Background
Chronic kidney disease (CKD) is a non-communicable disease (NCD) that not only affects adults but also children and babies, which can cause a decrease in quality of life and increased mortality. CKD and hypertension in adolescence and young adulthood are predisposing factors for cardiovascular and neurological diseases later in life[
1,
2,
3].
Early screening for CKD will have an impact on better prognosis, but requires appropriate, careful, and continuous modalities [
4]. Currently, the modalities established in developed countries include urinary screening and blood pressure measurements. Underdeveloped nephrons also play a role in CKD incidence [
4]. Disrupted development of nephrons is common in premature births. In premature conditions, the number of nephrons remains insufficient, and hypertension and glomerular hyperfiltration often occur. Monitoring premature babies requires uniformity so that potential incidents of acute kidney disease (AKD) and CKD are lately discovered. Early diagnosis of acute kidney injury (AKI) is important in preventing AKD [
5,
6].
In daily practice, premature babies require minimal handling treatment; therefore, urinary catheterization is not recommended, because it can cause iatrogenic urinary tract infection (UTI). This situation causes difficulties in the detection of oliguria in neonates. The modality commonly used in screening for AKI in neonates is SCr measurement. This examination is sometimes only meaningful if AKI occurs for > 48 h. This causes delayed treatment and affects the prognosis. In the adult and pediatric populations, there are other promising modalities for early detection of AKI, including examination of cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule-1 (KIM-1) [
7,
8,
9]. This protein examination is not yet common, and it still requires government and hospital policies to be prepared. The government requires valid research data to examine the expression of these proteins.
Research on NGAL, KIM-1, and cystatin C levels in neonates during the early detection of AKI has not been well published. Research on IL-18 in neonates has been published; however, procuring IL-18 kits is expensive. Therefore, they are difficult to be implemented in developing countries. Oligonephronemia-related prematurity is a predisposition factor for CKD not only in adulthood but also in childhood [
4]. Research to determine the relationship between NGAL and serum creatinine in AKI in the first 24 hours of birth is very necessary, to find out the best modality for early detection of AKI in at-risk neonates. Also, the evidence of of serum NGAL for the monitoring of AKI treatment in LBW neonates was needed. Although research on the significance of serum NGAL level in detecting AKI early in neonates has been published, there is still some controversies [
4,
10,
11,
12,
13,
14]. In addition, urinary NGAL has almost the same significance as serum NGAL; therefore, research on the use of serum NGAL in monitoring neonatal AKI is necessary. This study aimed to determine whether NGAL can be used as a modality for early detection and monitoring of improvement in AKI amongst LBW neonates.
Methods
A prospective-cohort study was conducted in Hasan Sadikin General Hospital, Bandung, West Java, from January–July 2024. The inclusion criteria were preterm infants born at 28–34 weeks of gestational age, without any dehydration. The preterm infants with congenital heart disease, infant lupus, congenital anomalies of the kidney and urinary tract (CAKUT), and sepsis were excluded, to avoid bias from additional risk factors of postrenal and prerenal AKI. Preterm infants met the inclusion criteria for urinary production monitoring, NGAL levels, and SCr levels during the first 24 hours. NGAL measurements using ElabscienceR Human NGAL ELISA kit. Statistical analysis was conducted with Kolmogorov-Smirnov test to test normality on numerical data, Wilcoxon-test to test the differences in changes in NGAL, SCr, and urine volume between D1 and D3, Cochran’s Q-test to determine the difference in positivity rate between modalities, Spearman’s-rank test to determine the correlation between SCr and urine volume with NGAL. Characteristic data are presented descriptively for categorical data using numbers and percentages, and numerical data are presented as median, IQR and minimum maximum, because the data were not normally distributed. The confidence interval was 95% with a significance level of 5% (p<0.05), and the analysis was performed using SPSS version 27.0.
The research project was approved by institutional review boards at Hasan Sadikin General Hospital, all methods were performed in accordance with the relevant guidelines and regulations.
Results
This study included 77 low-birthweight neonates with varying demographic and clinical characteristics (
Table 1). Based on KDIGO, NGAL, and nRIFLE criterias, AKI were found in 19, 63, and 7 neonates, respectively. Of the total subjects, the majority were male (58.4%), the median gestational age of neonates was 33 weeks, with a gestational age range of 28–34 weeks, indicating that most of the subjects were born prematurely. The birth weight of neonates in this study varied, with a median of 1600 g, in the range of 750–2150 g, indicating that the subjects consisted of neonates with birth weights that were very low to close to normal, and most of them were born spontaneously (54.5%). The health condition of the neonate after birth was assessed using the APGAR score, with a median value of 7 in the first minute, 8 in the second minute, and 9 in the fifth minute, which showed an improvement in condition over time. As many as 27.3% of mothers who gave birth experienced hypertension during pregnancy, which may contribute to premature birth and low birth weight in neonates. This study provides important insights into the demographic and clinical characteristics of LBW neonates, which may be useful for the development of better treatment and intervention strategies in the future.
Based on the analysis in
Table 2, which presents changes in urine volume: The creatinine levels, and NGAL between the first day (D1) and the third day (D3) in neonates with low birth weight, several important conclusions can be drawn: (1) Urine volume: There was a significant increase in urine volume from D1 to D3, with the median increasing from 1.4 mL/kg/hour (D1) 3.0 mL/kg/hour (D3). These changes indicate a significant increase in urinary output, which may reflect changes in kidney function or the body response to clinical intervention. A p-value of < 0.001 confirmed that this change was statistically significant. (2) Creatinine, a traditional marker of kidney function, did not show significant changes between D1 and D3. Median creatinine levels changed only slightly from 0.76 mg/dL in D1 to 0.74 mg/dL in D3, with a p-value of 0.655, indicating that this change was not statistically significant. This indicates that kidney filtering function remained stable during this observation period, (3) Neutrophil Gelatinase-Associated Lipocalin (NGAL): There was a significant increase in NGAL levels from D1 to D3, with the median increasing from 168 ng/mL on D1 to 507 ng/mL on D3. This increase, which was also statistically significant (p value < 0.001), indicates that NGAL responds more quickly to kidney changes or stress than creatinine.
It can be concluded that in neonates with low birth weight, there was a significant increase in urine volume and NGAL levels from the first to the third day. NGAL, which increased more rapidly and significantly than creatinine, suggests that NGAL is a more responsive and possibly more effective modality for early detection of kidney damage or kidney stress. Meanwhile, the stability of creatinine levels showed that, despite changes in kidney function detected by NGAL, kidney filtering function measured through creatinine remained stable during this observation period. These changes need to be considered in the clinical monitoring of low birth weight neonates, especially in the context of early detection and treatment of kidney damage.
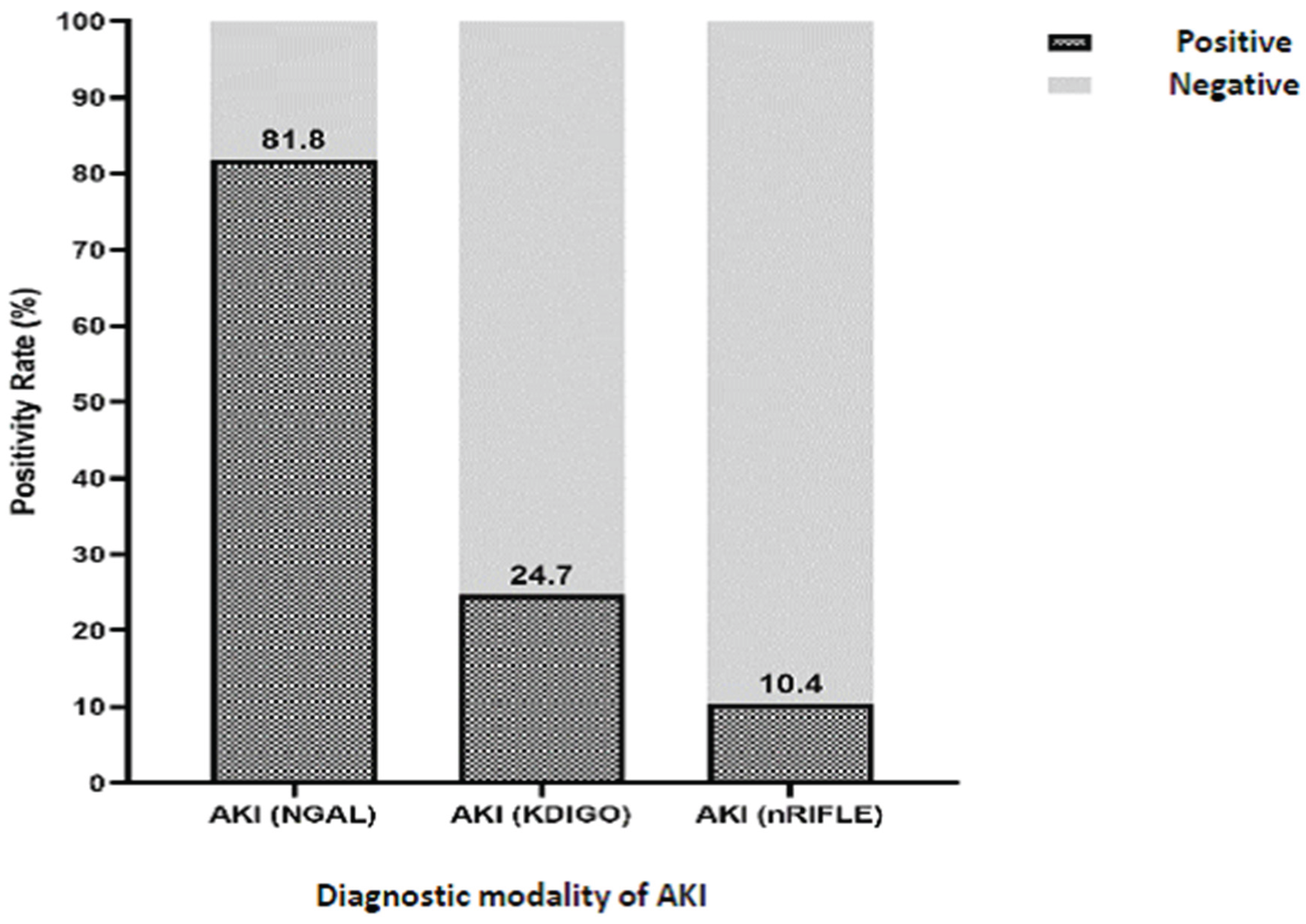
This study evaluated the positivity rate of Acute Kidney Injury (AKI) in neonates with low birth weight using three different diagnostic modalities: NGAL, KDIGO, and nRIFLE (
Table 3). The results obtained show significant differences in the positivity of each modality in detecting AKI.
NGAL emerged as the most sensitive diagnostic modality, with an AKI positivity rate of 81.8%. A 95% confidence interval range (71.8% to 88.8%) showed that NGAL consistently detected AKI in the majority of neonates examined (
Figure 1). This indicates that NGAL can be a very effective tool for early detection of AKI in neonatal populations with low birth weight. In contrast, KDIGO shows a lower positivity rate, which is 24.7%, with a 95% confidence interval between 16.4% to 35.4%. Although KDIGO can still detect AKI, its positivity is much lower compared to NGAL.
Overall, NGAL proved to be the most sensitive and responsive modality in detecting AKI in neonates with low birth weight, compared to KDIGO and nRIFLE. These findings highlight the importance of selecting the right diagnostic tools in the monitoring and managing AKI in this vulnerable neonatal population, to ensure early detection and timely intervention.
Table 4.
The correlation between serum creatinine changes with urine volume and NGAL.
Table 4.
The correlation between serum creatinine changes with urine volume and NGAL.
| Variables. |
Serum NGAL changes |
| r |
p-value |
| Serum creatinine changes |
-0.026 |
0.410 |
| Urinary volume changes |
-0.167 |
0.073 |
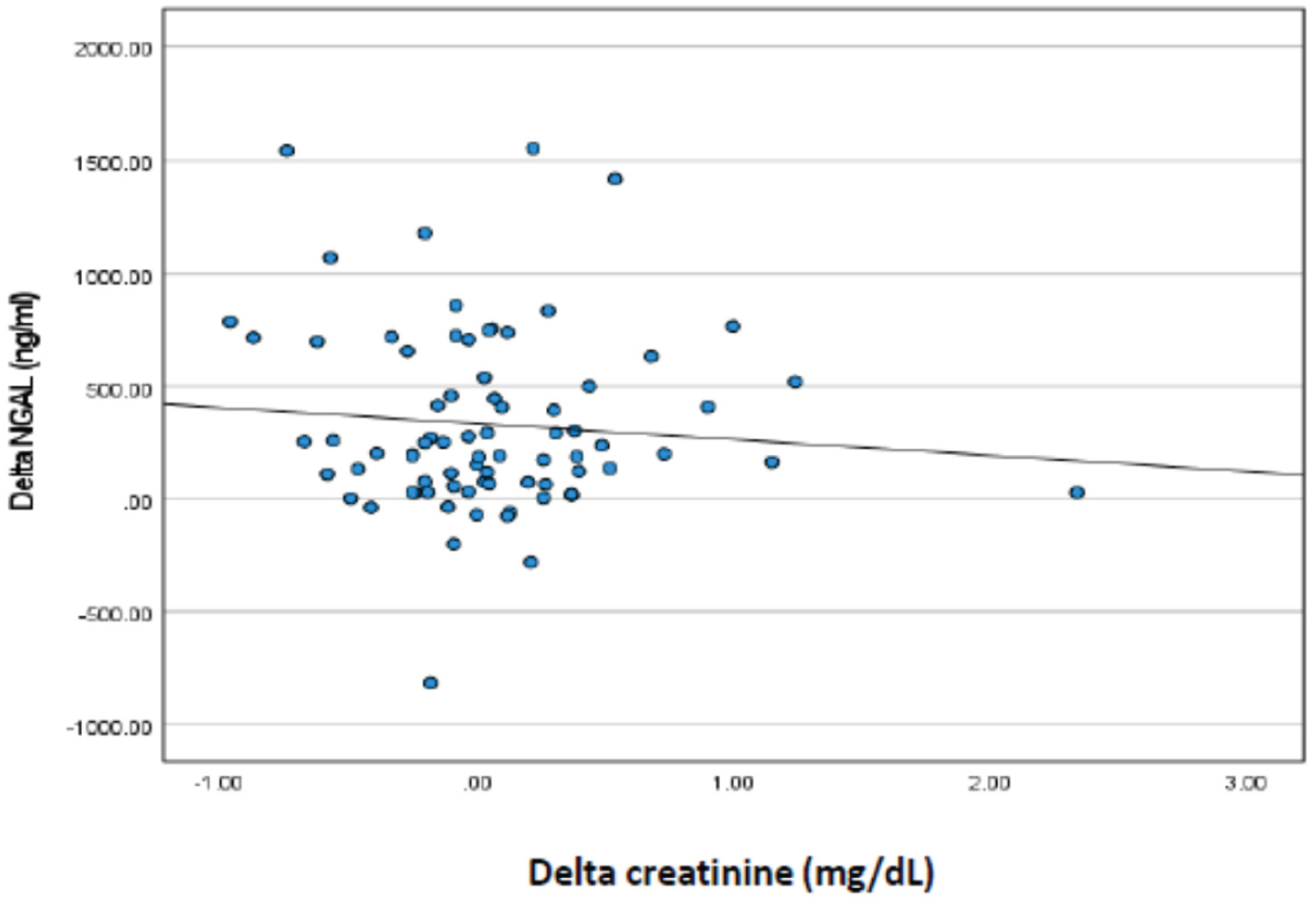
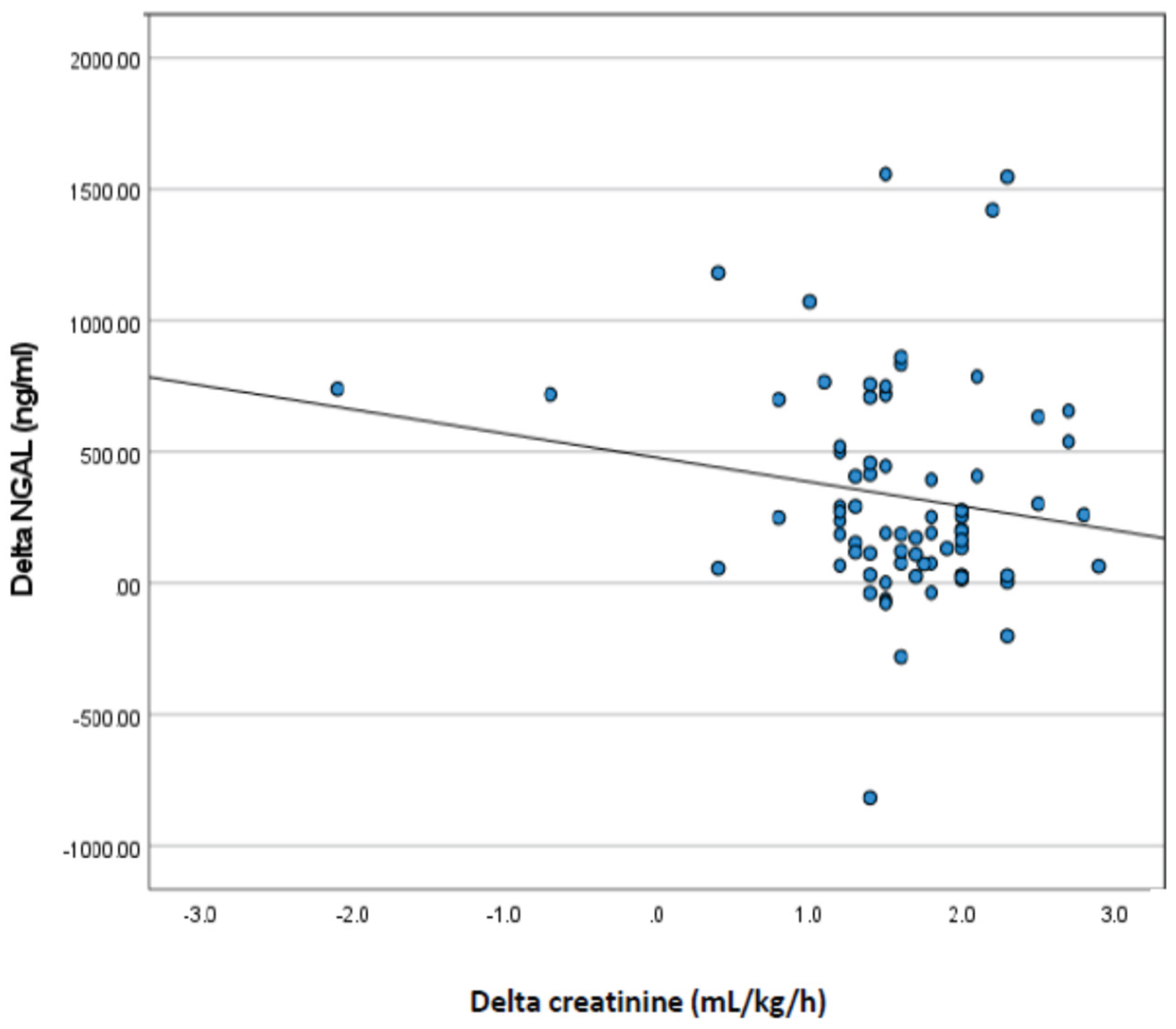
Based on
Table 3 and
Figure 2, the correlation between changes in creatinine and urine volume with changes in NGAL from day one (D1) to day three (D3) in neonates with low birth weight, the following conclusions can be drawn: Correlation of creatinine changes with NGAL changes: The Spearman (r) correlation value between creatinine change and NGAL change is -0.026 with a p value of 0.410. This suggests that there was almost no correlation between creatinine and NGAL changes. This indicates that the change in creatinine levels from D1 to D3 had no significant relationship with the change in NGAL levels in the neonates studied. Correlation of changes in urine volume with changes in NGAL: The Spearman correlation value (r) between the change in urine volume and the change in NGAL was -0,167 with (p = 0.073). Although this negative correlation was stronger than that with creatinine, it was not statistically significant (p > 0.05). This suggests that there is a slight tendency for an increase in urine volume to be associated with a decrease in NGAL, but this correlation is not strong enough to be considered significant. The results of this correlation analysis showed that changes in creatinine levels and urine volume from day one to day three were not significantly correlated with changes in NGAL levels in neonates with low birth weight. In other words, changes in NGAL levels, as a marker of kidney injury, were not directly related to changes in creatinine or urine volume during this observation period.
As mentioned on
Table 5, there was no statistically significant relationship was found between subject characteristics (sex, gestational age, birth weight, mode of delivery, and history of maternal hypertension) and NGAL levels in neonates with low birth weight (p > 0.05). These results suggest that NGAL levels are not influenced by the above characteristics; in other words, the characteristics (sex, gestational age, birth weight, mode of delivery, and history of maternal hypertension) are not confounding factors.
Discussion
The potency of CKD could be detected earlier, i.e., from history taking regarding neonatal history of low birth weight and prematurity [
1,
2,
3,
15]. Oligonephropathy-related prematurity is a predisposition factor for hypertension, not only in adulthood, but also during infancy. Delayed diagnosis and neglected hypertension have been known as a contributing factor of CKD and end-stage kidney disease (ESKD) [
16,
17]. Primary prevention of CKD should be performed as soon as possible, that is, routine urinary and blood pressure screening since childhood.
Table 1 shows that the subjects in this study were homogeneous in terms of sex, gestational age, birth weight, and delivery methods.
As shown in
Table 2, since the first 24 h, NGAL levels have increased (above 150 ng/mL) in neonates experiencing oliguria, while creatinine levels did not increase. However, neonates with AKI who improved after treatment, serum NGAL levels remained elevated, while serum creatinine levels did not increase. This indicates that the detection of AKI in neonates can be performed early by knowing urine production or by examining NGAL levels. Serum creatinine cannot be used to determine AKI earlier in LBW neonates. This may be because there is very little muscle mass and lean body mass in LBW neonates. In addition, this is also possible because the neonates in this study have received adequate management to improve AKI (this is proven by the urine volume, which is no longer oliguric). NGAL as a protein released by damaged tubular cells, continue to rise when urine volume starts to return to normal, indicating that although AKI has been improved, there are still many tubular cells that have not recovered from injury [
18]. The important thing in the present study is NGAL can be detected earlier but is not very good at monitoring AKI as it improves. Therefore, monitoring diuresis and creatinine levels, as well as electrolyte and acid-base balance, are still needed to monitor AKI which is being treated.
Table 3 shows that the positivity rate for serum first day NGAL was better than that for those serum creatinine, both using the KDIGO and nRIFLE criteria. This is in accordance with previous studies on adult and pediatric populations [
7,
8,
9,
13,
14,
18,
19,
20,
21,
22]. In a systematic review study by Xu et al. (2022) it was stated that NGAL was very good at detecting AKI conditions that required kidney replacement therapy (KRT). However, the systematic review only includes studies on adult populations (more than 18 years old), there are no studies on children, infants and neonates [
18]. Research according to Maisel et al. (2016) states that the NGAL cut off point value of more than 150 ng/dL is a strong predictor that AKI is at risk of worsening kidney failure, and Andriani et al. (2015) found that this cut off has very high specificity and sensitivity, i.e., 88 and 81%, respectively [
8,
11]. Research according to Batte et al. (2022) states that high NGAL values in children with sickle cell disease are associated with high mortality [
20]. According to Zhang et al., NGAL is significant as an early detector of AKI in the adult population [
9]. However, the present study is the first in examining this in LBW neonates.
Table 4 shows that there was no correlation between changes in urine volume and changes in creatinine and NGAL, which is not in accordance with previous studies which showed that NGAL and creatinine were correlated with urine volume. The different results obtained in this study may be because NGAL at 24 h was increased in LBW neonates with AKI who then underwent adequate management, so that AKI improved, as can be seen from the urine volume, which was no longer oliguric on the third day of examination. The good results of this therapy resulted in no increase in creatinine and urine volume, but not polyuria, while NGAL continued to increase. NGAL is a tubular protein, indicating that injury to the tubules has not yet healed completely. This strongly suggests that NGAL is good for the early detection of AKI in LBW neonates but is not good for monitoring improvement in AKI. Therefore, the combination of NGAL, urine volume monitoring, and serum creatinine levels is important for the management of AKI in LBW neonates. NGAL is important for early detection; therefore, treatment is not delayed, and monitoring urine volume and serum creatinine is needed to determine the success of AKI management.
Zou (2023) meta-analysis includes most of the pediatric population, there is an interesting thing, NGAL is very accurate in detecting AKI in asphyxiated neonates [
7]. However, there has been no research that specializes in preterm neonates 28–34 weeks of gestational age. Although previous studies regarding NGAL in neonates have been published, there is still controversy, especially according to Sarafidis et al. (2014) who stated that NGAL cannot be a predictor of AKI 1–2 days earlier than creatinine, while other studies said it could [
10]. Hanna et al. (2016) and research by Elmas et al. (2017) stated that urine NGAL can detect AKI earler in nonsepsis neonates and nonasphyxiated neonates [
12,
13]. The controversy between several studies requires a systematic review of the role of NGAL in early detection of AKI in preterm neonates. Therefore, this research is very essential, because it adds to the repertoire of systematic reviews, with something that is highlighted in this research, namely the suspicion that AKI occurs more often in preterm and low birth weight (LBW) neonates. The LBW state is associated with hypothermia and metabolic disorders, which are often superimposed on metabolic disorders due to early phase AKI.
This study is the first to compare changes in serum NGAL and creatinine levels in neonates with improved AKI. Meanwhile, this study has some limitation, i.e single center setting, the number of subjects was not representative to determine the cut-off point for NGAL in neonates with AKI.
Conclusions
The results in the form of a non-significant correlation between the two conclude that serum NGAL cannot represent AKI that has improved. Hence, the monitoring of creatinine and urinary production is still needed in the management of LBW neonates with AKI.
Funding
Internal Research Grant of Universitas Padjadjaran No. 1753/UN631/PT00/2024.
Institutional Review Board Statement
Participants provided signed informed consent prior to enrollment in the study. The research project was approved by institutional review boards at Hasan Sadikin General Hospital No.DP.04.03/D.XIV.6.5./112/2024. Informed consent were gained from a parent and/or legal guardian for study participation.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Warrens, H; Banerjee, D; Herzog, CA. Cardiovascular Complications of Chronic Kidney Disease: An Introduction; European cardiology: England, 2022; Vol. 17, p. e13. [Google Scholar] [CrossRef]
- Mayne, KJ; Lees, JS; Mark, PB. Cardiovascular complications of chronic kidney disease. Medicine (Baltimore) [Internet] 2023, 51(3), 190–5. Available online: https://www.sciencedirect.com/science/article/pii/S1357303922003267. [CrossRef]
- Jankowski, J; Floege, J; Fliser, D; Böhm, M; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation [Internet] Available from. 2021, 143(11), 1157–72. [Google Scholar] [CrossRef]
- V S, R P, V M. Renal Function and Renal Volume of Children Born with Very Low Birth Weight. J Evid Based Med Healthc. 2020, 7, 1541–4.
- Cho, MH. Pediatric Acute Kidney Injury: Focusing on Diagnosis and Management. Child Kidney Dis. 2020, 24(1), 19–26. [Google Scholar] [CrossRef]
- Krishnasamy, S; Sinha, A; Bagga, A. Management of Acute Kidney Injury in Critically Ill Children. Indian J Pediatr [Internet] Available from. 2023, 90(5), 481–91. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z; Chen, B; Tang, F; Li, X; Xiao, D. Predictive value of neutrophil gelatinase-associated lipocalin in children with acute kidney injury: A systematic review and meta-analysis. Front Pediatr 2023, 11(March). [Google Scholar] [CrossRef] [PubMed]
- Andriani, M; Puspita, Y. Sensitivitas dan Spesifisitas Neutrophil Gelatinase Associated Lipocalin sebagai Penanda Dini Acute Kidney Injur y pada Pasien ICU dan HCU. Anesth Crit Care 2015, 33(1), 272–8. [Google Scholar]
- Zhang, J; Han, J; Liu, J; Liang, B; Wang, X; Wang, C. Clinical significance of novel biomarker NGAL in early diagnosis of acute renal injury. Exp Ther Med. 2017, 14(5), 5017–21. [Google Scholar] [CrossRef]
- Sarafidis, K; Tsepkentzi, E; Diamanti, E; Agakidou, E; Taparkou, A; Soubasi, V; et al. Urine neutrophil gelatinase-associated lipocalin to predict acute kidney injury in preterm neonates. A pilot study. Pediatr Nephrol 2014, 29(2), 305–10. [Google Scholar] [CrossRef] [PubMed]
- Maisel, AS; Wettersten, N; van Veldhuisen, DJ; Mueller, C; Filippatos, G; Nowak, R; et al. Neutrophil Gelatinase-Associated Lipocalin for Acute Kidney Injury During Acute Heart Failure Hospitalizations: The AKINESIS Study. J Am Coll Cardiol 2016, 68(13), 1420–31. [Google Scholar] [CrossRef]
- Tabel, Y; Elmas, A; Ipek, S; Karadag, A; Elmas, O; Ozyalin, F. Urinary neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury in preterm infants. Am J Perinatol 2014, 31(2), 167–74. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M; Brophy, PD; Giannone, PJ; Joshi, MS; Bauer, JA; RamachandraRao, S. Early urinary biomarkers of acute kidney injury in preterm infants. Pediatr Res. 2016, 80(2), 218–23. [Google Scholar] [CrossRef]
- Song, Y; Sun, S; Yu, Y; Li, G; Song, J; Zhang, H; et al. Diagnostic value of neutrophil gelatinase-associated lipocalin for renal injury in asphyxiated preterm infants. Exp Ther Med 2017, 13(4), 1245–8. [Google Scholar] [CrossRef]
- Hilmanto, D. Disease-Associated Systemic Complications in Childhood Nephrotic Syndrome: A Systematic Review. 2022. (February). [Google Scholar] [CrossRef]
- Ku, E; Lee, BJ; Wei, J; Weir, MR. Hypertension in CKD: Core Curriculum 2019. Am J Kidney Dis [Internet] Available from. 2019, 74(1), 120–31. [Google Scholar] [CrossRef]
- Georgianos, PI; Agarwal, R. Hypertension in chronic kidney disease—treatment standard 2023. Nephrol Dial Transplant [Internet] Available from. 2023, 38(12), 2694–703. [Google Scholar] [CrossRef] [PubMed]
- Xu, C; Lin, S; Mao, L; Li, Z. Neutrophil gelatinase-associated lipocalin as predictor of acute kidney injury requiring renal replacement therapy: A systematic review and meta-analysis. Front Med. 2022, 9(September), 5–16. [Google Scholar] [CrossRef] [PubMed]
- Saputra, AN; Airlangga, PS; Rahman, BA; Kusuma, E; Kriswidyatomo, P; Sumartomo, C. Role of neutrophil gelatinase-associated lipocalin (NGAL) as an acute prerenal kidney injury marker: exploring factors associated with its postoperative levels in hypotension-controlled otorhinolaryngology surgery. Bali Med J. 2022, 11(3), 1844–8. [Google Scholar] [CrossRef]
- Batte, A; Menon, S; Ssenkusu, JM; Kiguli, S; Kalyesubula, R; Lubega, J; et al. Neutrophil gelatinase-associated lipocalin is elevated in children with acute kidney injury and sickle cell anemia, and predicts mortality. Kidney Int [Internet] Available from. 2022, 102(4), 885–93. [Google Scholar] [CrossRef]
- Lumlertgul, N; Amprai, M; Tachaboon, S; Dinhuzen, J; Peerapornratana, S; Kerr, SJ; et al. Urine Neutrophil Gelatinase-associated Lipocalin (NGAL) for Prediction of Persistent AKI and Major Adverse Kidney Events. Sci Rep [Internet] Available from. 2020, 10(1), 8718. [Google Scholar] [CrossRef]
- Jahaj, E; Vassiliou, AG; Pratikaki, M; Gallos, P; Mastora, Z; Dimopoulou, I; et al. Serum Neutrophil Gelatinase-Associated Lipocalin (NGAL) Could Provide Better Accuracy Than Creatinine in Predicting Acute Kidney Injury Development in Critically Ill Patients. J Clin Med [Internet] 2021, 10(22). Available online: https://www.mdpi.com/2077-0383/10/22/5379. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).