Introduction
Cardiovascular diseases (CVDs) represent a significant public health burden in South Korea. Over the past ten years, deaths attributable to CVD have risen by 25.2%, making it the second most common cause of mortality nationwide as of 2022. In addition, the increasing prevalence of hypertension and dyslipidemia among young adults is particularly concerning, as it may contribute to a future rise in CVD incidence. Each year, roughly 10,000 patients require intensive care unit admission following cardiovascular surgery, and this number showed an upward trend from 2010 to 2019. Surgical procedures associated with the highest death rates include those involving the aorta, coronary artery bypass grafting (CABG), and valve surgeries [
1,
2].
Cardiac rehabilitation (CR) is recognized for its benefits in enhancing exercise tolerance and quality of life and reducing the need for hospital readmissions and risk of death. Authoritative clinical practice guidelines from regions including Europe, America, and Korea have encouraged the integration of CR into the treatment plans of patients suffering from conditions such as congestive heart failure, valve-related diseases, coronary artery disease, and those recovering from heart surgeries [
3,
4,
5,
6].
CR is structured into three distinct stages. The initial stage, or phase 1, starts with the patient's early movement activities in the ICU and ward to improve their daily living skills. This early stage typically involves supervised exercises within the hospital's CR center, where the patient's heart rate (HR) and electrocardiography (ECG) are closely monitored. Approximately 1–3 months post-surgery, following hospital discharge, patients return to the hospital for a symptom-limited cardiopulmonary exercise test (CPET). Depending on the test outcomes, patients identified as having moderate-to-high risk are recommended to participate in a supervised, center-based phase 2 CR program that includes thorough HR and ECG monitoring. Conversely, those deemed to be at low risk may be advised to undertake CR at home. The final stage, phase 3, encourages patients to commit to sustained CR exercises and risk factor management within their home and community settings, promoting indefinite, self-managed care and monitoring [
7].
Symptom-limited treadmill-based CPET is widely recognized as a crucial component of the standard CR program. It evaluates a patient's exercise capacity and prognosis by monitoring blood pressure, HR, ECG, and gas exchange as exercise intensity progressively increases. This detailed assessment helps in tailoring the right exercise prescription for CR [
5]. Consequently, numerous clinical guidelines advocate for the administration of CPET prior to starting CR [
8]. For instance, the Japanese guidelines suggest the implementation of a symptom-limited exercise stress test between 14 and 21 days following a myocardial infarction (MI) [
9], and the ACC/AHA guidelines recommend that this test can be safely carried out between 3 and 6 weeks following MI [
10].
However, these recommendations primarily apply to the subacute phase, and evidence supporting the safety and feasibility of treadmill-based CPET in the early postoperative period remains limited. Although one previous study reported the feasibility of a low-level treadmill-based CPET within four days after CABG, the sample size was very small [
11]. Furthermore, nearly 20% of the patients were unable to undergo low-level CPET before discharge after MI because of medical instability, fear, or difficulty in walking on a treadmill [
12]. In addition, treadmill-based CPET has been associated with a significantly higher incidence of overall cardiac events, premature ventricular contractions, angina, and ventricular tachycardia compared with cycle ergometer–based testing [
13]. Cycle ergometer–based CPET, particularly using a recumbent cycle ergometer, may represent a safer alternative for patients in the early postoperative period. Unlike treadmill exercise, recumbent cycling is performed in a seated or semi-recumbent position, which minimizes balance demands and reduces the risk of falls. Moreover, as a non–weight-bearing modality, it requires less muscular effort, which makes it more suitable and potentially safer for patients recovering from cardiac surgery [
14].
Recent studies have highlighted the advantages of initiating CR shortly after cardiac surgery, showing improvements in survival and shorter hospital stays without an increased risk of adverse events. Sezai et al. demonstrated that patients who began CR within a median of 1–3 days after open-heart surgery experienced lower in-hospital mortality, reduced length of stay, and decreased medical costs compared with those who did not receive early CR. Likewise, Pack et al. reported that it is safe to enroll patients in CR within two weeks after discharge, provided that individualized evaluation and tailored exercise prescriptions are conducted within a structured CR program [
15,
16].
Despite these findings, most previous studies and medical practices have traditionally scheduled CPET at approximately one month postoperatively [
17]. Notably, guidelines regarding the safest timing for conducting CPET after cardiac surgery are lacking [
8]. This one-month period after surgery is critical, as patients are at risk of falling into a state of physical inactivity, which has been linked to increased long-term mortality [
18]. Implementing a safe and clinically informative submaximal CPET during the in-hospital phase 1 CR period may be crucial for the active application of early CR after cardiac surgery, as it can enhance both the safety and effectiveness of phase I CR and facilitate appropriate exercise prescription during the early transition to phase 2 CR. This study aimed to assess the safety, feasibility, and clinical utility of an early postoperative recumbent ergometer-based CPET performed within two weeks after open-heart surgery.
Methods
Study Population
This prospective study was carried out at OO University OO Hospital and included patients who underwent open-heart surgery. The study protocol was approved by the Institutional Review Board of OO University OO Hospital (approval No: 2021AN0091). Between November 2021 and February 2023, patients referred for inpatient CR to the Department of Physical Medicine and Rehabilitation after open-heart surgery were screened for eligibility. Referrals were primarily made by the Department of Cardiothoracic Surgery, and eligibility was determined according to predefined inclusion and exclusion criteria. Written informed consent was obtained from all participants prior to enrollment.
Eligible participants were adults aged over 18 years with a left ventricular ejection fraction (LVEF) greater than 35% who were capable of performing an exercise test. Patients diagnosed with acute coronary syndrome, valvular heart disease, or aortic dissection who had undergone open-heart surgery and were able to ambulate independently were included.
Patients were excluded if they were classified as high-risk due to a history of cardiac arrest or the presence of an implantable cardioverter defibrillator, had unstable medical conditions, or exhibited cognitive impairments that interfered with understanding the exercise protocol. Individuals with musculoskeletal or neurological disorders affecting the lower extremities that limited exercise participation, as well as those unable to walk independently, were also excluded.
Study Protocol
Starting on the fifth day after surgery, 20 patients participated in inpatient CR sessions. Sessions were held five times per week, lasted approximately one hour, and included warm-up exercises, resistance training, aerobic exercise, and cool-down activities. Before hospital discharge, which occurred around postoperative day nine, participants underwent a recumbent ergometer-based submaximal CPET along with the grip strength test, 6-minute walk test (6MWT), Korean activity scale/index (KASI), EuroQol-5 dimension, and short-form 36-item health survey (SF-36).
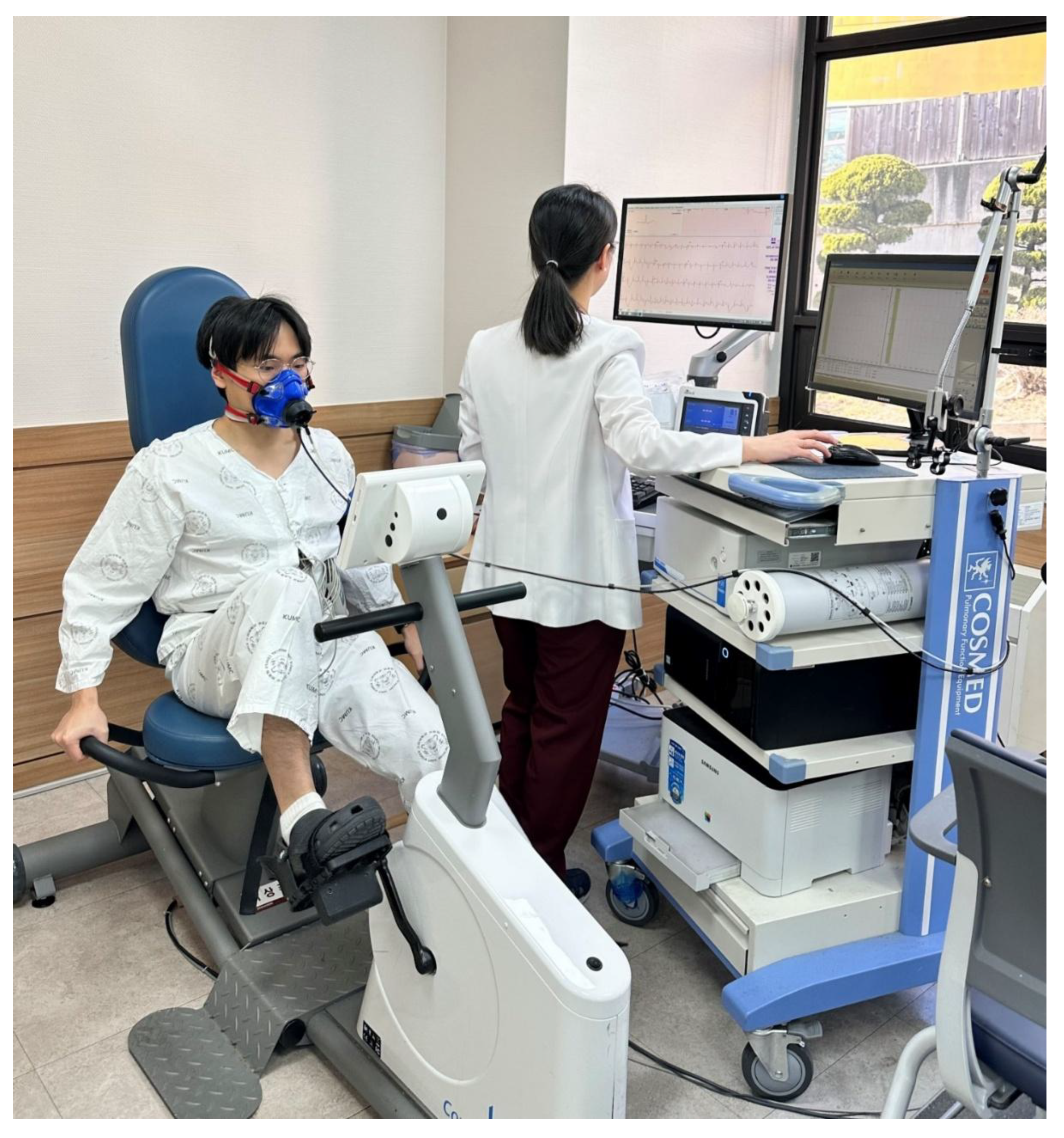
Although treadmill-based CPET is commonly used because patients may discontinue cycling tests due to quadriceps fatigue and difficulty maintaining cadence, [
19,
20] a recumbent ergometer was selected in this study to reduce physical strain on individuals in the early postoperative period (
Figure 1). Participants were positioned on a recumbent ergometer (Corival Recumbent CPET 969900, Lode, Groningen, Netherlands) and began pedaling at 60 rpm with an initial workload of 0 W. The workload was increased by 20 W every two minutes. Testing was terminated when absolute indications for discontinuation or predefined termination criteria were met (
Table 1). Throughout the test, HR, oxygen consumption (VO
2), metabolic equivalents of task (MET), respiratory exchange ratio (RER), rating of perceived exertion (RPE), blood pressure (BP), dyspnea and angina scale, total exercise duration, and maximal work capacity (Wmax) were recorded. Wmax was defined as the highest workload that could be sustained for at least 30 seconds.
The grip strength test, a widely used indicator of muscle strength and an important predictor of cardiovascular outcomes, hospitalization duration, functional status, and perioperative complication [
21,
22,
23], was measured using a digital dynamometer (JAMAR PLUS+ Digital Hand Dynamometer; Sammons Preston Rolyan, Bolingbrook, IL, USA). Participants performed two maximal efforts with each hand, and the highest value (kg) was recorded. Since most participants were right-handed, only right-hand grip strength was analyzed.
The 6MWT was conducted to assess functional capacity and daily activity performance. [
24] Participants walked along a 30-meter corridor for six minutes, aiming to cover the greatest possible distance while maintaining a perceived exertion level of 3 to 4 (moderate to somewhat strong) on the Borg CR scale 10. [
25] Rest was allowed if needed, and the test was discontinued in cases of dyspnea or chest pain that interfered with daily activities. Participants were informed of the elapsed time at one-minute intervals without encouragement. Heart rate, blood pressure, and oxygen saturation were measured before and after the test, and total walking distance was recorded.
The KASI [
26] is a Korean adaptation of the Duke Activity Status Index (DASI). The DASI is a 12-item questionnaire designed to evaluate functional capacity and quality of life and has been shown to correlate with peak oxygen uptake and major adverse cardiac events. [
27] Participants completed the KASI independently, and total scores were calculated based on their responses.
The EuroQol-5 dimension (EQ-5D) questionnaire assesses health-related quality of life in five dimensions : mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. [
28] Each dimension has three response levels (no problems, moderate problems, severe problems). Responses were converted into index scores using Korean population value sets, ranging from –0.171 to 1. A score of 1 indicates perfect health, 0 corresponds to death, and negative values represent health states worse than death. [
29,
30]
The SF-36 questionnaire was used to assess overall quality of life across eight domains: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. These domains produce scores ranging from 0 (worst health) to 100 (best health). The results can be summarized into the Physical Component Summary (PCS) and Mental Component Summary (MCS), both standardized to a mean of 50 with a standard deviation of 10 in the general population. [
31] The Korean version of the SF-36 was used to ensure cultural relevance. [
32]
Statistical Analysis
SPSS version 26 was used for the statistical analysis. Spearman’s correlation, adjusting for age and sex, was used to evaluate the correlation between 6MWT, self-reported questionnaire outcomes (KASI, EQ-5D, SF-36), and estimated peak VO
2 obtained during the exercise test. Correlation coefficients >0.6 were considered high, those <0.3 were categorized as poor, and values falling between these ranges were regarded as moderate. [
33] Statistical significance was set at a p-value < 0.05.
Results
Baseline Characteristics
Twenty patients were recruited between November 2021 and April 2023 and completed the protocol. The baseline characteristics of the study participants are presented in
Table 2. The average age of the patients was 62.50 years (15 males, 5 females). The median time to initiate inpatient CR was 5 days, while the median time to undergo CPET was 9 days postoperative. The most common reasons for surgery were infective endocarditis and valve diseases, each accounting for 25% of the cases, followed by stable angina (20%) and MI (15%). Ten patients underwent valve surgery, while eight patients underwent CABG surgery. The remaining two patients received total arch replacement and coronary artery fistulectomy.
Assessments of Functional Capacity
Table 3 displays the outcomes of functional capacity measured at an average of nine days after surgery. The average grip strength of the right hand was 29.93 kg (±1.71). The 6MWT was 350.15 meters (±18.85). The average scores for KASI and SF-36 were 10.26 (±0.77) and 106.48 (±3.13), respectively. The median value of EQ-5D was 0.72 (range: 0.68–0.72).
Table 3.
Functional capacity measure results (N=20).
Table 3.
Functional capacity measure results (N=20).
| Variables |
Values |
| Grip strength test (kg) |
29.93 ± 1.71 |
| 6MWT (m) |
350.15 ± 18.85 |
| KASI |
10.26 ± 0.77 |
| EQ-5D |
0.72 (0.68 ; 0.72) |
| SF - 36 |
|
| Total |
106.48 ± 3.13 |
| PCS |
47.14 ± 1.57 |
| MCS |
59.34 ± 2.18 |
Table 4.
Results of a recumbent ergometer-based submaximal CPET (N=20).
Table 4.
Results of a recumbent ergometer-based submaximal CPET (N=20).
| |
Values |
| CPET duration (second) |
411.75 ± 168.25 |
| Estimated peak VO2 (ml/kg/min) |
12.32 ± 0.75 |
| Percentage of VO2 max (%) |
46.65 ± 2.08 |
| Estimated peak MET |
3.52 ± 0.22 |
| Estimated peak RER |
1.01 (0.98 ; 1.12) |
| Wmax (Watt) |
63.00 ± 7.00 |
| Estimated peak RPE |
15.00 ± 0.51 |
| Estimated peak HR (beats/min) |
111.8 ± 3.76 |
| Percentage of age-predicted maximal HR (%) |
70.95 ± 2.09 |
| Estimated peak Systolic BP (mmHg) |
162.60 ± 4.47 |
Results of Recumbent Ergometer-Based Submaximal CPET
During the test, the average duration of the CPET was 411.75 ± 168.25 seconds. The average estimated peak VO2 was 12.32 ± 0.75 ml/kg/min, corresponding to 46.65 ± 2.08% of VO2 max. The average values for estimated peak MET and Wmax were 3.52 ± 0.22 and 63.00 ± 7.00 W, respectively. The estimated peak RER was 1.01 (range: 0.98–1.12), and the peak RPE was 15.00 ± 0.51. Additionally, the estimated peak HR was 111.8 ± 3.76 beats/min, equivalent to 70.95 ± 2.09% of the age-predicted maximal HR.
After adjusting for age and sex, Spearman's correlation analysis demonstrated a strong positive correlation between the estimated peak VO
2 and. Additionally, moderate positive correlations were observed between estimated peak VO
2 and 6MWT (r=0.52, p=0.026), KASI (r=0.59, p=0.011), and PCS of the SF-36 questionnaire (r=0.546, p=0.019). No significant correlation was found between the estimated peak VO
2 and other measurements (
Table 5).
Throughout the test, no patients experienced significant cardiac events, such as ST-segment elevation or depression, or anginal chest pain. The reasons for test termination were shortness of breath in 10 patients and leg discomfort in nine patients. One patient stopped the test because of a combination of shortness of breath and leg discomfort, and another patient stopped because of leg discomfort accompanied by a decrease in BP. In the patient who exhibited a drop in BP, the resting BP was 129/93 mmHg. During stage 3 of the test, the BP decreased from 144/86 mmHg at stage 2 to 116/89 mmHg, and the test was terminated according to the predefined termination criteria (
Table 1).
Discussion
Our findings further support the idea that initiating submaximal exercise testing within 2 weeks (an average of 9 days) after cardiac surgery can be safely performed. Hamm et al. suggested that patients who underwent exercise testing 15–28 days after acute MI had a significantly higher rate of cardiac arrest compared with those tested within 14 days after acute MI [
34]. Miller et al. found that performing an exercise test soon after MI is beneficial to promote patients' self-confidence, guide post-discharge exercise prescriptions, and predict post-hospital prognosis. However, they emphasized that such tests should be conducted in a well-controlled research environment due to safety concerns [
35]. A previous study on early symptom-limited exercise stress test after PTCA or CABG found that the main reasons for early termination of the test were chest pain, ST changes, hemodynamic instability, dyspnea, and musculoskeletal pain [
36]. In our study using a recumbent ergometer-based submaximal CPET, none of the patients experienced ST changes, hemodynamic instability, or chest pain.
A previous study found that the results of a submaximal exercise test using a treadmill, performed 5.0 ± 2.8 days after admission for unstable angina, were useful in predicting outcomes during the first year after hospital discharge [
37]. However, few studies have examined submaximal testing early after cardiac surgery. Although VO
2 max obtained from a maximal treadmill test is 6–17% higher than that obtained from a maximal cycle ergometer test due to the engagement of larger muscle groups during treadmill exercise, a linear increase in work rate on both treadmill and cycle ergometer can effectively demonstrate oxygen uptake patterns during CPET [
38,
39]. We chose the recumbent ergometer for the submaximal exercise test because it carries a lower risk of falls and allows for easier acquisition of ECG data with fewer artifacts.
Despite these limitations, our findings showed a high degree of consistency with previously reported results. Takeyama et al [
40] studied 28 patients who underwent CABG and divided them into training and control groups, with exercise training initiated one week after surgery. All patients underwent CPET using a cycle ergometer. Peak VO₂ measured during CPET was 13.1 ± 1.7 ml/kg/min in the training group (mean age 58.8 ± 6.3 years) and 13.7 ± 2.5 ml/kg/min in the control group (mean age 61.7 ± 8.7 years). In the present study, peak VO₂ measured at a median of 9 days after surgery was 12.32 ± 0.75 ml/kg/min. Considering the relatively higher mean age of our study population (62.5 years) and the greater proportion of female patients, the similarity in peak VO₂ values suggests a strong correlation with previous findings, supporting the validity of early submaximal CPET after cardiac surgery.
However, some methodological differences exist between the study by Takeyama et al. and our study. First, unlike our study, which utilized a recumbent ergometer, their study used an upright cycle ergometer. In our cohort of patients who had recently undergone open-heart surgery, a recumbent ergometer was used to minimize the risk of falls and enhance safety during early postoperative testing. Previous studies have demonstrated that upright cycle ergometers generally yield higher peak VO₂ values than recumbent ergometers at submaximal workloads [
41], which may partially account for the slightly higher oxygen consumption values observed by Takeyama et al. Additionally, although Takeyama et al conducted CPET early (one week after surgery), they did not examine the correlations between peak VO₂ and other functional measures. As a result, the reliability of CPET-derived aerobic capacity in the early postoperative phase could not be fully assessed in their study. To address this limitation, we evaluated correlations between CPET parameters and multiple functional and patient-reported outcomes, including the 6MWT, KASI, and the PCS domain of SF-36, thereby providing further evidence supporting the validity of early submaximal CPET after cardiac surgery.
The estimated peak VO
2 measurements in our study also showed significant correlations with the 6MWT, KASI, and PCS of the SF-36 questionnaires. Pauletti et al. demonstrated that 6MWT performed on postoperative day 5 after cardiac surgery can predict clinical outcomes and patient recovery [
42]. The 6MWT has been widely used in previous studies [
43] to evaluate aerobic capacity in patients undergoing cardiac surgery before and after initiation of aerobic exercise training, owing to its simplicity and the fact that it does not require specialized or expensive equipment. However, submaximal exercise testing offers additional advantages, as it can assess further physiological parameters such as oxygen consumption, HR, and MET. In addition, our findings are consistent with earlier reports demonstrating a moderate to high correlation between the physical function domain of SF-36 and 6MWT performance [
44]. These findings suggest that the estimated peak VO₂ can be used to assess patient’s physical function and quality of life. Importantly, the strong correlation between peak VO₂ obtained from recumbent ergometer-based submaximal CPET and other well-established functional and questionnaire-based assessments indicates that estimated peak VO₂ may be reliably used as a reference parameter for exercise prescription during the early postoperative phase.
Limitations
This study had several important limitations. First, the small sample size and high percentage of male participants may have reduced the statistical power of our findings. Second, the use of a recumbent ergometer rather than a treadmill may have resulted in an underestimation of peak oxygen consumption [
19]; however, this approach was deliberately chosen to minimize cardiovascular and musculoskeletal stress in patients during the early postoperative period following cardiac surgery.
Conclusion
The findings of this study indicate that CPET using a recumbent ergometer can be initiated safely and feasibly within two weeks after cardiac surgery. Early initiation of CPET does not increase the risk of adverse events, supporting its safety in the early postoperative period. Importantly, peak VO₂ values obtained during submaximal CPET were sufficiently reliable to guide exercise prescriptions. These findings support the use of early CPET to enable a more accurate, individualized exercise intensity prescription during phase I or early phase II cardiac rehabilitation, which may improve both cardiorespiratory fitness and functional outcomes in patients following cardiac surgery.
Author Contributions
Conceptualization, Bo Ryun Kim. and Yeon Mi Kim.; Methodology, Bo Ryun Kim.; Software, Bo Ryun Kim.; Validation, Yeon Mi Kim, Bo Ryun Kim and Sung Bom Pyun.; Formal Analysis, Yeon Mi Kim.; Investigation, Bo Ryun Kim. And Yeon Mi Kim.; Resources, Ho Sung Son., Jae Seung Jung, Hee Jung Kim; Data Curation, X.X.; Writing – Original Draft Preparation, Yeon Mi Kim; Writing – Review & Editing, Bo Ryun Kim. and YeonMi Kim; Visualization, Yeon Mi Kim.; Supervision, Ho Sung Son.; Project Administration, Sung Bom Pyun.; Funding Acquisition, Bo Ryun Kim.
Funding
This Study was Supported by a Grant from Republic of Korea University and the National Research Foundation of Republic of Korea (NRF) Grant Funded by the Republic of Korean \Government (MSIT) (No. RS-2024-00336696).
Institutional Review Board Statement
Trial Registration: This Study was Registered with the Clinical Research Information Service (CRIS) under Trial Registration Number KCT0006439 on August 13, 2021.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Abbreviations
| CVD |
Cardiovascular disease |
| CABG |
coronary artery bypass grafting |
| CR |
cardiac rehabilitation |
| HR |
heart rate |
| ECG |
electrocardiogram |
| CPET |
cardiopulmonary exercise test |
| CBCR |
center-based cardiac rehabilitation |
| HBCR |
home-based cardiac rehabilitation |
| 6MWT |
6-min walk test |
| EQ-5D |
EuroQol-5 dimension |
| SF-36 |
short-form 36-item health survey |
| PCS |
Physical component summary |
| MCS |
Mental component summary |
| KASI |
Korean Activity Scale/Index |
| MET |
metabolic equivalent of task |
| Wmax |
maximal work capacity |
| RPE |
rate of perceived exertion |
References
- Nam, S.W.; Song, I.A.; Oh, T.K. Trends in Cardiovascular Surgery in South Korea: A Nationwide Cohort Study from 2010 to 2019. J Cardiothorac Vasc Anesth 2023, 37, 1651–1658. [Google Scholar] [CrossRef]
- Kang, H.J.; Wang, J.; Cho, E.J.; Kim, H.L.; Lee, C.J.; Park, J.H.; Kang, J.; Suh, J.; Choi, K.H.; Lee, S.Y.; et al. Addressing Cardiovascular Diseases Challenges in South Korea: Strategies to Improve Outcomes. Korean Circ J 2025, 55, 557–583. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Fonarow, G.C.; Breathett, K.; Jurgens, C.Y.; Pisani, B.A.; Pozehl, B.J.; Spertus, J.A.; Taylor, K.G.; Thibodeau, J.T.; Yancy, C.W. 2020 ACC/AHA clinical performance and quality measures for adults with heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circulation: Cardiovascular Quality and Outcomes 2020, 13, e000099. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). European Heart Journal 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Kim, C.; Sung, J.; Lee, J.H.; Kim, W.S.; Lee, G.J.; Jee, S.; Jung, I.Y.; Rah, U.W.; Kim, B.O.; Choi, K.H.; et al. Clinical Practice Guideline for Cardiac Rehabilitation in Korea. Ann Rehabil Med 2019, 43, 355–443. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Marrouche, N.F.; Aguinaga, L.; Albert, C.M.; Arbelo, E.; Choi, J.-I.; Chung, M.K.; Conte, G.; Dagher, L.; Epstein, L.M. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic: Developed in partnership with and endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), the European Heart Rhythm Association (EHRA), the Heart Rhythm Society (HRS), and the Latin American Heart Rhythm Society (LAHRS). EP Europace 2021, 23, 313–313. [Google Scholar] [PubMed]
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.E.; Schmid, J.P.; Vigorito, C.; et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. European Journal of Preventive Cardiology 2021, 28, 460–495. [Google Scholar] [CrossRef] [PubMed]
- Price, K.J.; Gordon, B.A.; Bird, S.R.; Benson, A.C. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? European Journal of Preventive Cardiology 2016, 23, 1715–1733. [Google Scholar] [CrossRef]
- Nohara, R.; Adachi, H.; Goto, Y.; Hasegawa, E.; Ishihara, S.; Itoh, H.; Kimura, Y.; Maehara, K.; Makita, S.; Matsuo, H.; et al. Guidelines for Rehabilitation in Patients With Cardiovascular Disease (JCS 2012) - Digest Version. Circulation Journal 2014, 78, 2022–2093. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Beasley, J.W.; Duvernoy, W.F.C.; Mark, D.B.; McCallister, B.D.; Winters, W.L.; Ritchie, J.L.; Cheitlin, M.D.; Eagle, K.A.; Gardner, T.J.; et al. ACC/AHA guidelines for exercise testing: Executive summary - A report of the American College of Cardiology American Heart Association task force on practice guidelines (Committee on exercise testing). Circulation 1997, 96, 345–354. [Google Scholar] [CrossRef]
- Christopherson, D.J.; Shively, M.; Sivarajan, E.S. Low-level exercise testing before and after coronary artery bypass surgery. Int J Nurs Stud 1984, 21, 241–253. [Google Scholar] [CrossRef]
- Krone, R.J.; Gillespie, J.A.; Weld, F.M.; Miller, J.P.; Moss, A.J. Low-level exercise testing after myocardial infarction: usefulness in enhancing clinical risk stratification. Circulation 1985, 71, 80–89. [Google Scholar] [CrossRef]
- Ren, C.; Zhu, J.X.; Shen, T.; Song, Y.X.; Tao, L.Y.; Xu, S.L.; Zhao, W.; Gao, W. Comparison Between Treadmill and Bicycle Ergometer Exercises in Terms of Safety of Cardiopulmonary Exercise Testing in Patients With Coronary Heart Disease. Front Cardiovasc Med 2022, 9. [Google Scholar] [CrossRef]
- Le, J.N.; Zhou, R.; Tao, R.; Dharmavaram, N.; Dhingra, R.; Runo, J.; Forfia, P.; Raza, F. Recumbent Ergometer vs Treadmill Cardiopulmonary Exercise Test in HFpEF: Implications for Chronotropic Response and Exercise Capacity. J Card Fail 2023, 29, 407–413. [Google Scholar] [CrossRef]
- Sezai, A.; Shimokawa, T.; Kanaoka, K.; Fukuma, N.; Sekino, H.; Shiraishi, H.; Sumita, Y.; Nakai, M.; Iwanaga, Y.; Furukawa, Y.; et al. Efficacy of Early Cardiac Rehabilitation After Cardiac Surgery - Verification Using Japanese Diagnosis Procedure Combination Data. Circ Rep 2022, 4, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Pack, Q.R.; Dudycha, K.J.; Roschen, K.P.; Thomas, R.J.; Squires, R.W. Safety of Early Enrollment into Outpatient Cardiac Rehabilitation After Open Heart Surgery. American Journal of Cardiology 2015, 115, 548–552. [Google Scholar] [CrossRef]
- Nilsson, H.; Nylander, E.; Borg, S.; Tamás, É.; Hedman, K. Cardiopulmonary exercise testing for evaluation of a randomized exercise training intervention following aortic valve replacement. Clinical Physiology and Functional Imaging 2019, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cha, S.; Kang, S.; Han, K.; Paik, N.J.; Kim, W.S. High prevalence of physical inactivity after heart valve surgery and its association with long-term mortality: A nationwide cohort study. European Journal of Preventive Cardiology 2021, 28, 749–757. [Google Scholar] [CrossRef]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef]
- Lockwood, P.A.; Yoder, J.E.; Deuster, P.A. Comparison and cross-validation of cycle ergometry estimates of VO2max. Med Sci Sports Exerc 1997, 29, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Hand-grip dynamometry predicts future outcomes in aging adults. Journal of geriatric physical therapy 2008, 31, 3–10. [Google Scholar] [CrossRef]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife hand grip strength as a predictor of old age disability. Jama 1999, 281, 558–560. [Google Scholar] [CrossRef]
- Fu, L.Y.; Zhang, Y.Y.; Shao, B.H.; Liu, X.J.; Yuan, B.; Wang, Z.Q.; Chen, T.A.; Liu, Z.G.; Liu, X.C.; Guo, Q. Perioperative poor grip strength recovery is associated with 30-day complication rate after cardiac surgery discharge in middle-aged and older adults-a prospective observational study. Bmc Cardiovascular Disorders 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L. The six-minute walk test. Respiratory care 2003, 48, 783–785. [Google Scholar]
- Borg, G.A.V. Psychophysical Bases of Perceived Exertion. Medicine and Science in Sports and Exercise 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Sung, J.; On, Y.K.; Kim, H.S.; Chae, I.H.; Sohn, D.W.; Oh, B.H.; Lee, M.M.; Park, Y.B.; Choi, Y.S.; Lee, Y.W. Development of Korean activity scale/index (KASI). Korean Circulation Journal 2000, 30, 1004–1009. [Google Scholar] [CrossRef]
- Shaw, L.J.; Olson, M.B.; Kip, K.; Kelsey, S.F.; Johnson, B.D.; Mark, D.B.; Reis, S.E.; Mankad, S.; Rogers, W.J.; Pohost, G.M.; et al. The value of estimated functional capacity in estimating outcome: results from the NHBLI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol 2006, 47, S36–43. [Google Scholar] [CrossRef] [PubMed]
- Rabin, R.; Charro, F.d. EQ-SD: a measure of health status from the EuroQol Group. Annals of medicine 2001, 33, 337–343. [Google Scholar] [CrossRef]
- Kim, M.-H.; Cho, Y.-S.; Uhm, W.-S.; Kim, S.; Bae, S.-C. Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Quality of life research 2005, 14, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Nam, H. EQ-5D Korean valuation study using time trade of method. In Seoul: Korea Centers for Disease Control and Prevention; 2007. [Google Scholar]
- Ware, J.E. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- Han, C.W.; Lee, E.J.; Iwaya, T.; Kataoka, H.; Kohzuki, M. Development of the Korean version of short-form 36-item health survey: Health related QOL of healthy elderly people and elderly patients in Korea. Tohoku Journal of Experimental Medicine 2004, 203, 189–194. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: correlational analysis. Singapore Med J 2003, 44, 614–619. [Google Scholar] [PubMed]
- Hamm, L.F.; Crow, R.S.; Stull, G.A.; Hannan, P. Safety and characteristics of exercise testing early after acute myocardial infarction. Am J Cardiol 1989, 63, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.H.; Borer, J.S. Exercise testing early after myocardial infarction. Risks and benefits. Am J Med 1982, 72, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.P.; Bum, Yong; Kim, Duk You; Kim, Young Joo. The Safety of Early Exercise Stress Test after Coronary Intervention. Journal of Korean Academy of Rehabilitation Medicine 2010, 34, 6. [Google Scholar]
- Butman, S.M.; Olson, H.G.; Gardin, J.M.; Piters, K.M.; Hullett, M.; Butman, L.K. Submaximal exercise testing after stabilization of unstable angina pectoris. J Am Coll Cardiol 1984, 4, 667–673. [Google Scholar] [CrossRef]
- Haglund, E.K.; Bremander, A.B. Aerobic capacity in patients with rheumatoid arthritis: a comparison of two submaximal test methods. Musculoskeletal Care 2009, 7, 288–299. [Google Scholar] [CrossRef]
- Stringer, W.W. Cardiopulmonary exercise testing: current applications. Expert Rev Respir Med 2010, 4, 179–188. [Google Scholar] [CrossRef]
- Takeyama, J.; Itoh, H.; Kato, M.; Koike, A.; Aoki, K.; Fu, L.T.; Watanabe, H.; Nagayama, M.; Katagiri, T. Effects of physical training on the recovery of the autonomic nervous activity during exercise after coronary artery bypass grafting: effects of physical training after CABG. Jpn Circ J 2000, 64, 809–813. [Google Scholar] [CrossRef]
- Wehrle, A.; Waibel, S.; Gollhofer, A.; Roecker, K. Power Output and Efficiency During Supine, Recumbent, and Upright Cycle Ergometry. Front Sports Act Living 2021, 3, 667564. [Google Scholar] [CrossRef]
- Pauletti, H.O.; Gomes, W.J.; Rocco, I.S.; Viceconte, M.; Garcia, B.C.M.; Marcondi, N.O.; Bublitz, C.B.; Costa, A.D.S.; Paiva, T.P.; Spina, G.D.; et al. Early Six-Minute Walk Test May Predict Midterm Outcomes Following Coronary Artery Bypass Grafting. Braz J Cardiovasc Surg 2023, 38, e20220459. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Indraratna, P.; Tardo, D.T.; Peeceeyen, S.C.; Peoples, G.E. Safety and efficacy of aerobic exercise commenced early after cardiac surgery: A systematic review and meta-analysis. Eur J Prev Cardiol 2019, 26, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Alison, J.A.; Kenny, P.; King, M.T.; McKinley, S.; Aitken, L.M.; Leslie, G.D.; Elliott, D. Repeatability of the six-minute walk test and relation to physical function in survivors of a critical illness. Phys Ther 2012, 92, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).