1. Introduction
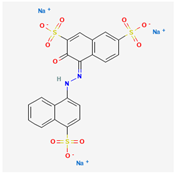
Amaranth (R2; C20H11N2Na3O10S3; E123, CAS no. 915-67-3) is a fully synthetic, water-soluble azo dye (IUPAC: trisodium; 3-hydroxy-4-[(4-sulfonatonaphthalen-1-yl)diazenyl]naphthalene-2,7-disulfonate) that is widely used as a food colorant and cosmetic ingrediants [
1,
2,
3]. R2, despite its name originating from the red-colored amaranth plant, is a fully synthetic dye. The physicochemical properties of R2 are presented in
Table 1. Chemically, it belongs to the class of anionic azo colorants, characterized by the presence of –N=N– linkages and fused aromatic rings (Scheme 1) [
4]. Although azo dyes themselves are not typically carcinogenic, the reductive cleavage of their azo bonds can release aromatic amines, several of which are recognized carcinogens. For this reason, azo-based colorants are often considered environmental contaminants with potential implications for both human health and ecological safety [
5,
6,
7,
8,
9]. Also, R2 is regulated as a cosmetic colorant in Korea. Under Korea MFDS standards, R2 is permitted solely as a cosmetic colorant, but it is not allowed in products specifically labeled for infants or for children ≤13 years [
10]. In the European Union, R2 is listed in Annex IV as an allowed colorant under Regulation (EC) No 1223/2009; however, its use as a hair-dye substance is prohibited (Annex II, entry 1350). Thus, within the EU it is managed as a cosmetic colorant—not as a hair dye ingredient [
11,
12].
The cosmetics market has shown steady growth; however, continuous concerns regarding the safety of cosmetics have highlighted the need for further investigation and research. Among cosmetic colorants, issues related to tar pigments have been particularly noteworthy. For example, R2 and New Coccine (R102, CAS no. 2611-82-7) have been prohibited in children’s foods to strengthen child safety protections, and their use has also been banned in oral hygiene products, such as mouthwashes and toothpastes, due to the potential for adverse health effects in children. Although no harmful effects have been directly demonstrated in humans, animal studies have raised concerns regarding carcinogenic potential and long-term exposure, leading to restrictions on the use of R102 in the United States since 1976 [
13]. In addition, Sunset Yellow FCF (CAS no. 1325-37-7) has been reported to induce renal tumors, urticaria, allergic reactions, and chromosomal damage. Reflecting these safety concerns, the European Food Safety Authority [
14] established an acceptable daily intake (ADI) of 4 mg/kg bw/day for this compound when used as a food additive [
15]. Despite such regulatory restrictions and toxicological findings for certain synthetic dyes, experimental evidence suggests that not all tar pigments pose the same level of risk. According to ECHA (2025), a rabbit skin-irritation study conducted in accordance with OECD Test Guideline 404 (n = 9 per group) using distilled water or an ointment as the vehicle found that topical R2 at 0.1% and 1% was non-irritating and produced no evidence of systemic toxicity [
11]. Consistently, ECHA (2025) reported that, in an OECD TG 439 assay using the EpiDerm™ reconstructed human epidermis, undiluted R2 (25 mg, 1 h) yielded a mean tissue viability of 101.7% relative to the DPBS negative control (30 μL), whereas the 5% SDS positive control reduced viability to approximately 4.5% of control; negative-control OD values ranged from 1.195 to 1.430 after 20–25 DPBS rinses [
12].
Thus, the safety of cosmetics is evaluated through risk assessment, which requires comprehensive information on the chemical structure, physicochemical properties, toxicological profiles, and patterns of human exposure to the ingredients [
16,
17,
18]. Exposure assessment is the process of quantitatively and/or qualitatively estimating the amount or level of a hazardous substance to which humans may be exposed through the use of cosmetics or other products. In this context, the systemic exposure dose (SED) is considered, and the determination of dermal absorption is a critical parameter in calculating SED values [
19,
20,
21]. Therefore, the evaluation of dermal absorption is essential for conducting a reliable risk assessment of cosmetic ingredients.
However, to date, no dermal absorption studies on R2 have been reported. Therefore, the present study aimed to evaluate its dermal absorption using an in vitro Franz diffusion cell system. The Franz diffusion cell is widely employed for dermal absorption and penetration studies, as it provides a controlled and standardized environment for assessing the permeation of drugs and cosmetic compounds through skin or artificial membranes, thereby generating reliable data for product development [
22,
23]. It is particularly valuable for formulation optimization, enabling direct comparisons of how different ingredients or vehicles influence permeation and absorption rates [
24,
25]. In addition, it facilitates comparative studies across multiple formulations or products, assisting researchers in identifying the most effective options. The versatility of this system allows adaptation to different skin types or conditions, making it suitable for evaluating a wide range of formulations, including creams, gels, ointments, and patches [
26,
27,
28]. Moreover, modern Franz diffusion systems support automation, which increases throughput, improves reproducibility, and reduces human error during sample collection and analysis [
29]. Accordingly, the objective of this study was to determine the dermal absorption of R2 as part of its risk assessment using the in vitro Franz diffusion cell system.
2. Materials and Methods
2.1. Chemicals
R2 was purchased from Sigma Aldrich Co. (St. Louis, MO, USA). Formic acid was obtained from Merck Millipore Co. (Kenilworth, NJ, USA). Phosphate buffered saline (PBS) was purchased from Sigma Aldrich (St. Louis, MO, USA). Distilled water (D.W) and acetonitrile (ACN) were obtained from Honeywell Burdick & Jackson Co. (St. Harvey, MI, USA).
2.2. Preparation of Test Formulations
Two different formulations of R2 were prepared and used as test preparations in this study skin lotion represents a typical oil-in-water (O/W) emulsion, while cream corresponds to a conventional water-in-oil (W/O) emulsion. The detailed compositions of each formulation are provided in
Table 2. Both formulations contained 1.0% R2.
2.3. HPLC Instruments and Conditions
High-performance liquid chromatography (HPLC) analysis of R2 was performed using a Shimadzu system (Japan) equipped with a CBM-20A system controller, LC-20AD isocratic pump, SIL-20A autosampler, SPD-20A UV detector (523 nm), and CTO-20A column oven. Chromatographic separation was achieved on a KR100-5C18 column (250 × 4.6 mm, 5 μm; Kromasil, Sweden) coupled with a Security Guard Cartridge RP-1 (4 × 3.0 mm; Phenomenex, CA, USA). The mobile phase consisted of 90% methanol containing 0.1 M ammonium acetate (pH 6.8) and 10% distilled water, delivered at a flow rate of 1.0 mL/min. The column oven was maintained at 40 °C, and the injection volume for all samples was 20 μL.
2.4. Stability Test
For the stability evaluation, R2 was prepared at a concentration of 10 μg/mL by diluting 10 mg of the compound in PBS. The solution was vortexed thoroughly and aliquoted into Eppendorf tubes (50 μL, n = 3). Each sample was labeled according to the designated incubation times (0, 6, 12, 24, and 48 h) and placed in a water bath (Shaking Water Bath, JS Research, Gong-Ju, Korea) maintained at 32 °C and 60 rpm. At the respective time points, samples were retrieved, followed by the addition of 200 μL of methanol (MeOH). After vortexing, the mixtures were filtered and subsequently subjected to analysis.
2.5. Analytical Method Validation
2.5.1. Calibration Standards and Quality Control (QC) Samples: Accuracy and Precision
Matrix-matched calibration standards were prepared separately for each matrix. For SKIN, calibration levels were 0.1, 0.5, 1, 3, 5, and 10 μg/mL, prepared by spiking 5 μL of working standard into 95 μL of SKIN blank (final 100 μL). For WASH, stratum corneum (SC), and receptor fluid (RF), calibration levels were 0.2, 0.5, 1, 3, 5, and 10 μg/mL, prepared identically (5 μL standard + 95 μL of the corresponding matrix blank). Calibration curves were constructed for each matrix. Each calibration solution was subsequently diluted 10-fold, yielding final concentrations of 0.01 or 0.02, 0.05, 0.1, 0.3, 0.5, and 1 μg/mL. Quality control (QC) samples were prepared at four concentration levels: the lower limit of quantification (LLOQ, 0.1 or 0.2 μg/mL), low QC (LOQ, 0.6 μg/mL), medium QC (MOQ, 4 μg/mL), and high QC (HOQ, 8 μg/mL). All prepared solutions were filtered prior to analysis, and stock solutions, calibration standards, and QC samples were stored at −20 °C until use. Method validation for R2 was evaluated based on linearity, sensitivity, selectivity, accuracy, and precision, using calibration curves and QC samples. Calibration curves were constructed by weighted (1/x) linear regression of the peak area ratios across the concentration range. Intra-day accuracy and precision were determined by analyzing triplicate samples in each matrix at 0.2, 0.6, 4, and 8 μg/mL within a single day. The accuracy and precision were calculated using the following formula:
Inter-day accuracy and precision were assessed in the same manner over three consecutive days, using the same concentration levels. Accuracy and precision should be within 20% of the LLOQ concentration and within 15% of the LOQ, MOQ and HOQ according to the Ministry of Food and Drug Safety’s bio-sampling method verification guidelines [
30].
2.6. Preparation of Rat Skin
Sprague-Dawley (SD) rats (male, 8 weeks, 228 ± 7 g) were supplied by Samtako Co. (Osan, Korea). For the in vitro study, 8-week-old rats were sacrificed using CO2 to obtain full thickness skin samples, which were stored at −20 °C. The dorsal hair of the rats was shaved using electronic hair removers (SM-129, JOAS, Namyangju, Korea) for the 7 × 7 cm2 dermal application.
2.7. Franz Diffusion Cell and Sample Collection
This study followed the Korea Ministry of Food and Drug Safety (MFDS) guideline and OECD Guideline 428 for in vitro skin absorption method [
31,
32]. The Franz diffusion cell system was used for the dermal absorption test. The Franz diffusion cell system was composed of a Vision microette auto-sampler, circulating waterbath, stirring drive, stirring control, and autofill (Hanson, Chatsworth, CA, USA). The prepared skin was thawed at room temperature and hydrated in saline for 5 minutes. The skin was fixed between the donor and receptor chamber, and checked to ensure there were no air bubbles between the receptor fluid (PBS) and the skin. Pre-weighed formulations were applied to the dosing area. Each formulation of 200 mg (113 mg/cm
2, 3.39 mg/cm
2 of R2) was applied to 1.77 cm
2 of the donor chambers. Sampling times were set at 0, 1, 2, 4, 8, 12, and 24 hours following dose application. After 24 hours of dosing, the remaining formulation was collected using alcohol swabs (WAHS, BDTM Alcohol Swabs, Becton Dickinson Corp, NJ, USA), to measure unabsorbed R2. The tape stripping method was used to remove the remaining formulation from the stratum corneum. The stratum corneum was removed by applying 2 × 2 cm pieces of tape (Scotch™, 3M, Maplewood, MN, USA) sequentially 15 times. The SKIN process involves cutting the skin (epidermis and dermis) into 8 pieces using surgical scissors and placing it in solvent to be extracted. The WASH, SC, and SKIN used in each step were put in 20 mL PBS, sonicated for an hour, and stored at –20 °C for 24 hours before analysis.
2.8. Statistical Analysis
All data are presented as the mean ± standard deviation (SD). Statistical calculations were performed using Excel 2019 (Microsoft for Windows) and GraphPad Prism software version 5.04 (San Diego, California, USA).
3. Results
3.1. Stability Test
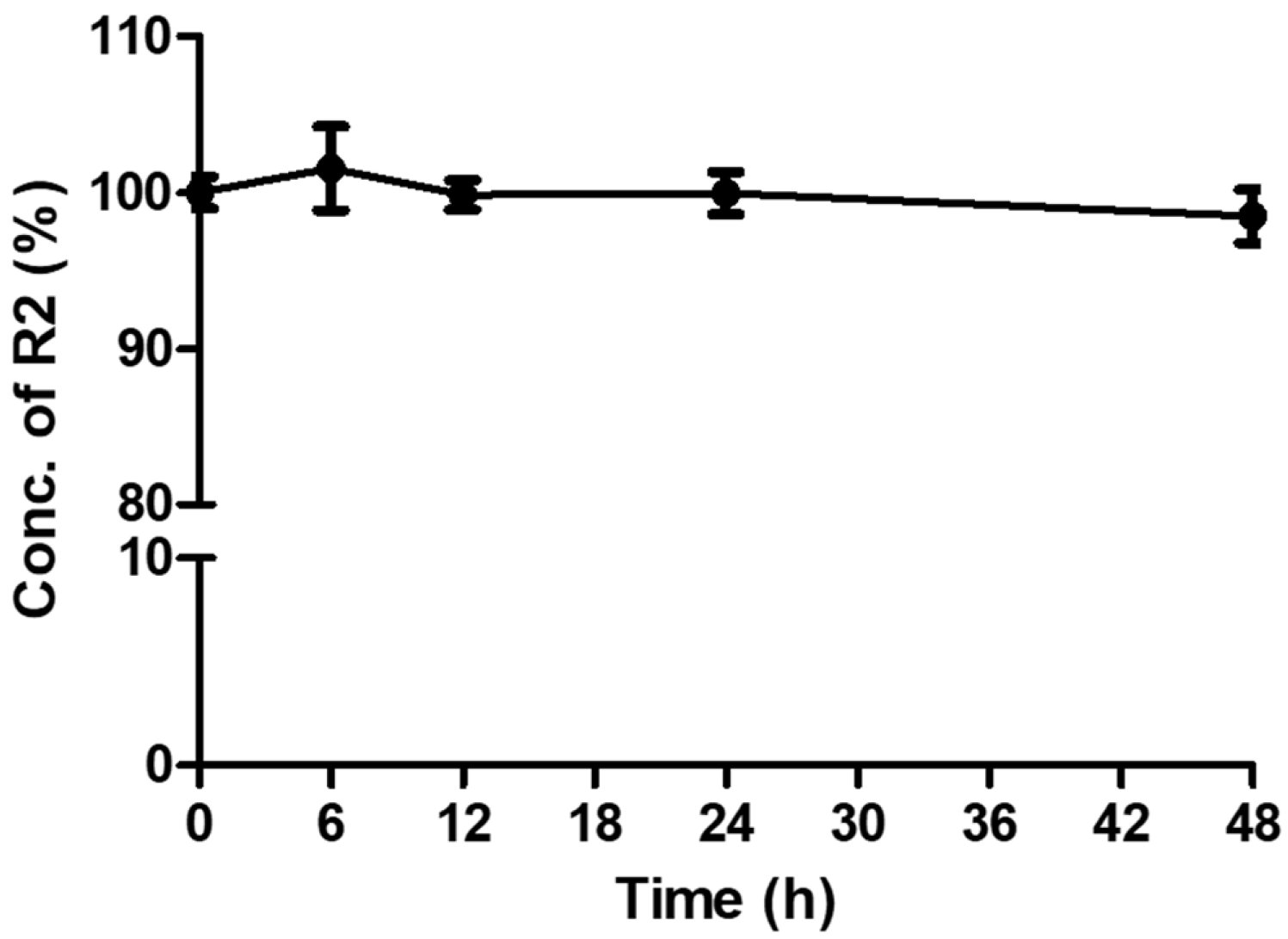
Stability testing verified that sink conditions for R2 were achieved under the planned skin permeation test conditions. No time-dependent change in concentration was detected at 0, 6, 12, 24, or 48 h, and R2 remained stable for 48 h in phosphate-buffered saline (PBS) under sink conditions (
Figure 1). Accordingly, R2 was selected for the in vitro skin permeation experiment (n = 3).
3.2. Analytical Method Validation of R2
Calibration curves were constructed using seven concentration levels in accordance with the Korea Ministry of Food and Drug Safety (2013) guideline for bioanalytical method validation. The retention time of R2 was 2.01 minutes, and no disturbing peaks were observed that interfered with the analysis. Following the pretreatment described above, HPLC analysis of R2-spiked samples yielded the calibration curve shown, which is representative, displaying excellent linearity (r
2 > 0.9996). Matrix-matched calibration curves showed good linearity over 0.1–10 μg/mL for SKIN and 0.2–10 μg/mL for WASH, SC, and RF. Intra- and inter-day accuracy and precision were evaluated to determine the reliability of the current analytical method. Intra- and inter-day accuracy and precision were assessed at four QC levels (0.2, 0.6, 4.0, and 8.0 μg/mL of R2) (
Table 3). All results complied with MFDS (2013) bioanalytical validation criteria—accuracy within ± 20% and CV ≤ 20% at the LLOQ (0.1 μg/mL for WASH/SC/RF; 0.2 μg/mL for SKIN) and within ± 15% / ≤ 15% at the other levels. Matrix-matched performance was: WASH, accuracy 95.5–99.8% with CV 0.6–5.5%; SC, 98.0–106.9% with CV 0.4–5.8%; SKIN, 100.5–104.4% with CV 0.5–3.9%; and RF, 99.4–102.9% with CV 0.3–5.8%. Collectively, these data confirm the reliability of the method for quantifying R2 [
30].
3.3. In Vitro Dermal Absorption of R2
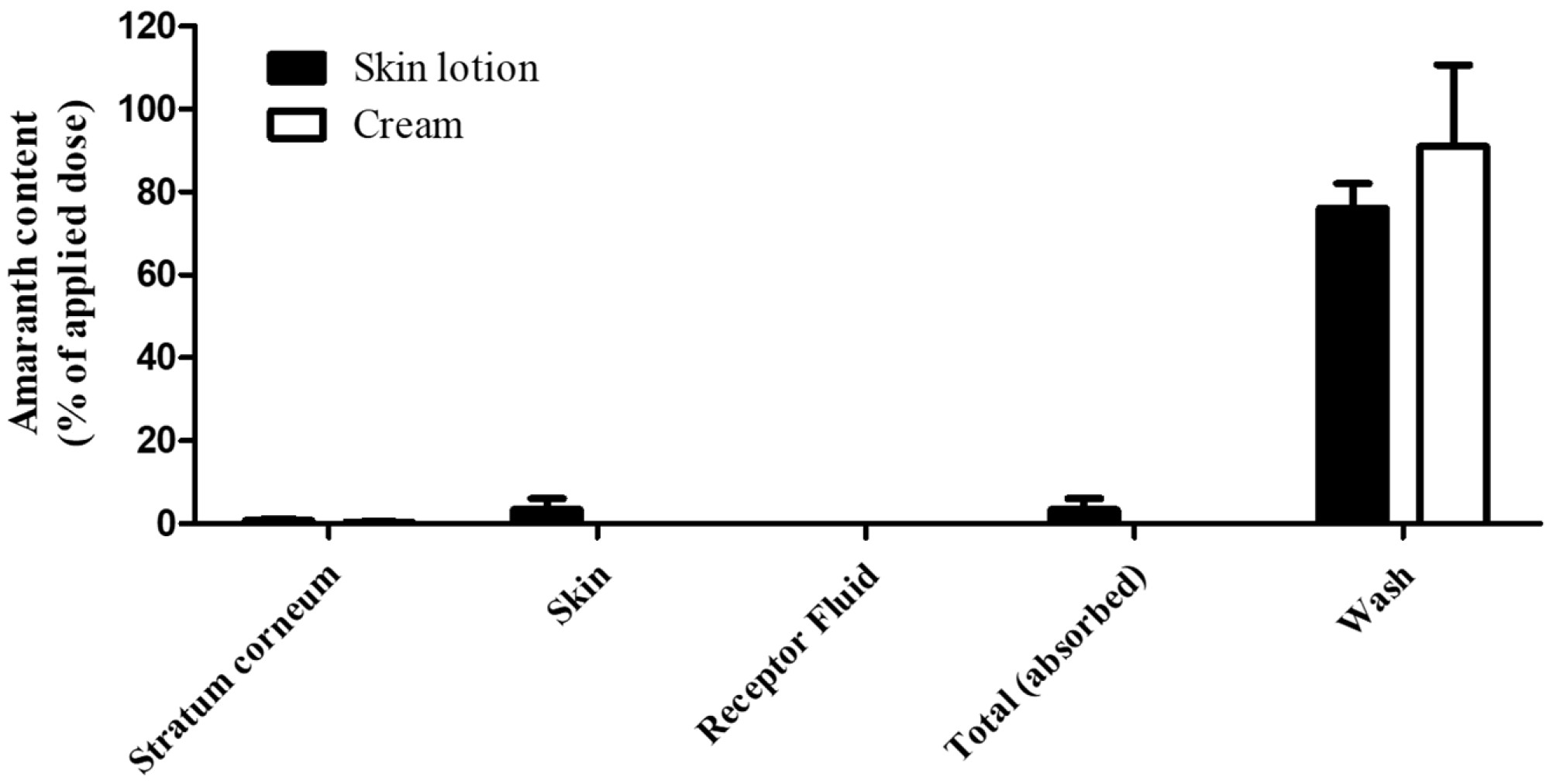
After completion of the permeation study, residual R2 in each compartment was quantified. In the skin lotion formulation, recoveries were 76.1 ± 5.9% (WASH), 0.8 ± 0.3% (SC), 3.4 ± 2.7% (SKIN), and 0 ± 0% (RF), yielding a total recovery of 80.3 ± 8.2%. In the cream formulation, the corresponding values were 91.0 ± 19.6% (WASH), 0.3 ± 0.2% (SC), 0 ± 0% (SKIN), and 0 ± 0% (RF), with a total recovery of 91.4 ± 19.4% (
Table 4). The amounts reaching the receptor fluid (RF) and the calculated permeation rates for each R2--containing formulation are summarized in
Figure 2. The total dermal absorption of R2 was 3.4 ± 2.7% (34.5 ± 27.0 μg/cm
2) for skin lotion and 0 ± 0% (0 μg /cm
2) for cream (
Figure 2).
4. Discussion
The skin absorption rate is a critical parameter in cosmetic risk assessment, as it directly determines the extent to which a cosmetic ingredient enters the body through the skin, thereby influencing systemic toxicity and the overall safety profile of the product. First, the skin absorption rate is essential for estimating the systemic exposure dosage (SED), which serves as the foundation for cosmetic safety evaluations. Accurate SED values indicate how much of an ingredient may reach systemic circulation and target organs under realistic usage conditions [
24]. Second, this parameter plays a pivotal role in establishing the margin of safety (MoS), a core concept in regulatory toxicology. By quantifying the absorbed fraction, regulators and researchers can confirm that human exposure remains well below levels associated with adverse effects. Finally, data derived from skin absorption studies are directly applied to MoS calculations for individual cosmetic ingredients, ensuring compliance with regulatory requirements and minimizing potential health risks to consumers. Collectively, these considerations underscore the indispensable role of skin absorption data in risk assessment frameworks for cosmetics.
Rat skin is commonly employed in percutaneous absorption studies owing to practical advantages such as ease of availability, small size, and low cost. However, it exhibits markedly higher permeability compared to human skin, often by an order of magnitude or more. For example, mean in vitro permeation flux through rat skin has been reported to be approximately elevenfold greater than that of human skin across diverse physicochemical classes of compounds, and for certain lipophilic molecules, this difference can reach up to 50-fold [
33,
34]. One of the principal reasons for this discrepancy is the thinner stratum corneum in rats, which provides a weaker barrier relative to human skin [
35,
36]. Surface properties also differ, as rat skin tends to be hairier than human skin, although hairless strains are sometimes utilized to better approximate human permeation characteristics [
34]. Given these differences, rat skin models are generally regarded as providing a “worst-case scenario” for human risk assessment. Consequently, dermal absorption data generated in rats require careful interpretation, and appropriate adjustment factors or safety margins should be applied when extrapolating to human exposure estimates [
33,
34,
36].
In this study, rat skin was used instead of human skin in order to obtain dermal absorption data under more stringent conditions, thereby providing a conservative estimate of safety. As noted above, R2 has been prohibited in children’s confectionery and in oral formulations such as mouthwashes and oral wipes due to safety concerns [
13]. In the United States, its use has been banned since 1976, and it is also prohibited as a food additive to strengthen consumer safety management [
37].
This study demonstrated the dermal absorption characteristics of R2 using franz diffusion cells under two representative cosmetic formulations (skin lotion and cream). Currently, no clear regulatory limits on the permissible concentration of R2 in cosmetics are established in Korea or the European Union. In particular, the European SCCS has prohibited its use as a hair dye ingredient, and therefore no reference concentration can be directly cited. For this reason, we selected two representative leave-on formulations that are considered to reflect realistic consumer exposure scenarios, and a test concentration of 1% was applied. This level was chosen based on the general range of incorporation for tar dyes in cosmetic formulations, representing a conservative approach for safety assessment [
38]. The results of the in vitro dermal absorption studies revealed distinct distribution patterns depending on the formulation. In the skin lotion, the majority of R2 remained on the skin surface and was removed by washing (76.1 ± 5.9%), while only a small fraction was retained in the stratum corneum (0.8 ± 0.3%) and viable skin layers (3.4 ± 2.7%). No R2 was detected in the receptor fluid. In the cream formulation, 91.0 ± 19.6% of the applied dose was removed by washing and 0.3 ± 0.2% was retained in the stratum corneum, whereas neither the viable skin layers nor receptor fluid showed detectable levels. Accordingly, the final dermal absorption rates were determined to be 3.4 ± 2.7% for the skin lotion and 0% for the cream, with total recoveries of 80.3 ± 8.2% and 91.4 ± 19.4%, respectively. These findings indicate that the vast majority of the applied dose remains on the skin surface and does not penetrate into systemic circulation. The low dermal absorption of R2 can be further explained by its physicochemical properties. According to SCCS guidance (2023), compounds with a molecular weight above 500 Da, log P ≤ –1 or ≥ 4, melting point > 200 °C, and topological polar surface area (TPSA) > 120 Ų are expected to exhibit limited skin penetration [
39]. R2 possesses all these characteristics, with a molecular weight of 604.5 Da, log P of –5.13, melting point > 300 °C, and TPSA of 242 Ų. These properties are consistent with the observed minimal absorption in our experiments, supporting the conclusion that systemic exposure to R2 via dermal routes is negligible under realistic cosmetic use conditions.
Taken together, the present findings highlight that dermal absorption of R2 is minimal under cosmetic use conditions, consistent with predictions based on its physicochemical profile. Although the use of R2 as a cosmetic colorant remains restricted in several jurisdictions, the conservative test design and low absorption rates observed here provide valuable data for risk assessment.
5. Conclusions
In this study, the dermal absorption of Amaranth (R2), a synthetic azo dye used as a cosmetic colorant, was evaluated using an in vitro Franz diffusion cell system with rat skin under two representative formulations (skin lotion and cream) at a concentration of 1%. A validated HPLC method ensured reliable quantification, and the results indicated minimal absorption. In the lotion formulation, most of the applied dose was removed by washing, with only 3.4 ± 2.7% (34.5 ± 27.0 μg/cm2) detected in skin and none in the receptor fluid. In the cream formulation, absorption was negligible (0%, 0 μg /cm2), with nearly all of the substance remaining on the skin surface. These findings are consistent with the physicochemical properties of R2 (MW = 604.5 Da, log P = –5.13, melting point > 300 °C, TPSA = 242 Ų), which predict poor skin penetration. Collectively, this study provides the first validated dermal absorption data for R2 and offers valuable information for future safety assessments and regulatory considerations.
Author Contributions
Data collection, formal analysis, and writing—original draft preparation, J.D.L.; data curation and formal analysis, H.Y.K.; writing—review and editing, G.-W.H. and K.-B.K.; supervision, K.-B.K.; project administration, K.-B.K.; funding acquisition, K.-B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants (24202MFDS268 and 25202MFDS002) from the Ministry of Food and Drug Safety, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article material.
Acknowledgments
The authors would like to express their sincere appreciation to the laboratory staff at the Center for Human Risk Assessment, Dankook University, for their assistance with data processing throughout the study. We are also grateful to Gi-Wook Hwang for his valuable support in manuscript writing, as well as for his critical review and editorial assistance. Special thanks are extended to Kyu-Bong Kim for his comprehensive oversight of the research, including final review and editing of the manuscript, overall project management, and securing the funding that enabled this work.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Rovina, K.; Siddiquee, S.; Shaarani, S.M. Toxicology, extraction and analytical methods for determination of amaranth in food and beverage products. Trends Food Sci Technol 2017, 65, 68–79. [Google Scholar] [CrossRef]
- Shabani, A.M.; Dadfarnia, S.; Dehghani, Z. On-line solid phase extraction system using 1,10-phenanthroline immobilized on surfactant-coated alumina for the flame atomic absorption spectrometric determination of copper and cadmium. Talanta 2009, 79, 1066–1070. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Y.; Yang, X.; Zhao, J. Sensitive determination of amaranth in drinks by highly dispersed CNT in graphene oxide “water” with the aid of small amounts of ionic liquid. Food Chem. 2015, 179, 318–324. [Google Scholar] [CrossRef]
- Fajardo, A.S.; Martins, R.C.; Martinez-Huitle, C.A.; Quinta-Ferreira, R.M. Treatment of amaranth dye in aqueous solution using one cell or two cells in series with active and non-active anodes. Electrochim. Acta 2016, 210, 96–104. [Google Scholar] [CrossRef]
- Brown, M.A.; De Vito, S.C. Predicting azo dye toxicity. Crit. Rev. Environ. Sci. Technol. 1993, 23, 249–324. [Google Scholar] [CrossRef]
- Chung, K.-T.; Cerniglia, C.E. Mutagenicity of azo dyes: Structure–activity relationships. Mutat. Res. Rev. Genet. Toxicol. 1992, 277, 201–220. [Google Scholar] [CrossRef]
- Lopez-de-Alba, P.; Lopez-Martínez, L.; Cerdà, V.; De Leon, L. Simultaneous determination of tartrazine, sunset yellow and allura red in commercial soft drinks by multivariate spectral analysis. Quim. Anal. 2001, 20, 63–72. [Google Scholar]
- Ghanbari, K.; Roushani, M.; Farzadfar, F.; Goicoechea, H.C.; Jalalvand, A.R. Developing a four-dimensional voltammetry as a powerful electroanalytical methodology for simultaneous determination of three colorants in the presence of an uncalibrated interference. Chemom. Intell. Lab. Syst. 2019, 189, 27–38. [Google Scholar] [CrossRef]
- Jian, J.; Li-Ping, G. Sensitive voltammetric sensor for amaranth based on ordered mesoporous carbon. Chin. J. Anal. Chem. 2013, 41, 681–686. [Google Scholar]
- MFDS. Regulation on the Review of Functional Cosmetics. Notice No. 2023-61, 2025. Available online: https://www.mfds.go.kr/brd/m_211/view.do?seq=14791 (accessed on 22 August 2025).
- EC (European Commission). CosIng: Annex II—List of substances prohibited in cosmetic products, 2025. Available online: https://ec.europa.eu/growth/tools-databases/cosing/reference/annexes/list/II (accessed on 22 August 2025).
- EC (European Commission). CosIng: Annex IV—List of colorants permitted in cosmetic products, 2025. Available online: https://ec.europa.eu/growth/tools-databases/cosing/reference/annexes/list/IV (accessed on 22 August 2025).
- MFDS. Administrative notice of the draft amendment to the types, standards, and testing methods of pigments in cosmetics. Notice No. 2015-373, 2015. Available online: https://www.mfds.go.kr/brd/m_209/view.do?seq=29425 (accessed on 22 August 2025).
- EFSA. Reconsideration of the temporary ADI and refined exposure assessment for Sunset Yellow FCF (E 110). EFSA J. 2014, 12, 3765. [Google Scholar] [CrossRef]
- Amchova, P.; Siska, F.; Ruda-Kucerova, J. Food safety and health concerns of synthetic food colors: An update. Toxics 2024, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, K.B.; Lee, J.Y.; Kwack, S.J.; Kwon, Y.C.; Kang, J.S.; et al. Risk assessment of 5-chloro-2-methylisothiazol-3(2H)-one/2-methylisothiazol-3(2H)-one (CMIT/MIT) used as a preservative in cosmetics. Toxicol. Res. 2019, 35, 103–117. [Google Scholar] [CrossRef]
- Lee, J.D.; Lee, J.Y.; Kwack, S.J.; Shin, C.Y.; Jang, H.J.; Kim, H.Y.; et al. Risk assessment of triclosan, a cosmetic preservative. Toxicol. Res. 2019, 35, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Lim, D.; Choi, H.; Choi, S.; Choi, S.; Hong, J.; et al. Risk assessment of cyclohexasiloxane D6 in cosmetic products. Toxicol. Res. 2024, 40, 421–429. [Google Scholar] [CrossRef]
- Sung, C.R.; Kim, K.B.; Lee, J.Y.; Lee, B.M.; Kwack, S.J. Risk assessment of ethylhexyl dimethyl PABA in cosmetics. Toxicol. Res. 2019, 35, 131–136. [Google Scholar] [CrossRef]
- Kim, D.; Seok, J.K.; Kim, M.; Choi, S.; Hong, J.; Yoon, Y.A.; et al. Safety assessment of cocamidopropyl betaine, a cosmetic ingredient. Toxicol. Res. 2024, 40, 361–375. [Google Scholar] [CrossRef]
- MFDS. Cosmetics Risk Assessment Guidelines. 2023. Available online: https://www.mfds.go.kr/brd/m_1060/view.do?seq=15405 (accessed on 22 August 2025).
- Kim, Y.J.; Kim, H.Y.; Lee, J.D.; Kim, H.Y.; Im, J.E.; Kim, K.B. Analytical method development and dermal absorption of pyrogallol, a hair dye ingredient. Toxics 2022, 10, 570. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.Y.; Lee, J.D.; Kim, H.Y.; Im, J.E.; Kim, K.B. Analytical method development and dermal absorption of gallic acid, a hair dye ingredient. Toxicol. Res. 2024, 40, 449–456. [Google Scholar] [CrossRef]
- Im, J.E.; Kim, H.Y.; Lee, J.D.; Park, J.J.; Kang, K.S.; Kim, K.B. Effect of application amounts on in vitro dermal absorption test using caffeine and testosterone. Pharmaceutics 2021, 13, 641. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, A.; Mahmood, S.; Thakur, A.; Mirza, M.A.; Bhatia, A. Franz diffusion cell and its implication in skin permeation studies. J. Dispersion Sci. Technol. 2024, 45, 943–956. [Google Scholar] [CrossRef]
- Lee, J.D.; Kim, J.Y.; Jang, H.J.; Lee, B.M.; Kim, K.B. Percutaneous permeability of 1-phenoxy-2-propanol, a preservative in cosmetics. Regul. Toxicol. Pharmacol. 2019, 103, 56–62. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.Y.; Lee, J.D.; Kim, H.Y.; Im, J.E.; Kim, K.B. Analytical method development and dermal absorption of 2-amino-5-nitrophenol (2A5NP), a hair dye ingredient under oxidative condition. Toxicol. Res. 2022, 39, 231–238. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, Y.J.; Lee, J.D.; Kim, H.R.; Seo, D.W. Analytical method development and dermal absorption of 4-amino-3-nitrophenol (4A3NP), a hair dye ingredient under oxidative or non-oxidative conditions. Toxics 2024, 12, 340. [Google Scholar] [CrossRef]
- Sebe, I.; Zsidai, L.; Zelkó, R. Novel modified vertical diffusion cell for testing of in vitro drug release (IVRT) of topical patches. HardwareX 2022, 11, e00293. [Google Scholar] [CrossRef]
- MFDS. Guideline on Bioanalytical Method Validation. 2013. Available online: http://www.mfds.go.kr/index.do?mid=1162&seq=7560 (accessed on 22 August 2025).
- MFDS. Guideline for In Vitro Skin Absorption Method. 2009. Available online: http://www.mfds.go.kr/index.do?mid=1161&seq=4788 (accessed on 22 August 2025).
- OECD. Guideline for the Testing of Chemicals: Skin Absorption—In Vitro Method (Test No. 428). Organisation for Economic Co-operation and Development, 2004. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg428-508.pdf (accessed on 22 August 2025).
- Jung, E.C.; Maibach, H.I. Animal models for percutaneous absorption. J. Appl. Toxicol. 2015, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; et al. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Marquet, F.; Grandclaude, M.C.; Ferrari, E.; Champmartin, C. Capacity of an in vitro rat skin model to predict human dermal absorption: Influences of aging and anatomical site. Toxicol. In Vitro 2019, 61, 104623. [Google Scholar] [CrossRef]
- Kocsis, D.; Klang, V.; Schweiger, E.M.; Varga-Medveczky, Z.; Mihály, A.; Pongor, C.; et al. Characterization and ex vivo evaluation of excised skin samples as substitutes for human dermal barrier in pharmaceutical and dermatological studies. Skin Res. Technol. 2022, 28, 664–676. [Google Scholar] [CrossRef]
- FDA. Food and Drug Administration’s Regulation of Food Additives. U.S. Food and Drug Administration, 1977. Available online: https://www.gao.gov/products/100521 (accessed on 22 August 2025).
- CIR. Final Safety Assessment of Coal Tar as Used in Cosmetics. 2008. Available online: https://cir-reports.cir-safety.org/cir-ingredient-status-report (accessed on 22 August 2025).
- SCCS (Scientific Committee on Consumer Safety). SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, 12th revision, SCCS/1647/22, 2023. Available online: https://health.ec.europa.eu/publications/sccs-notes-guidance-testing-cosmetic-ingredients-and-their-safety-evaluation-12th-revision_en (accessed on 22 August 2025).
- EC. CosIng: Cosmetic Ingredient Database—Amaranth (CI 16185), 2025. Available online: https://ec.europa.eu/growth/tools-databases/cosing/details/32726 (accessed on 23 August 2025).
- NIH. PubChem compound summary for amaranth (CID 13506): Computed properties, 2025. National Institutes of Health. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/13506 (accessed on 22 August 2025).
- ECHA. Registration dossier: Trisodium 3-hydroxy-4-(4′-sulphonatonaphthylazo)naphthalene-2,7-disulphonate, 2025. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/17264 (accessed on 22 August 2025).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).