Submitted:
17 January 2026
Posted:
19 January 2026
You are already at the latest version
Abstract
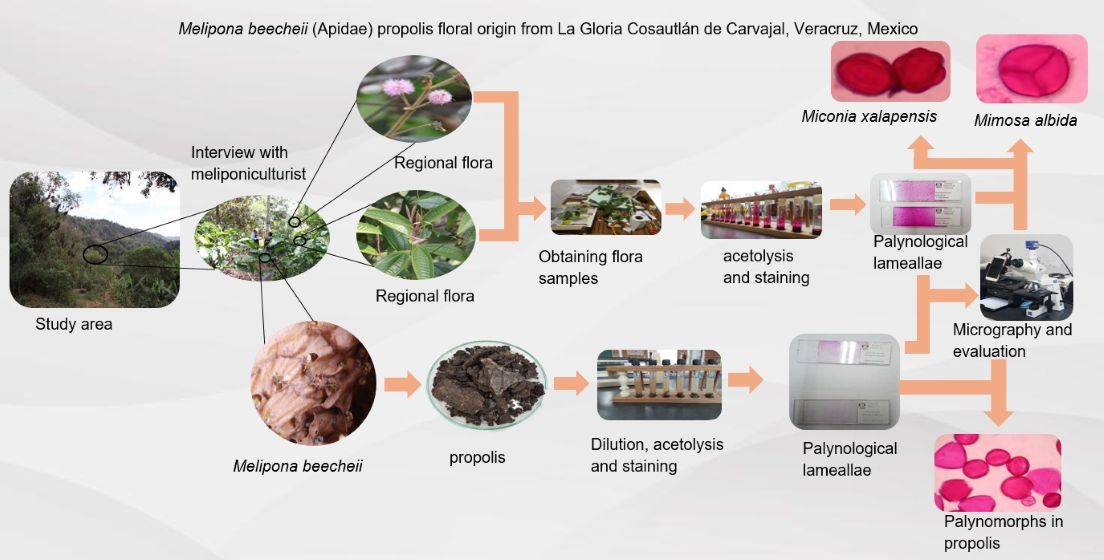
This study aimed to identify and characterize the Melipona beecheii propolis botanical origin from La Gloria, Cosautlán de Carvajal, Veracruz, Mexico. We compiled a floristic inventory of 75 foraging species, and their pollen and propolis from nests. The pollen was processed by acetolysis, and each species was analyzed by using optical microscopy resulting in diagnostic micrographs. We counted 1500 pollen grains from the processed propolis, where we identified 25 pollen types; Mimosa albida, Miconia xalapensis, Sambucus nigra, Coffea arabica, Struthantus aff. quercicola, Trichilia havanensis, and Pimenta dioica, among others. Based on our results we observed that M. beecheii preferred M. albida for propolis elaboration, even though also used other trees and shrubs with numerous inflorescences, small flowers, and pollen between 9 and 35 µm, which contain phytochemicals with significant biological activity. Finally, it is important to mention that M. beecheii is an excellent pollinator that contributes to the regeneration and conservation of biodiversity; its knowledge and management could be incorporated into meliponicultural practices as an ecosystem service that will help to human population conservation in its origin place.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
3. Results
3.1. Foraging Flora Determination
3.2. Propolis Floral Origin
3.3. Palynomorphs Role
3.4. Palynomorphs Size
3.5. Palynomorphs from the Main Propolis Species
3.6. Resource Availability
3.7. Characteristics of Mostly Foraged Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Challenger, A.; Soberón, J. Los ecosistemas terrestres. In Capital natural de México; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, 2008; Vol. 1, pp. 87–108.
- Ravelo, P. K.; Hernández M. F.R.; Paneque, T. I.; Toledo, L.E.; Gutiérrez, H. Relación de la población natural de abejas de la tierra (Melipona beecheii) con la flora en el valle San Andrés. Rev. Cuba. Cienc. For. CFORES 2014, 2, 3.
- Arnold, N.; Zepeda, R.; Márquez Dávila, M.; Aldasoro Maya, M. Las abejas sin aguijón y su cultivo en Oaxaca, México con catálogo de especies; 1; 1° ed.; El Colegio de la Frontera Sur: México, 2018; pp. 1-147.
- Bradbear, N. fao.org/4/y5110s/y5110s00.htm Available online: https://www.fao.org/4/y5110s/y5110s00.htm (accessed on 29 April 2025).
- Villalón, M.Y.; Leal-Ramos, A.; León, L.E. Acciones estratégicas para el fomento de Melipona beecheii en la polinización de agroecosistemas cafetaleros. Av. Cuba 2014, 16, 296–305.
- Baena-Díaz, F.; Chévez, E.; Porter-Bolland, L.; Baena-Díaz, F.; Chévez, E.; Porter-Bolland, L. ¿Qué sabemos de las abejas sin aguijón (Hymenoptera: Apidae, Meliponini) en México? Diversidad, Ecología y polinización. Acta Zool. Mex. 2023, 39. [CrossRef]
- Sakagami, S.F. 4 - Stingless Bees. In Social Insects; Hermann, H.R., Ed.; Academic Press, 1982; pp. 361–423.
- Michener, C. The Bees of the World; 1; 2da ed.; The Johns Hopkins University Press: United States os America, 2007; pp. 1-972.
- Kent, R.B. Mesoamerican Stingless Beekeeping. J. Cult. Geogr. 1984, 1, 14-28. [CrossRef]
- Crane, E. The Past and Present Status of Beekeeping with Stingless Bees. Bee World 1992, 73, 29–42. [CrossRef]
- Pimentel, E.G. Importancia de las abejas sin aguijón, en las familias del área rural de Guatemala. Rev. Cienc. Multidiscip. CUNORI 2017, 1, 119–120. [CrossRef]
- México, Un País de Abejas Available online: https://www.inecol.mx/index.php/divulgacion/ciencia-hoy/mexico-un-pais-de-abejas (accessed on 1 November 2025).
- Propóleos Available online: https://atlasnacionaldelasabejasmx.github.io/atlas/cap4.html (accessed on 23 May 2025).
- Sotelo Santos, L.E.; Alvarez Asomoza, C. The Maya Universe in a Pollen Pot: Native Stingless Bees in Pre-Columbian Maya Art. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S.R.M., Roubik, D.W., Eds.; Springer International Publishing: Cham, 2018; pp. 299–309.
- Schwarz, H.F.; Bacon, A.L. Stingless Bees (Meliponidae) of the Western Hemisphere: Lestrimelitta and the Following Subgenera of Trigona: Trigona, Paratrigona, Schwarziana, Parapartamona, Cephalotrigona, Oxytrigona, Scaura, and Mourella. Bulletin of the AMNH; v. 90. 1948.
- Jong, H. de The Land of Corn and Honey: The Keeping of Stingless Bees (Meliponiculture) in the Ethno-Ecological Environment of Yucatan (Mexico) and El Salvador. Univ. Utrecht 1999.
- Quezada-Euán, J.J.G.; Nates-Parra, G.; Maués, M.M.; Roubik, D.W.; Imperatriz-Fonseca, V.L. The Economic and Cultural Values of Stingless Bees (Hymenoptera: Meliponini) among Ethnic Groups of Tropical America. Sociobiology 2018, 65, 534–557. [CrossRef]
- Chapman, A.M.; Montemayor, F. Puertos de Intercambio En Mesoamérica Prehispánica. 1° ed.; Instituto Nacional de Antropología e Historia, México, 1959: pp. 1-71.
- Guaita Gavilanes, M.G.; Martinez Castillo, M.; Hernandez Zavala, A.; Guaita Gavilanes, M.G.; Martinez Castillo, M.; Hernandez Zavala, A. La miel de abejas sin aguijón: una medicina diferente. Epistem. Sonora 2023, 17, 49–59. [CrossRef]
- Quezada-Euán, J.J.G.; Paxton, R.J.; Palmer, K.A.; Itzá, W. de J.M.; Tay, W.T.; Oldroyd, B.P. Morphological and Molecular Characters Reveal Differentiation in a Neotropical Social Bee, Melipona Beecheii (Apidae: Meliponini). Apidologie 2007, 38, 247–258. [CrossRef]
- Roubik, D.W.; De Camargo, J.M.F. The Panama Microplate, Island Studies and Relictual Species of Melipona (Melikerria) (Hymenoptera: Apidae: Meliponini). Syst. Entomol. 2012, 37, 189–199. [CrossRef]
- Cane, J. Habitat Fragmentation and Native Bees: A Premature Verdict? Conserv. Ecol. 2001, 5, 1-10. [CrossRef]
- España, A.D.H.; Moran, O.A.P.; Kumul, R.G.C. Compuestos bioactivos presentes en geopropóleos de Melipona beecheii y su potencial uso en la medicina tradicional. Desde el Herbario CICY. 2023, 15, 33-37.
- Barth, O.M. Melissopalynology in Brazil: A Review of Pollen Analysis of Honeys, Propolis and Pollen Loads of Bees. Sci. Agric. 2004, 61, 342–350. [CrossRef]
- Propóleos. Available online: https://atlas-abejas.agricultura.gob.mx/cap4.html (accessed on 1 November 2025).
- Ghisalberti, E.L. Propolis: A Review. Bee World 1979, 60, 59–84. [CrossRef]
- Marcucci, M.C. Propolis: Chemical Composition, Biological Properties and Therapeutic Activity. Apidologie 1995, 26, 83–99. [CrossRef]
- Chuttong, B.; Lim, K.; Praphawilai, P.; Danmek, K.; Maitip, J.; Vit, P.; Wu, M.-C.; Ghosh, S.; Jung, C.; Burgett, M.; et al. Exploring the Functional Properties of Propolis, Geopropolis, and Cerumen, with a Special Emphasis on Their Antimicrobial Effects. Foods 2023, 12, 3909. [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid.-Based Complement. Altern. Med. ECAM 2013, 2013, 964149. [CrossRef]
- Petit, J.-L. Propóleo Para Cuidar Frutales. Fertil. Tierra Rev. Agric. Ecológica 2004, 15, 13–15.
- Roman, S.; Palos, E.; Mateescu, C. Treatment of Some Gynaecological Diseases with Apitherapeuti-Cal Products. Proc XXXII Int Congr Apic. Rio Jan. Braz. Apimondia Buchar. Rom. 1989, 215–216.
- Stojko, R.; Stojko, A. Uso de Preparados Apiterapéuticos En La Ginecología. Proc 33rd Int Congr Apic. Beijing China Apimondia Buchar. Rom. 1993, 142–143.
- Tushevskii, V.F.; Porokhnyak, L.A.; Tikhonov, A.I.; Budnikova, T.M. Morphological Aspects of the Hepatoprotective Effect of Propolis Tablets. 1991. 70-71.
- Campos, J.F.; Santos, H.F. dos; Bonamigo, T.; Domingues, N.L. de C.; Souza, K. de P.; Santos, E.L. dos Stingless Bee Propolis: New Insights for Anticancer Drugs. Oxid. Med. Cell. Longev. 2021, 2021, 2169017. [CrossRef]
- Pazin, W.M.; Mônaco, L. da M.; Soares, A.E.E.; Miguel, F.G.; Berretta, A.A.; Ito, A.S. Antioxidant Activities of Three Stingless Bee Propolis and Green Propolis Types. J. Apic. Res. 2017, 56 ,40-49. [CrossRef]
- Portal, A.S.; Cordova, C.M.M. de; Portal, A.S.; Cordova, C.M.M. de Propolis from Meliponinae: A Highway from Ancient Wisdom to the Modern Medicines. In Melittology - New Advances; IntechOpen, 2023.
- Rodríguez Pérez, B.; Canales Martínez, M.M.; Penieres Carrillo, J.G.; Cruz Sánchez, T.A. Composición química, propiedades antioxidantes y actividad antimicrobiana de propóleos mexicanos. Acta Univ. 2020, 30, 1-29. [CrossRef]
- Ruiz Ruiz, J.C.; Pacheco López, N.A.; Rejón Méndez, E.G.; Samos López, F.A.; Medina Medina, L.; Quezada-Euán, J.J.G. Phenolic Content and Bioactivity as Geographical Classifiers of Propolis from Stingless Bees in Southeastern Mexico. Foods 2023, 12, 1434. [CrossRef]
- Hernández Melgar, A.G. Caracterización de Los Compuestos Mayoritarios de Una Muestra de Geopropóleo, Producido Por Melipona Beecheii En Coatepec Veracruz. Licenciatura, Facultad de Química, México, UNAM, 2015.
- Ramón-Sierra, J.; Peraza-López, E.; Rodríguez-Borges, R.; Yam-Puc, A.; Madera-Santana, T.; Ortiz-Vázquez, E. Partial Characterization of Ethanolic Extract of Melipona Beecheii Propolis and in Vitro Evaluation of Its Antifungal Activity. Rev. Bras. Farmacogn. 2019, 29, 319–324. [CrossRef]
- Yam-Puc, A.; Santana-Hernández, A.A.; Yah-Nahuat, P.N.; Ramón-Sierra, J.M.; Cáceres-Farfán, M.R.; Borges-Argáez, R.L.; Ortiz-Vázquez, E. Pentacyclic Triterpenes and Other Constituents in Propolis Extract from Melipona Beecheii Collected in Yucatan, México. Rev. Bras. Farmacogn. 2019, 29, 358–363. [CrossRef]
- Farré, R.; Frasquet, I.; Sánchez, A. El própolis y la salud. Ars Pharm. Internet 2004, 45, 21–43.
- Vargas-Sánchez, R.D.; Peñalba-Garmendia, M.C.; Sánchez-Escalante, J.J.; Torrescano-Urrutia, G.R.; Sánchez-Escalante, A. Pollen Profile of Propolis Produced on the Eastern Edge of the Sonoran Desert in Central Sonora, Mexico. Acta Botánica Mex. 2016, 114, 69–85.
- Ramírez, E.; Pacheco-Palomo, K.; Moguel Ordóñez, Y.; Moreno, R.; Godínez-García, L. Angiosperm Resources for Stingless Bees (Apidae, Meliponini): A Pot-Pollen Melittopalynological Study in the Gulf of Mexico. In Pot-Pollen in Stingless Bee Melittology; 2018; pp. 111–130..
- Herrera-López, M.G.; Richomme, P.; Peña-Rodríguez, L.M.; Calvo-Irabien, L.M. Bee Species, Botanical Sources and the Chemical Composition of Propolis from Yucatan, Mexico. J. Chem. Ecol. 2023, 49, 408–417. [CrossRef]
- Pérez-Morfi, A.; Ramírez-Arriaga, E.; Canto, A. Comparative Analysis of Melipona Beecheii Pollen Foraging Preferences in Deciduous, Semi-Deciduous, and Semi-Evergreen Tropical Forests of the Yucatan Peninsula. Apidologie 2025, 56, 1–21. [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen, 5° ed.; Universidad Nacional Autónoma de México, México, 2004; pp. 1-97.
- INEGI MAPA DIGITAL DE MÉXICO 2014. http://gaia.inegi.org.mx/mdm6/?v=bGF0OjE5LjMxMzk0LGxvbjotOTYuODk0MTYsejo3LGw6YzM1MHxjdXN2Ng== (archived on 15 July 2024).
- Rzedowski, J. Análisis preliminar de la flora vascular de los bosques mesófilos de montaña de México. Acta Bot. Mex. 1996, 25–44.
- SEFIPLAN SISTEMA DE INFORMACIÓN MUNICIPAL. Available online: http://ceieg.veracruz.gob.mx/wp-content/uploads/sites/21/2016/05/Cosautl%C3%A1n-de-Carvajal.pdf (accessed on 29 April 2025).
- Bernard, H.R. Métodos de Investigación En Antropología. Entrevistas No Estructuradas Semiestructuradas, 2da ed.; Altamira PRESS, USA, 1995, 147–166.
- Izquierdo, G.M. Informantes y muestreo en investigación cualitativa. Investig. Andina 2015, 17, 1148–1150.
- Leff, E. Complejidad, racionalidad ambiental y diálogo de saberes. Congr. Int. Interdiscip. Particip. Animac. E Interv. Socioeducativa Barcelona, España, 2006. 1-12.
- González González, J.; Novelo Maldonado, E. Algas. In Manual de Herbario. Administración y manejo de colecciones, técnicas de recolección y preparación de ejemplares botánicos, 1° ed.; Lot & F. Chiang., Eds.; Universidad Nacional Autónoma de México, México, 1986: 47-54.
- Araujo-Mondragón, F.; Redonda-Martínez, R.; Araujo-Mondragón, F.; Redonda-Martínez, R. Flora melífera de la región centro-este del municipio de Pátzcuaro, Michoacán, México. Acta Botánica Mex. 2019. [CrossRef]
- May, T.; Rodríguez, S. Plantas de Interés Apícola En El Paisaje: Observaciones de Campo y La Percepción de Apicultores En República Dominicana. Rev. Geogr. Am. Cent. 2012, 1, 133-162.
- SAGARPA NORMA Oficial Mexicana NOM-003-SAG/GAN-2017, Propóleos, producción y especificaciones para su procesamiento. https://www.dof.gob.mx/normasOficiales/6794/sagarpa11_C/sagarpa11_C.html (archived 12 November 2024).
- Cornejo-Tenorio, G.; Manríquez, G.I.; Colín, S.S. Flora de Los Tuxtlas: Guía Ilustrada, 1° ed.; Universidad Nacional Autónoma de México, 2019; pp.1-500.
- EncicloVida Búsqueda por región Available online: https://enciclovida.mx/explora-por-region (accessed on 29 April 2025).
- Gómez-Pompa, A.; Krömer, T.; Castro-Cortés, R. Atlas de la flora de Veracruz: Un patrimonio natural en peligro. Universidad Veracruzana. Available online: https://libros.uv.mx/index.php/UV/catalog/book/FC135 (accessed on 29 April 2025).
- Villegas, D.G.; Bolaños, M.A.; Miranda, J.A.; Sandoval, H.R.; Lizama, M.J.M. Flora Nectarífera y Polinífera En El Estado de Veracruz. México, 2° ed.; SAGARPA, México, 2003; pp. 1-130.
- Plants of the World Online | Kew Science Available online: https://powo.science.kew.org/ (accessed on 31 October 2025).
- International Plant Names Index Available online: https://www.ipni.org/ (accessed on 31 October 2025).
- de Rivas, S. Polen y Esporas Introducción a La Palinología y Vocabulario Palinológico. 1°ed.; H. Blume, México, 1978; 1-219.
- Erdtman, G. Handbook of Palynology: Morphology, Taxonomy, Ecology. An Introduction to the Study of Pollen Grains and Spores; 1° ed.; København Munksgaard, 1969; pp. 1-486.
- González Gutierrez, K.; Mosquera Mosquerall, H. Estandarización de la acetolisis de Erdtman (1969) para el análisis palinológico de muestras fecales de murciélagos polinizadores (Phyllostomidae: Glossophaginae-Lonchophyllinae). Revista Tumbaga 2016, 1, 49-61.
- Sánchez, L. Métodos Palinológicos de Análisis. Doc. No Publ. Cent. Investig. Apícolas Trop. CINAT-UNA Heredia Costa Rica 2001.
- Soejarto, D.D.; Fonnegra, R. Polen: Diversidad en formas y tamaños. Actual. Biológicas 1972, 1, 2–13. [CrossRef]
- Muller, J. Form and Function in Angiosperm Pollen. Ann. Mo. Bot. Gard. 1979, 66, 593–632. [CrossRef]
- Moreno Patiño, J.E.; Roubik, D.W. Pollen and Spores of Barro Colorado Island.1° ed.; Missouri Botanical Garden, St. Louis, Missouri, USA, 1991; pp. 1-278.
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, S.; Le Thomas, A. Glossary of Pollen and Spore Terminology. Rev. Palaeobot. Palynol. 2007, 143, 1–81. [CrossRef]
- Barrantes-Vásquez, A.; Sánchez-Chaves, L.; Hernández-Sánchez, G.; Flores, W.M.-; Barrantes-Vásquez, A.; Sánchez-Chaves, L.; Hernández-Sánchez, G.; Flores, W.M.- Principales plantas de importancia alimenticia para la abeja nativa sin aguijón Trigona fulviventris (Guérin-Méneville) en Pocosol, Guanacaste, Costa Rica. Rev. For. Mesoam. Kurú 2019, 16, 13–23. [CrossRef]
- Sandoval, E. Técnicas Aplicadas al Estudio de La Anatomía Vegetal, 1° ed.; UNAM, México, 2005; pp. 1-273.
- Alfaro Bates, R.; González Acereto, J.A.; Ortiz Diaz, J.J.; Burgos Pérez, A.I.; Martínez Hernández, E.; Ramírez Arriaga, E. Caracterización palinológica de mieles de la península de Yucatán; 1° ed.; Coordinación General de Extensión, UNAM: Yucatán, México, 2010;1-144.
- Flores, R. Identificación de Granos de Polen de Algunas Familias Comunes En El Área Central de La República de Guatemala., Licenciatura. Universidad de San Carlos de Guatemala-USAC: Guatemala, 1989.
- Moriano Huillca, M.O. Palinología de la flora actual en el área de influencia del sitio arqueológico de Salapunku, Santuario Histórico de Machupicchu, Cusco. Licenciatura. Cusco, Perú, 2022.
- Palacios-Chávez, R.; Arreguín-Sánchez, M.D. la L.; Quiroz-García, D.L. Morfologia de los granos de polen de la subfamilia Caesalpinioideae (Leguminosae) del Valle de Mexico. POLIBOTÁNICA 1996, 1, 16–21.
- Ramos Díaz, A.; Noriega Trejo, R.; Sánchez Contreras, Á.; San Román Ávila, D.; Góngora Chin, R.; Rodríguez Buenfil, I. Catálogo de los principales tipos polínicos encontrados en las mieles producidas en la Península de Yucatán, 1° ed.; FOMIX: Yucatán, México, 2015; 1-114.
- Villanueva-Gutiérrez, R.; Roubik, D.W.; Colli-Ucán, W.; Tuz-Novelo, M. The Value of Plants for the Mayan Stingless Honey Bee Melipona Beecheii (Apidae: Meliponini): A Pollen-Based Study in the Yucatán Peninsula, Mexico. In Pot-Pollen in Stingless Bee Melittology; Vit, P., Pedro, S.R.M., Roubik, D.W., Eds.; Springer International Publishing: Cham, 2018; pp. 67–76.
- Fonte, L.; Milera, M.; Demedio, J.; Blanco, D. Selectividad de Pecoreo de La Abeja Sin Aguijón Melipona Beecheii Bennett En La EEPF “Indio Hatuey”, Matanzas. Pastos Forrajes 2012, 35, 333–342.
- Mendieta Izquierdo, G. Informantes y Muestreo En Investigación Cualitativa. Investig. Andina 2015, 17, 1148–1150.
- Catálogo de Árboles Para Las Abejas Available online: https://issuu.com/inanaac/docs/catalogo_inana_digital (accessed on 30 April 2025).
- Ramírez-Arriaga, E.; Navarro-Calvo, Lidia Amelia; and Díaz-Carbajal, E. Botanical Characterisation of Mexican Honeys from a Subtropical Region (Oaxaca) Based on Pollen Analysis. Grana 2011, 50, 40–54. [CrossRef]
- Engel, M.S.; Dingemans-Bakels, F. Nectar and pollen resources for stingless bees (Meliponinae, Hymenoptera) in Surinam (South America). Apidologie 1980, 11, 341–350. [CrossRef]
- Iwama, S.; Melhem, T.S. The pollen spectrum of the honey of Tetragonisca angustula angustula latreille (APIDAE, Meliponinae). Apidologie 1979, 10, 275–295. [CrossRef]
- Ramalho, M.; Kleinert-Giovannini, A.; Imperatriz-Fonseca, V.L. Utilization of floral resources by species of Melipona (Apidae, Meliponinae): floral preferences. Apidologie 1989, 20, 185–195. [CrossRef]
- Sommeijer, M.J.; Rooy, G. a. D.; Punt, W.; Bruijn, L.L.M.D. A comparative study of foraging behavior and pollen resources of various stingless bees (Hym., Meliponinae) and honeybees (HYM., apinae) in Trinidad, WEst-indies. Apidologie 1983, 14, 205–224. [CrossRef]
- Montoya-Pfeiffer, P.M.; León-Bonilla, D.; Nates-Parra, G. Catálogo de polen en mieles de Apis mellifera provenientes de zonas cafeteras en la Sierra Nevada de Santa Marta, Magdalena, Colombia. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2014, 38, 364–384.
- Padilla Vargas, P.J. Etnobotánica de las especies utilizadas por la abeja Scaptotrigona Mexicana en Cuetzalan del Progreso, Puebla, México. Master of Sciences, Santa Cruz Xoxocotlán, Oaxaca, México, 2015.
- Council for Agricultural Research and Economics (CREA) Available online: https://www.fao.org/mountain-partnership/members/detail/council-for-agricultural-research-and-economics-crea/en (accessed on 31 October 2025).
- Feuer, S.M.; Kuijt, J. Fine Structure of Mistletoe Pollen VI. Small-Flowered Neotropical Loranthaceae. Ann. Mo. Bot. Gard. 1985, 72, 187. [CrossRef]
- Sánchez-Dzib, Y. de los A.; Sosa-Nájera, S.; Lozano-García, M. del S. Morfología Polínica de Especies de la Selva Mediana Subperennifolia en la Cuenca del Río Candelaria, Campeche. Bol. Soc. Botánica México 2009, 83–104.
- Majeed, I.; Rizwan, K.; Ashar, A.; Rasheed, T.; Amarowicz, R.; Kausar, H.; Zia-Ul-Haq, M.; Marceanu, L.G. A Comprehensive Review of the Ethnotraditional Uses and Biological and Pharmacological Potential of the Genus Mimosa. Int. J. Mol. Sci. 2021, 22, 7463. [CrossRef]
- Quezada García, M.W.; Rivera Martinez, M. del P. Determinación de fitoconstituyentes de la raíz y hojas de mimosa albida procedentes de Conache – La Libertad. Licencicatura, Universidad Nacional de Trujillo, Trujillo, Perú, 2015.
- Quiñonez-Bastidas, G.N.; Navarrete, A. Mexican Plants and Derivates Compounds as Alternative for Inflammatory and Neuropathic Pain Treatment—A Review. Plants 2021, 10, 865. [CrossRef]
- Hernández-Dávila, O.A.; Laborde, J.; Sosa, V.J.; Díaz-Castelazo, C. Interaction Network between Frugivorous Birds and Zoochorous Plants in Cloud Forest Riparian Strips Immersed in Anthropic Landscapes. Avian Res. 2022, 13, 100046. [CrossRef]
- Pérez-Cadavid, A.; Rojas-Soto, O.R.; Bonilla-Moheno, M.; Pérez-Cadavid, A.; Rojas-Soto, O.R.; Bonilla-Moheno, M. Effect of Seed Ingestion by Birds on the Germination of Understorey Species in Cloud Forest. Rev. Mex. Biodivers. 2018, 89, 1167–1175. [CrossRef]
- Cunha, W.R.; Martins, C.; Ferreira, D. da S.; Crotti, A.E.M.; Lopes, N.P.; Albuquerque, S. In Vitro Trypanocidal Activity of Triterpenes from Miconia Species. Planta Med. 2003, 69, 470–472. [CrossRef]
- Gontijo, D.C.; Gontijo, P.C.; Brandão, G.C.; Diaz, M.A.N.; de Oliveira, A.B.; Fietto, L.G.; Leite, J.P.V. Antioxidant Study Indicative of Antibacterial and Antimutagenic Activities of an Ellagitannin-Rich Aqueous Extract from the Leaves of Miconia Latecrenata. J. Ethnopharmacol. 2019, 236, 114–123. [CrossRef]
- Jara, M.R.A.; Molero, H.R.L.; Landeo, E.C.; Segovia, J.C.P. Contenido de fenoles totales, flavonoides y capacidad antioxidante de tres especies endémicas del género Miconia del departamento de Ayacucho – 2019. Investigación 2020, 28, 245–251. [CrossRef]
- Spessoto, M.A.; Ferreira, D.S.; Crotti, A.E.M.; Silva, M.L.A.; Cunha, W.R. Evaluation of the Analgesic Activity of Extracts of Miconia Rubiginosa (Melastomataceae). Phytomedicine 2003, 10, 606–609. [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive Properties of Sambucus Nigra L. as a Functional Ingredient for Food and Pharmaceutical Industry. J. Funct. Foods 2018, 40, 377–390. [CrossRef]
- Liu, D.; He, X.-Q.; Wu, D.-T.; Li, H.-B.; Feng, Y.-B.; Zou, L.; Gan, R.-Y. Elderberry (Sambucus Nigra L.): Bioactive Compounds, Health Functions, and Applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced Research on the Antioxidant and Health Benefit of Elderberry (Sambucus Nigra) in Food – a Review. J. Funct. Foods 2015, 18, 941–958. [CrossRef]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-Influenza Activity of Elderberry (Sambucus Nigra). J. Funct. Foods 2019, 54, 353–360. [CrossRef]
- ALAsmari, K.M.; Zeid, I.M.A.; Al-Attar, A.M. Medicinal Properties of Arabica Coffee (Coffea Arabica) Oil: An Overview. Adv. Life Sci. 2020, 8, 20–29. [CrossRef]
- Babova, O.; Occhipinti, A.; Maffei, M.E. Chemical Partitioning and Antioxidant Capacity of Green Coffee (Coffea Arabica and Coffea Canephora) of Different Geographical Origin. Phytochemistry 2016, 123, 33–39. [CrossRef]
- Bisht, S.; Sisodia, S.S. Coffea Arabica: A Wonder Gift to Medical Science. J. Nat. Pharm. 2010, 1, 58-65. [CrossRef]
- Pérez-Hernández, L.M.; Chávez-Quiroz, K.; Medina-Juárez, L.Á.; Gámez Meza, N. Phenolic Characterization, Melanoidins, and Antioxidant Activity of Some Commercial Coffees from Coffea Arabica and Coffea Canephora. J. Mex. Chem. Soc. 2012, 56, 430–435.
- Alcántara-Quintana, L.E.; Arjona-Ruiz, C.; de Loera, D.; Gamboa-León, R.; Terán-Figueroa, Y. In Vitro Inhibitory and Proliferative Cellular Effects of Different Extracts of Struthanthus Quercicola: A Preliminary Study. Evid. Based Complement. Alternat. Med. 2022, 2022, 9679739. [CrossRef]
- Arjona-Ruiz, C.; Juarez-Flores, B.; Gamboa-León, R.; de Loera, D. Antidiabetic Activity and Hepatotoxic Effect of Aqueous Extracts of Struthanthus Quercicola. Rev. Bras. Farmacogn. 2022, 32, 472–477. [CrossRef]
- Nápoles, N.E.R.; Puentes, D.A.; Arias, Y.T. Relations between morphological-functional and ecological characteristics of Cuban native species of Meliaceae: strategy for forestry management. Acta Botánica Cuba. 2016, 215, 2-23.
- Biesmeijer, J.; Roberts, S.; Reemer, M.; Ohlemüller, R.; Edwards, M.; T, P.; Ap, S.; Sg, P.; R, K.; Cd, T.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351-354. [CrossRef]
- Ambi, A.; Bryan, J.; Borbon, K.; Centeno, D.; Liu, T.; Chen, T.P.; Cattabiani, T.; Traba, C. Are Russian Propolis Ethanol Extracts the Future for the Prevention of Medical and Biomedical Implant Contaminations? Phytomedicine 2017, 30, 50–58. [CrossRef]
- Rojczyk, E.; Klama-Baryła, A.; Łabuś, W.; Wilemska-Kucharzewska, K.; Kucharzewski, M. Historical and Modern Research on Propolis and Its Application in Wound Healing and Other Fields of Medicine and Contributions by Polish Studies. J. Ethnopharmacol. 2020, 262, 113159. [CrossRef]
- Ortiz Reyes, L.Y.; Quiroz-García, D.L.; Arreguín-Sánchez, M.L.; Fernández Nava, R. Origen botánico y caracterización fisicoquímica de la miel de meliponinos (Apidae:Meliponini) de Teocelo, Veracruz, México. Polibotánica 2022, 153–170. [CrossRef]
- Fischer, G.; Conceição, F.R.; Leite, F.P.L.; Dummer, L.A.; Vargas, G.D.; Hübner, S. de O.; Dellagostin, O.A.; Paulino, N.; Paulino, A.S.; Vidor, T. Immunomodulation Produced by a Green Propolis Extract on Humoral and Cellular Responses of Mice Immunized with SuHV-1. Vaccine 2007, 25, 1250–1256. [CrossRef]
- Sosa López, Á.A.; Cabrera, M.G.; Álvarez, M.Y. Vegetación de origen como parámetro de caracterización microbiana de los propóleos. J. Selva Andina Biosph 2016, 4, 3- 23.
- Bascompte, J.; Jordano, P. Plant-Animal Mutualistic Networks: The Architecture of Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 567–593. [CrossRef]
- Cortés, L.E.U.C.; Rivas, J.A.M. Conocimiento local de las abejas sin aguijón (APIDAE: meliponini) entre los mayas lacandones de Metzabok, Chiapas, México. ETNOBIOLOGÍA 2023, 21, 71–85.





| Botanical family | Scientific name | Popular name in Mexico | Percentage (%) | Way of life |
| ALTINGIACEAE | Liquidambar styraciflua L. | Liquidámbar | 0.30 % | Tree |
| ADOXACEAE | Sambucus nigra L. | Sauco | 3.20 % | Tree |
| CANNABACEAE | Trema micrantha (L.) Blume | No reports | 1.30 % | Tree |
| CARICACEAE | Carica papaya L. | Papaya | 0.30 % | Herbaceous |
| CLETHRACEAE | Clethra mexicana DC. | Marangola | 0.40 % | Tree |
| CUPRESSACEAE | Hesperocyparis lusitanica (Mill.) Bartel | Pino | 0.70 % | Tree |
| EUPHORBIACEAE | Ricinus communis L. | Higuerilla | 1.20 % | Bush |
| FABACEAE |
Diphysa americana (Mill.) M. Sousa Mimosa albida Humb. & Bonpl. ex Willd. |
Quibracho Dormilona grande |
0.30 % 60.20 % |
Tree Bush |
| HYPERICACEAE | Vismia baccifera (L.) Triana & Planch. | Chotillo | 0.30 % | Tree |
| LAMIACEAE | Salvia serotina L. | No reports | 0.50 % | Herbaceous |
| LAURACEAE | Persea schiedeana Nees | Chinine | 0.30 % | Tree |
| LORANTHACEAE | Struthanthus aff. quercicola | Correhuela | 2.40 % | Hemiparasite |
| MELASTOMATACEAE | Miconia xalapensis (Bonpl.) D. Don ex DC. | Tezhuate | 15.60 % | Bush |
| MALVACEAE | Heliocarpus appendiculatus Turcz | Jonote blanco | 1.90 % | Tree |
| MELIACEAE |
Cedrela odorata L. Trichilia havanensis Jacq. |
Cedro rojo Rama tinaja |
1.20 % 2.30 % |
Tree Bush |
| PRIMULACEAE | Ardisia compressa Kunth | Capulín de mayo | 0.20 % | Bush |
| MYRTACEAE |
Psidium guajava L. Pimenta dioica (L.) Merr. |
Guayaba Pimienta |
0.30 % 2.00 % |
Tree Tree |
| PINACEAE | Pinus spp. | Pino | 0.20 % | Tree |
| POACEAE | Zea mays L. | Maíz | 0.50 % | Herbaceous |
| RUBIACEAE |
Coffea arabica L. Hamelia patens Jacq. |
Café Costa Rica Pasmillo |
2.60 % 1.70 % |
Bush Bush |
| RUTACEAE | Citrus spp. | Cítricos | 0.10 % | Tree |
| Ene | Feb | Mzo | Abr | May | Jun | Jul | Ago | Sep | Oct | Nov | Dic | |
| Mimosa albida | ||||||||||||
| Miconia xalapensis | ||||||||||||
| Sambucus nigra | ||||||||||||
| Coffea arabica | ||||||||||||
| Struthantus aff. quercicola | ||||||||||||
| Trichilia havanensis | ||||||||||||
| Pimenta dioica |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.





