robotic Liver Resections: Current State-of-the-Art and Future Perspectives - Springer Link. https://link.springer.com/article/10.1007/s40137-025-00469-5.
- Augmented Reality and Image-Guided Robotic Liver Surgery - MDPI. https://www.mdpi.com/2072-6694/13/24/6268.
- Robotic Liver Resections: Current State-of-the-Art and Future Perspectives - ResearchGate. https://www.researchgate.net/publication/396198790_Robotic_Liver_Resections_Current_State-of-the-Art_and_Future_Perspectives.
- Leveraging augmented reality for dynamic guidance in 3-dimensional laparoscopic and robotic liver surgery: a prospective case series study. https://astr.or.kr/DOIx.php?id=10.4174%2Fastr.2025.109.1.44.
- Augmented Reality and Navigation Technologies in Liver Surgery | Nature Research Intelligence. https://www.nature.com/research-intelligence/nri-topic-summaries/augmented-reality-and-navigation-technologies-in-liver-surgery-micro-1206576.
- The role of 3D printing, virtual and augmented reality in liver surgery: The technological imperative! | Gastroenterology and Functional Medicine. https://www.hksmp.com/journals/gfm/article/view/383.
- Application value of mixed reality in hepatectomy for hepatocellular carcinoma. https://www.wjgnet.com/1948-9366/full/v14/i1/36.htm.
- Enhancing liver surgery and transplantation: the role of 3D printing and virtual reality. https://journal.hep.com.cn/artis/EN/10.20517/ais.2024.07.
- Cost-effectiveness and clinical impact of robotic-assisted hepatectomy - PubMed Central. https://pmc.ncbi.nlm.nih.gov/articles/PMC11997018.
- The value of Augmented Reality in surgery — A usability study on laparoscopic liver surgery. https://www.sciencedirect.com/science/article/pii/S1361841523002037.
- (PDF) Integrating Mixed Reality, Augmented Reality, and Artificial Intelligence in Complex Liver Surgeries: Enhancing Precision, Safety, and Outcomes - ResearchGate. https://www.researchgate.net/publication/391830083_Integrating_Mixed_Reality_Augmented_Reality_and_Artificial_Intelligence_in_Complex_Liver_Surgeries_Enhancing_Precision_Safety_and_Outcomes.
- Augmented reality in hepatobiliary-pancreatic surgery: a technology at your fingertips | Cirugía Española (English Edition) - Elsevier. https://www.elsevier.es/en-revista-cirugia-espanola-english-edition--436-articulo-augmented-reality-in-hepatobiliary-pancreatic-surgery-S2173507723000388.
- Augmented reality in liver surgery - HAL-Inria. https://inria.hal.science/hal-04414274/document.
- Augmented reality during laparoscopic liver resection: a work in progress that is terribly necessary - Laurent. https://ls.amegroups.org/article/view/4686/html.
- Advanced liver surgery training in collaborative VR environments | Computers and Graphics. [CrossRef]
- Augmented Reality Integration for Surgical Enhancement in Hepatic Surgery - Review of the Current Literature - PubMed. https://pubmed.ncbi.nlm.nih.gov/41196075.
- Enhancing liver surgery and transplantation: the role of 3D printing and virtual reality. https://www.oaepublish.com/articles/ais.2024.07.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





