Submitted:
13 January 2026
Posted:
14 January 2026
You are already at the latest version
Abstract
Keywords:
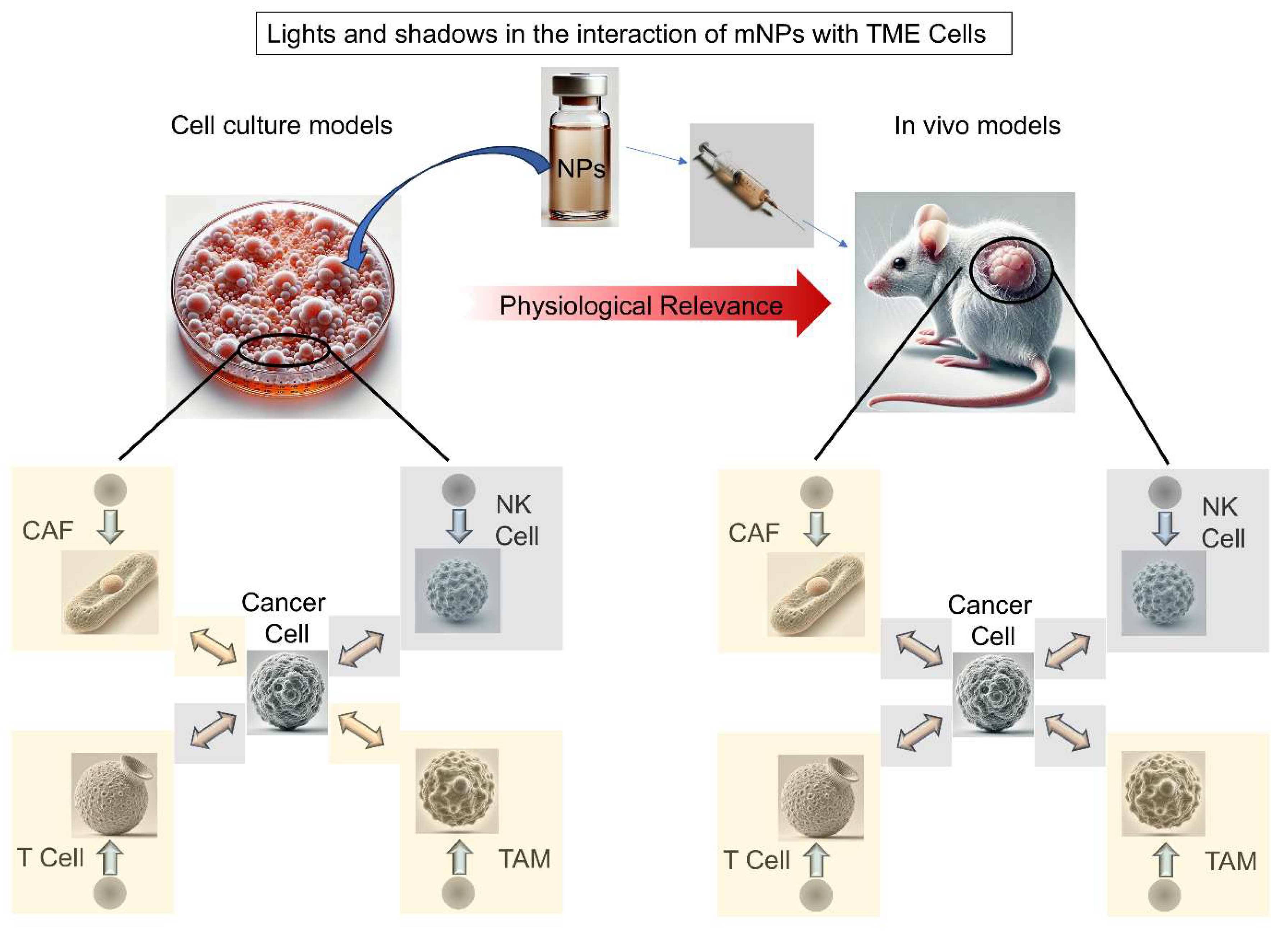
Introduction
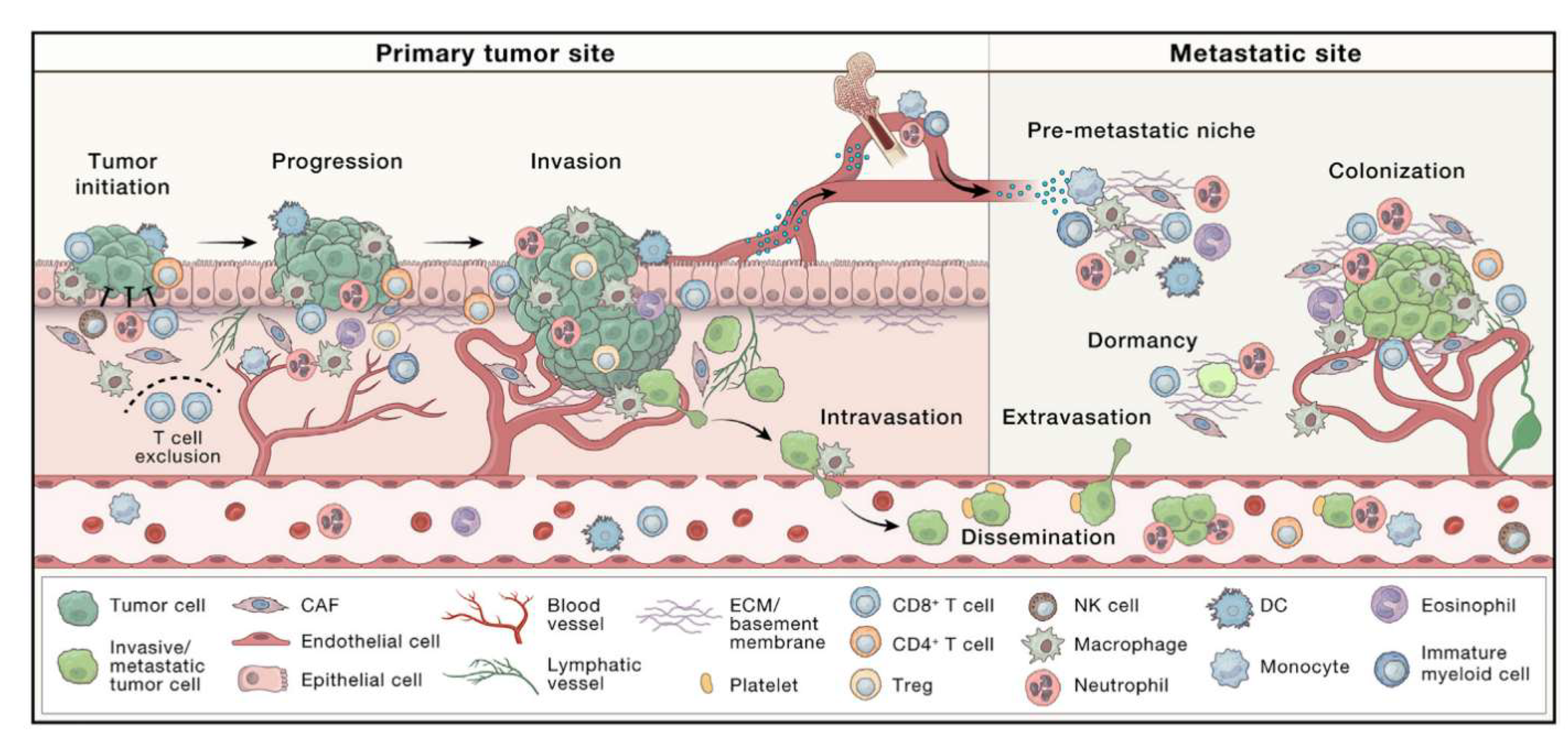
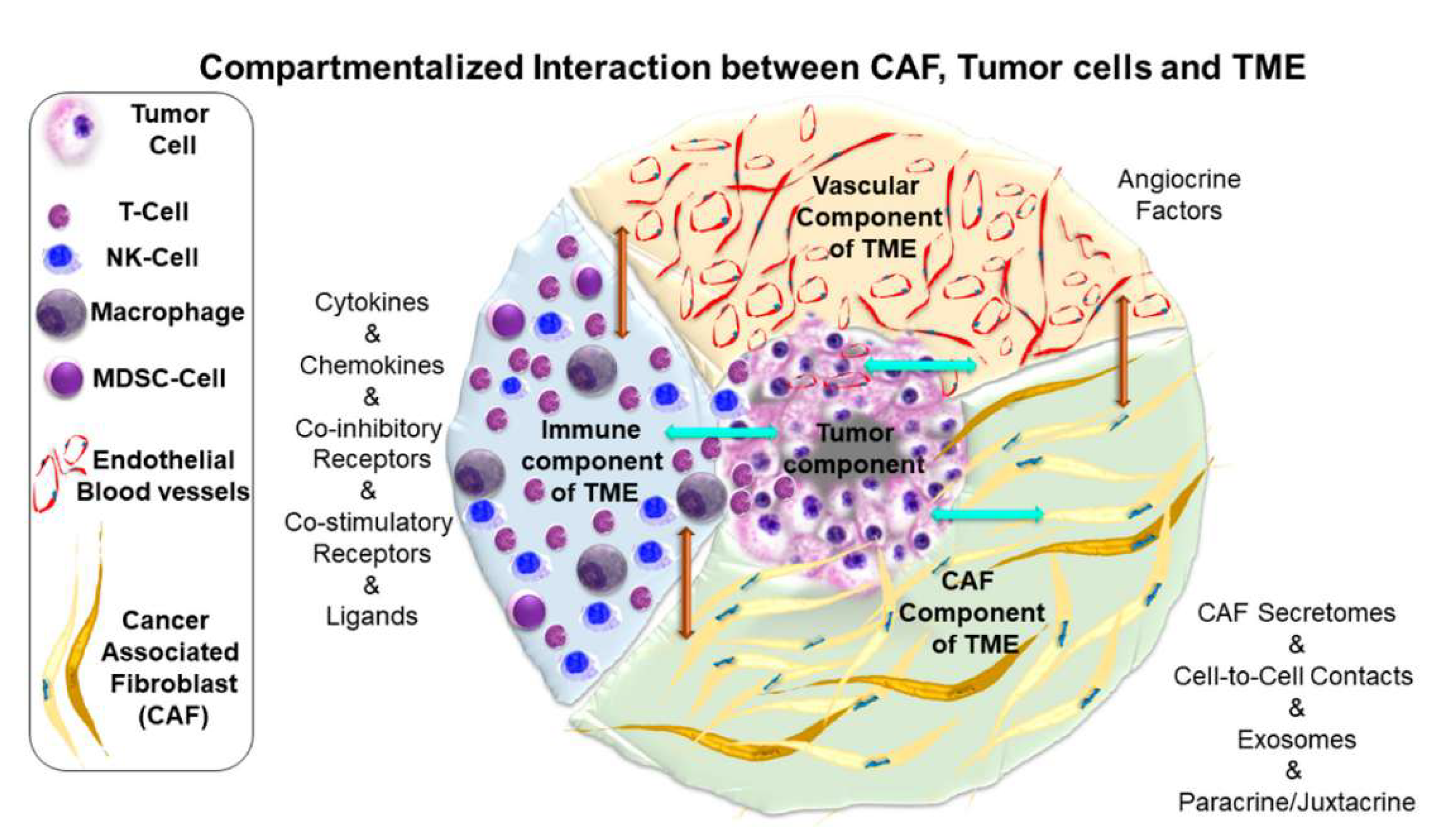
1.1. Cancer and The Tumor Microenvironment
1.2. Cancer Associated Fibroblast
1.3. Tumor Associated Macrophages
1.4. Immune cells of the TME
2. Nanotechnology in Cancer Treatment
2.1. Nanotechnology and the TME
2.1.1. mNPs and CAFs
Gadolinium (Gd) mNPs and CAFs
Gold (Au) mNPs and CAFs
Silver (Ag) and Core-Shell Au@Ag mNPs and CAFs
Multicomponent mNPs and CAFs
Summary and Reflections About mNPs and CAFs
| NPs | Cancer type | Cell culture |
Animal model |
Pathway / Mechanism |
Effects | Ref |
| Gd@C82(OH)22 NPs | Human pancreatic, fibrosarcoma and primary lung CAFs | 2D of primary cells (human) in all cases | Subcutaneous | TNFR2/p38 MAPK | Increase the synthesis of collagen types I and III | [90] |
| AuNPs (15 nm) | Colorectal cancer | 2D of SW620 cancer cells (human) | Subcutaneous | Akt | Decrease of collagen I, CAF density and stromal factors in vivo. Increase the drug-uptake (cisplatin) | [91] |
| AuNPs (20 nm) | Pancreatic cancer | 2D of primary CAFs (human) and CAF-19 cells (human) | N.A. | Lipogenesis-related genes | Lipid accumulation in CAFs, transforming them to a quiescent state | [93] |
| AuNPs (3 nm to 80 nm) | Oral squamous cell carcinoma | 2D of primary CAFs (human) | Subcutaneous | Reduction in expression of critical proteins and interleukins | Delay in tumor growth by co-inoculation of CAFs and cancer cells | [94] |
| AuNPs-PEG-RGD | Cervical cancer | 2D of Hs.895.T CAFs (human), Hs.895.Sk fibroblast (human) and HeLa cancer cells (human) | N.A. | N.A. | No effects over CAFs | [96] |
| AuNPs-PEG-RGD | Pancreatic cancer | 2D of CAF-98 cells (human), primary NPF-98 cells (human) and MIA-PaCa-2 and PANC-1cancer cells (both human) | Subcutaneous | N.A. | Higher retention in CAFs in cell culture and within tumor in vivo | [98] |
| AuNPs-PEG-RGD | Pancreatic cancer | 3D of CAF-98 cells (human) and MIA-PaCa-2 cancer cells (human) | N.A. | N.A. | No effects due to presence of CAFs | [101] |
| AuNPs (8 nm), AgNPs and Au@Ag NPs (11 nm) | Breast cancer | Co-culture of primary CAFs (human), NIH/3T3 fibroblasts (murine) and 4T1 cancer cells (murine) and human MCF-7 cancer cells (human) | Orthotopic | N.A. | CAFs exposed to NPs or the resulting conditioned media mitigated cancer cell migration | [102] |
| GIONFs | Desmoplastic cholangiocarcinoma | 2D and Co-culture of hTERT-HSC cells (human), RAW264.7 TAM cells (murine) and EGI-1 cancer cells (human) |
Subcutaneous | N.A. | Reduction of tumor stiffness and complete tumor regression | [104] |
2.1.2. mNPs and TAMs
Au mNPs and TAMs
Multicomponent mNPs and TAMs
Metal-Organic Framework (MOF) NPs and TAMs
IONPs and TAMs
Other mNPs and TAMs
Summary and Reflections About mNPs and TAMs
| NPs | Cancer type | Cell culture |
Animal model |
Pathway / Mechanism |
Effects | Ref |
| Human Serum Albumin (HSA)−Au(III) thiosemicarbazone NPs | Gastric cancer | 2D of RAW264.7 TAM cells (murine) and MGC-803 cancer cells (human) | Subcutaneous | NF-κB, iNOS, MsR2, STAT3, p-STAT3 and PD-1 | Remarkable tumor accumulation and potent antitumor effects | [105] |
| AuNPs conjugated with 5-fluorouracil (16 nm) |
Colorectal cancer and peritoneal metastasis | 2D of RAW264.7 TAM cells (murine) and CT26 cancer cells (murine) | Subcutaneous and a model of metastasis | N.A. | Following intraperitoneal administration of NPs, noticeable increase in TAMs (polarized to M1 phenotype) and CD3+T lymphocyte and high uptake of NPs by TAMs, in the metastatic model | [106] |
| Furin-responsive aggregated AuNPs loaded with doxorubicin and hydroxychloroquine (in the range 40-50 nm) |
Breast cancer | 2D of RAW264.7 TAM cells (murine), primary BMDM cells (murine) and MCF-7 cancer cells (human) | Subcutaneous | TNF-α, IL-6 and IL-10 | Polarization of TAMs and tumor growth delay | [107] |
| Polyaniline-based glyco-coated AuNPs (18-32 nm) |
Lung cancer | 2D of RAW264.7 TAM cells (murine), 3T3-L1 cells (murine) and MRC-5 cells (human) | Subcutaneous and orthotopic | Cell culture: NF-κB, iNOS, STAT6 and ARG1. Different pattern of interleukins secretion | Polarization of TAMs in cell culture and in vivo; tumor growth delay. Increase in CD8+ T cells and DC within the tumor and a reduction in Tregs | [108] |
| AuNPs (62 nm) |
Prostate cancer | 2D and Co-culture of THP-1 cancer cells (human), LNCaP cancer cells (human) and PC3 cancer cells (human) |
Orthotopic | IL-10, TGF-β, ARG1 IL-6, TNF-α, iNOS, CD163, LC3-II, GAPDH, SQSTM1, GAPDH. ATG5, ATG7, ATG12 and BECN1 | Polarization of TAMs | [109] |
| Au-manganese oxide NPs | Fibrosarcoma | 2D of primary murine TAMs | Subcutaneous (to obtain TAMs) | O2−, NO, ROS and HIF-1α Different pattern of interleukins secretion | Polarization of TAMs | [111] |
| antiPD-L1-SPIOs@PLGA@Au (>300 nm) |
Melanoma | 2D and Co-culture of BMDM (murine), Human Umbilical Vein Endothelial Cells and B16F10 cancer cells (murine) | Orthotopic | Increased in ROS levels | Application of radiotherapy, lead to polarization of TAMs. Increase in CD4+ and CD8+ T cells within the tumor. Tumor growth delay | [112] |
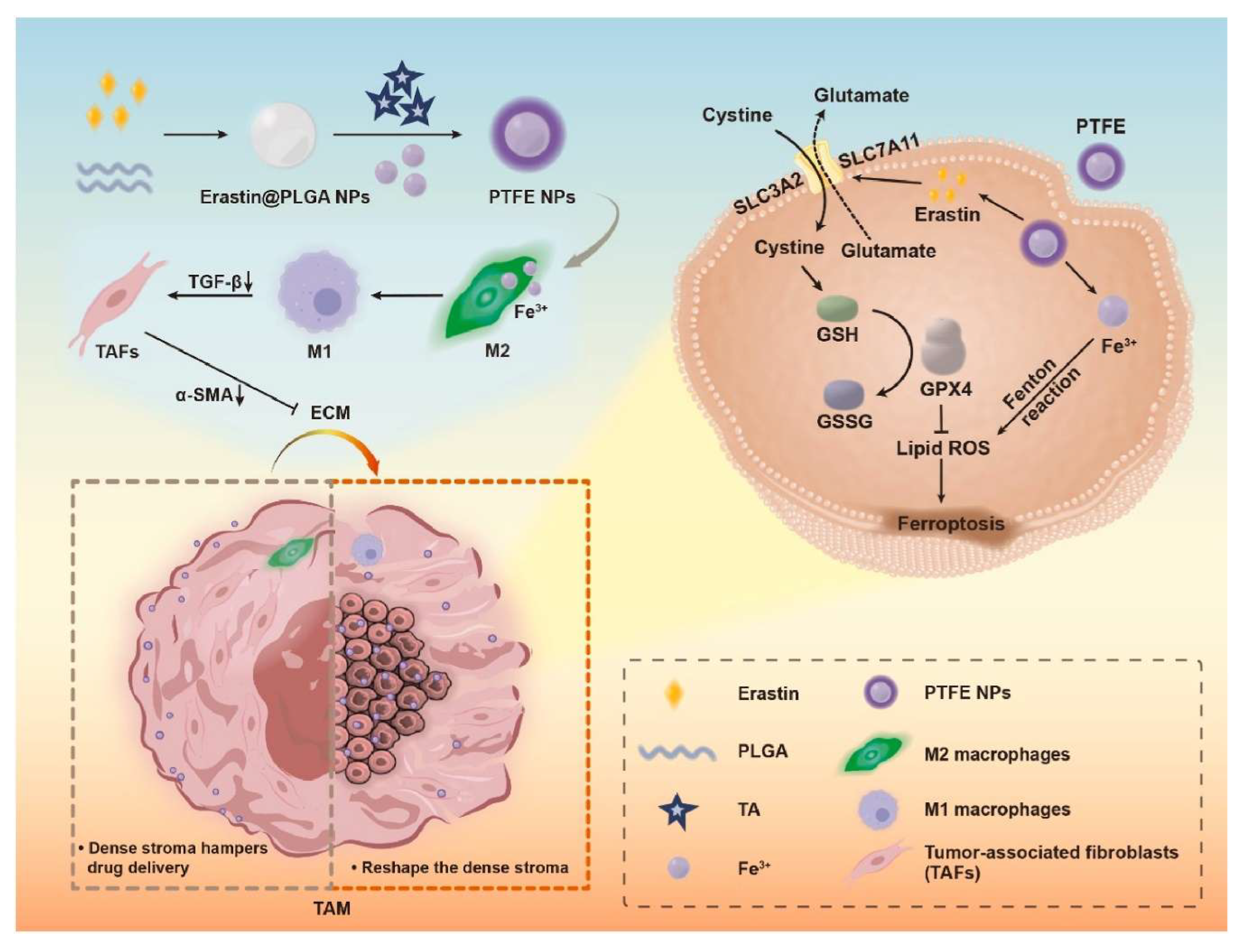
| Iron-containing metal-organic framework (MOF) NPs loaded with erastin | Pancreatic cancer | 3D of RAW264.7 TAM cells (murine), NIH3T3 cells (murine) and KPC1199 cancer cells (murine) | Subcutaneous | Antitumoral effect by composition and polarization of TAMs by different ways | The polarization of TAMs transforms CAFs to a quiescent state, both of which lead to delayed tumor growth in vivo | [113] |
| F-IONPs | Glioblastoma | 2D of RAW264.7 TAM cells (murine), CCD-986sk cells (human) and u87 cancer cells (human) | Subcutaneous | N.A. | Delineation of tumor margins | [115] |
| Hyaluronic acid-modified doxorubicin IONPs (>200nm) |
Breast cancer | 2D of RAW264.7 TAM cells (murine) and 4T1 cancer cells (murine) | Orthotopic | N.A. | Higher uptake efficiency and cytotoxic in cell culture. Both antitumor and anti-metastatic effects in vivo | [116] |
| Arginine-loaded hollow IONPs (>200nm) | Breast cancer | 2D and Co-culture of RAW264.7 TAM cells (murine) and 4T1 cancer cells (murine) | Subcutaneous | Cell culture: TNF-α, iNOS and NO. In vivo: TNF-α and NO |
Treated TAMs impact cancer cell viability both in cell culture and in vivo. In vivo, there is an increase in CD4+ and CD8+ T cells within the tumor and a reduction in Tregs | [117] |
| IONPs encapsulated with an inhibitor of CSF-1 in liposomes functionalized with TAT | Colorectal cancer | 2D of BMDM (murine) and CT26 cancer cells (murine) | Subcutaneous | CD86, CD206, iNOS, TNF-α and ARG1 | Polarization of TAMs. Tumor growth delay |
[118] |
| Polyaniline-coated IONPs (38 nm) |
Breast cancer | 2D and 3D of fibroblast (hMF) (human), primary monocytes (human) and MCF-7 cancer cells (human) |
N.A. | CD86 | Polarization of TAMs | [119] |
| IONPs coated with a catechol ligand and functionalized with HA | Breast cancer | 2D of RAW264.7 TAM cells (murine) and 4T1 cancer cells (murine) | Orthotopic | Cell culture: CXCL11, CD68, CD80, iNOS, IL-1β and TNF-α |
Polarization of TAMs. Tumor growth delay |
[120] |
| Enzyme-responsive mannose-grafted IONPs | Breast cancer and hepatic cancer | 2D of J774A TAM cells (murine), NIH/3T3 fibroblasts (murine), MCF-7 cancer cells (human) and HepG2 cancer cells (human) | N.A. | IL-6 and ARG1 | Keep the M1 phenotype at low dose. At high dose, keep the M2 phenotype | [121] |
| nanodisc-shaped IONPs | Head and neck squamous carcinoma | 2D of RAW264.7 TAM cells (murine) and SCC7 cancer cells (murine) | Subcutaneous | CD86, CD206, TNF-α, IL-1β, ARG1 and IL-4. | Polarization of TAMs in cell culture. Tumor growth delay in vivo. | [123] |
| PEGylated IONPs | Murine and human osteosarcoma | N.A. | Orthotopic | N.A. | Polarization of TAMs (induced by anti-CD47 rather than by IONPs) | [124] |
| Macrophages exposed to IONPs, AONPs, ZnONPs (≈30 nm) |
Melanoma | 2D and Co-culture of RAW264.7 TAM cells (murine), BMDCs (murine), 4T1 cancer cells (murine), CT26 cancer cells (murine) and B16F10-OVA cancer cells (murine) |
Orthotopic | CD86 and iNOS. | Polarization of TAMs. Tumor growth delay | [125] |
| Bone-targeting immunostimulatory metal-organic framework (BT-isMOF) NPs functionalized with zoledronic acid (ZOL) and CpG oligonucleotides | Breast cancer (Bone metastasis in vivo) | 2D of RAW264.7 TAM cells (murine), BMDM cells (murine) and MDA-MB-231 cancer cells (human) | Orthotopic (Metastatic model) | N.A. | Polarization of TAMs. Decrease of bone metastatic osteolysis and reduction of tumor growth and progression |
[126] |
| Chromium nanoparticles (Cr NPs) and siYTHDF1 were loaded onto chitosan, coated with carboxymethyl mannose, and functionalized with DSPE-modified RGD. | Hepatic cancer | 2D of RAW264.7 TAM cells (murine), BMDM cells (murine), THP-1 cancer cells (human) and Hepa1-6 cancer cells (murine) | Subcutaneous | NOS2, TNF-α, IL-1β, IL-12 ARG1, IL10, TGF-β, STAT3 and STAT1. | Polarization of TAMs. Tumor growth delay. Increase in CD4+ and CD8+ T cells within tumors |
[127] |
2.1.3. mNPs and Other Non-Malignant Cells of the TME
2.1.4. Direct and Indirect Effects of mNP on the TME
AuNPs and the Tumor Immune Microenvironment
AgNPs and the Tumor Immune Microenvironment
IONPs and the Tumor Immune Microenvironment
Manganese (Mn) mNPs the Tumor Immune Microenvironment
Other mNPs and the Tumor Immune Microenvironment
Multicomponent mNPs and the Tumor Immune Microenvironment
| NPs | Cancer type | Cell culture |
Animal model |
Pathway / Mechanism |
Effects | Ref |
| PLGA microspheres co-encapsulated with hollow gold nanoshells | Melanoma and lymphoma | 2D of splenic lymphocytes (murine), B16F10 cancer cells (murine) and EG7-OVA cancer cells (murine) | Orthotopic and subcutaneous | N.A. | Tumor growth delay in the primary and metastatic mass by NPs and phototherapy leading to activation of DC and T cells | [132] |
| Glycoadjuvant AuNPs | Melanoma | 2D of BMDC (murine) and B16-OVA cancer cells (murine) | Orthotopic | Cell culture: MHC II and CD86. In vivo: IFN-γ and TNF-α |
Tumor growth delay and inhibition of metastasis. Polarization of TAMs toward a M1 phenotype. Increased CD8+ T cells. Decreased T reg cells. Decreased MDSCs. |
[133] |
| Zwitterion-functionalized dendrimer-entrapped AuNPs loaded with CpG | Breast cancer | 2D and Co-culture of BMDCs (murine) and 4T1 cancer cells (murine) | N.A. | N.A. | Maturation of BMDCs and activation of DC. Anti tumoral effect | [134] |
| β-D-Glucose-reduced AgNPs | Breast cancer | N.A. | Subcutaneous | TNF-α, IFN-γ, IL-6, IL-2, IL-4 and IL-10 | Tumor growth delay. Increased levels of CD8+ cells, memory T cells and innate effector T cells. Decreased levels of CD4+ cells and Treg | [135] |
| AgNPs (5 nm and 50 nm) coated with PVP or citrate | Renal carcinoma | N.A. | Subcutaneous | N.A. | Tumor growth delay. Increased levels of CD8+ cells |
[136] |
| IONPs functionalized with PDA, subsequently with RGD and AA; with GOx physically absorbed | Colorectal Cancer | Co-culture of BMDCs (murine) and CT26 cancer cells (murine) | Subcutaneous | Ferroptosis of cancer cells induced BMDCs maturation in cell culture | BMDCs maturation in cell culture. Tumor growth delay |
[137] |
| MnO2 + Irom atoms (Fe3+) + Doxorubicin; encapsulated within PEG-polyphenols | Melanoma | 2D and Co-culture of BMDCs (murine) and B16–F10 cancer cells (murine) |
Orthotopic | CD11c, CD80, CD86, IL-6 and TNF-α | Tumor growth delay and metastatic control | [138] |
| Mn molybdate nanodots | Colorectal, melanoma and breast cancer | Co-culture of BMDCs (murine), CT26 cancer cells (murine) B16F10 cancer cells (murine), and 4T1 cancer cells (murine) | Subcutaneous and Orthotopic | N.A. | DCs maturation, TAM polarization toward the M1 phenotype, increased CD8+ T cells. Decreased Tregs and MDSCs, Tumor growth delay |
[139] |
| MnO2 NPs | Breast cancer | N.A. | Subcutaneous | CCL3 and TNF-α | Recruitment of neutrophils and their subsequent polarization. Increased CD8+ T cells. Tumor growth delay |
[140] |
| Mineralized MOF, encapsulating Perforin and Granzyme B, coupled with a lysosome-targeting aptamer (CD63-aptamer) | Breast cancer | 2D of T cells (murine) and 4T1 cancer cells (murine) | Orthotopic | Novel ATVs to improve NPs drug targeting and increase cancer cell specificity | Increasing cancer cell death in cell culture. Tumor growth delay and higher recruitment of CD8+ T cells | [141] |
| MOFs loaded with GOx and an indoleamine 2,3-dioxygenase inhibitor (1-methyltryptophan) | Melanoma and breast cancer | N.A. | Orthotopic | N.A. | Increased the number of CD8+ T cell, matured DC, B cells and NK cells and decreased Treg | [142] |
| MOFs functionalized with bovine serum albumin (BSA) and folic acid (FA), and loaded with triptolide (TPL), Fe3+ and tannic acid (TA) | Melanoma | N.A. | Orthotopic | N.A. | Increased matured DC; CD8+ and CD4+ cells | [143] |
| TiO2 NPs functionalized with a Ruthenium complex, followed by conjugation with siRNA | Head and neck squamous cell carcinoma | 2D of PBMCs (human) and HN6 cancer cells (human) | Subcutaneous (patient derived cells) and Orthotopic (induced model) |
IFN-γ | Tumor growth delay | [144] |
| anti-PD-L1- magnetic gold nanohut | Hepatocellular carcinoma | 2D of Hep55.1c cancer cells (murine) | Orthotopic | N.A. | Direct treatment with NPs and remodeling of the TME in vivo | [145] |
| ACNVax | Breast cancer | N.A. | Subcutaneous | BCL-6, IFN-γ, TNF-α, CXCR4, CXCR5, CCR7, L-selectin, CD11a, VLA-4, IL-21, CCL19, CCL21a, CXCL13 and CCL2 | Tumor growth delay. Increased of B cells, CD4+ T cells, CD8+ T cells and memory T cells |
[147] |
Conclusion
Acknowledgments
Conflicts of Interest
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol Cancer Res 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Vitale, I.; Shema, E.; Loi, S.; Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med 2021, 27, 212–224. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Proietto, M.; Crippa, M.; Damiani, C.; Pasquale, V.; Sacco, E.; Vanoni, M.; Gilardi, M. Tumor heterogeneity: preclinical models, emerging technologies, and future applications. Front Oncol 2023, 13, 1164535. [Google Scholar] [CrossRef]
- Onken, J.S.; Fekonja, L.S.; Wehowsky, R.; Hubertus, V.; Vajkoczy, P. Metastatic dissemination patterns of different primary tumors to the spine and other bones. Clin Exp Metastasis 2019, 36, 493–498. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Guha, P.; Heatherton, K.R.; O’Connell, K.P.; Alexander, I.S.; Katz, S.C. Assessing the Future of Solid Tumor Immunotherapy. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- Trosko, J.E. On the potential origin and characteristics of cancer stem cells. Carcinogenesis 2021, 42, 905–912. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal 2020, 18, 59. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Medicine 2023, 12, 11149–11165. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, A.; Chelstowska, B.; Olejarz, W.; Nowicka, G. Communication in the Cancer Microenvironment as a Target for Therapeutic Interventions. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther 2021, 221, 107753. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr Biol 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Pradip, D.; Jennifer, A.; Nandini, D. Cancer-Associated Fibroblasts in Conversation with Tumor Cells in Endometrial Cancers: A Partner in Crime. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Y.; Wu, B.; Li, Y.; Liu, Z.; Liu, Z.; Gao, Y.; Gao, L.; Song, Q.; Zheng, Z.; et al. Irradiation enhances the malignancy-promoting behaviors of cancer-associated fibroblasts. Front Oncol 2022, 12, 965660. [Google Scholar] [CrossRef]
- Louault, K.; Li, R.R.; DeClerck, Y.A. Cancer-Associated Fibroblasts: Understanding Their Heterogeneity. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Ansems, M.; Span, P.N. The tumor microenvironment and radiotherapy response; a central role for cancer-associated fibroblasts. Clin Transl Radiat Oncol 2020, 22, 90–97. [Google Scholar] [CrossRef]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef]
- Berzaghi, R.; Gundersen, K.; Dille Pedersen, B.; Utne, A.; Yang, N.; Hellevik, T.; Martinez-Zubiaurre, I. Immunological signatures from irradiated cancer-associated fibroblasts. Front Immunol 2024, 15, 1433237. [Google Scholar] [CrossRef]
- Davidson, S.; Coles, M.; Thomas, T.; Kollias, G.; Ludewig, B.; Turley, S.; Brenner, M.; Buckley, C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol 2021, 21, 704–717. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front Immunol 2019, 10, 1835. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.T.; Yang, J. Epithelial–mesenchymal transition in tumor metastasis. Molecular Oncology 2017, 11, 28–39. [Google Scholar] [CrossRef]
- Dumont, N.; Liu, B.; Defilippis, R.A.; Chang, H.; Rabban, J.T.; Karnezis, A.N.; Tjoe, J.A.; Marx, J.; Parvin, B.; Tlsty, T.D. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia 2013, 15, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Duda, D.G.; Duyverman, A.M.; Kohno, M.; Snuderl, M.; Steller, E.J.; Fukumura, D.; Jain, R.K. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A 2010, 107, 21677–21682. [Google Scholar] [CrossRef]
- Hellevik, T.; Berzaghi, R.; Lode, K.; Islam, A.; Martinez-Zubiaurre, I. Immunobiology of cancer-associated fibroblasts in the context of radiotherapy. J Transl Med 2021, 19, 437. [Google Scholar] [CrossRef]
- Ohlund, D.; Elyada, E.; Tuveson, D. Fibroblast heterogeneity in the cancer wound. J Exp Med 2014, 211, 1503–1523. [Google Scholar] [CrossRef]
- Dominguez, C.X.; Muller, S.; Keerthivasan, S.; Koeppen, H.; Hung, J.; Gierke, S.; Breart, B.; Foreman, O.; Bainbridge, T.W.; Castiglioni, A.; et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15(+) Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov 2020, 10, 232–253. [Google Scholar] [CrossRef]
- Ohlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Guo, T.; Xu, J. Cancer-associated fibroblasts: a versatile mediator in tumor progression, metastasis, and targeted therapy. Cancer Metastasis Rev 2024, 43, 1095–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: molecular mechanisms and interventional targets. Signal Transduct Target Ther 2024, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol 2019, 12, 76. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Karimova, A.F.; Khalitova, A.R.; Suezov, R.; Markov, N.; Mukhamedshina, Y.; Rizvanov, A.A.; Huber, M.; Simon, H.U.; Brichkina, A. Immunometabolism of tumor-associated macrophages: A therapeutic perspective. Eur J Cancer 2025, 220, 115332. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Mo, H.; Hu, X.; Gao, R.; Zhao, Y.; Liu, B.; Niu, L.; Sun, X.; Yu, X.; et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell 2021, 39, 1578–1593 e1578. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol 2020, 11, 583084. [Google Scholar] [CrossRef]
- Basak, U.; Sarkar, T.; Mukherjee, S.; Chakraborty, S.; Dutta, A.; Dutta, S.; Nayak, D.; Kaushik, S.; Das, T.; Sa, G. Tumor-associated macrophages: an effective player of the tumor microenvironment. Front Immunol 2023, 14, 1295257. [Google Scholar] [CrossRef]
- Pollard, J.W. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol 2008, 84, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Pittet, M.J.; Michielin, O.; Migliorini, D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol 2022, 19, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, S.; Pandha, H.S.; Morgan, R. Antiangiogenic effects of zoledronate on cancer neovasculature. Future Oncol 2011, 7, 1325–1333. [Google Scholar] [CrossRef]
- Lin, E.Y.; Pollard, J.W. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res 2007, 67, 5064–5066. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Chen, X.W.; Yu, T.J.; Zhang, J.; Li, Y.; Chen, H.L.; Yang, G.F.; Yu, W.; Liu, Y.Z.; Liu, X.X.; Duan, C.F.; et al. CYP4A in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene 2017, 36, 5045–5057. [Google Scholar] [CrossRef]
- Su, S.; Liu, Q.; Chen, J.; Chen, J.; Chen, F.; He, C.; Huang, D.; Wu, W.; Lin, L.; Huang, W.; et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 2014, 25, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Sangaletti, S.; Di Carlo, E.; Gariboldi, S.; Miotti, S.; Cappetti, B.; Parenza, M.; Rumio, C.; Brekken, R.A.; Chiodoni, C.; Colombo, M.P. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer Res 2008, 68, 9050–9059. [Google Scholar] [CrossRef]
- Barker, T.H.; Baneyx, G.; Cardo-Vila, M.; Workman, G.A.; Weaver, M.; Menon, P.M.; Dedhar, S.; Rempel, S.A.; Arap, W.; Pasqualini, R.; et al. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J Biol Chem 2005, 280, 36483–36493. [Google Scholar] [CrossRef]
- Bradshaw, A.D. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal 2009, 3, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.L.; Patel, R.K.; Anderson, A.N.; Bowden, S.G.; Whalen, R.; Giske, N.R.; Wong, M.H. Circulating Cells with Macrophage-like Characteristics in Cancer: The Importance of Circulating Neoplastic-Immune Hybrid Cells in Cancer. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Bates, M.; Mohamed, B.M.; Ward, M.P.; Kelly, T.E.; O’Connor, R.; Malone, V.; Brooks, R.; Brooks, D.; Selemidis, S.; Martin, C.; et al. Circulating tumour cells: The Good, the Bad and the Ugly. Biochim Biophys Acta Rev Cancer 2023, 1878, 188863. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Deng, Y.; Im, J.H.; Muschel, R.J.; Zou, Y.; Li, J.; Lang, R.A.; Pollard, J.W. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 2009, 4, e6562. [Google Scholar] [CrossRef]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, M.I.N. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Del Prete, A.; Salvi, V.; Soriani, A.; Laffranchi, M.; Sozio, F.; Bosisio, D.; Sozzani, S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cellular & Molecular Immunology 2023, 20, 432–447. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Anandasabapathy, N. The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol 2021, 52, 101481. [Google Scholar] [CrossRef] [PubMed]
- Stokic-Trtica, V.; Diefenbach, A.; Klose, C.S.N. NK Cell Development in Times of Innate Lymphoid Cell Diversity. Front Immunol 2020, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Coenon, L.; Geindreau, M.; Ghiringhelli, F.; Villalba, M.; Bruchard, M. Natural Killer cells at the frontline in the fight against cancer. Cell Death Dis 2024, 15, 614. [Google Scholar] [CrossRef]
- Terren, I.; Borrego, F. Role of NK Cells in Tumor Progression. Exp Suppl 2022, 113, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Dorner, B.G.; Smith, H.R.; French, A.R.; Kim, S.; Poursine-Laurent, J.; Beckman, D.L.; Pingel, J.T.; Kroczek, R.A.; Yokoyama, W.M. Coordinate expression of cytokines and chemokines by NK cells during murine cytomegalovirus infection. J Immunol 2004, 172, 3119–3131. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, B.; Bello, A.B.; Moon, J.J.; Arai, Y.; Lee, S.H. Regenerative Functions of Regulatory T Cells and Current Strategies Utilizing Mesenchymal Stem Cells in Immunomodulatory Tissue Regeneration. Tissue Eng Regen Med 2025, 22, 167–180. [Google Scholar] [CrossRef]
- Goldmann, O.; Nwofor, O.V.; Chen, Q.; Medina, E. Mechanisms underlying immunosuppression by regulatory cells. Front Immunol 2024, 15, 1328193. [Google Scholar] [CrossRef]
- Terme, M.; Chaput, N.; Combadiere, B.; Ma, A.; Ohteki, T.; Zitvogel, L. Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells. J Immunol 2008, 180, 4679–4686. [Google Scholar] [CrossRef]
- Zhang, A.; Fan, T.; Liu, Y.; Yu, G.; Li, C.; Jiang, Z. Regulatory T cells in immune checkpoint blockade antitumor therapy. Mol Cancer 2024, 23, 251. [Google Scholar] [CrossRef]
- Miggelbrink, A.M.; Jackson, J.D.; Lorrey, S.J.; Srinivasan, E.S.; Waibl-Polania, J.; Wilkinson, D.S.; Fecci, P.E. CD4 T-Cell Exhaustion: Does It Exist and What Are Its Roles in Cancer? Clin Cancer Res 2021, 27, 5742–5752. [Google Scholar] [CrossRef] [PubMed]
- Davern, M.; Lysaght, J. Cooperation between chemotherapy and immunotherapy in gastroesophageal cancers. Cancer Lett 2020, 495, 89–99. [Google Scholar] [CrossRef]
- Pallares, R.M.; Abergel, R.J. Nanoparticles for targeted cancer radiotherapy. Nano Research 2020, 13, 2887–2897. [Google Scholar] [CrossRef]
- Marotta, C.B.; Haber, T.; Berlin, J.M.; Grubbs, R.H. Surgery-Guided Removal of Ovarian Cancer Using Up-Converting Nanoparticles. ACS Appl Mater Interfaces 2020, 12, 48371–48379. [Google Scholar] [CrossRef]
- Kitsios, K.; Sharifi, S.; Mahmoudi, M. Nanomedicine Technologies for Diagnosis and Treatment of Breast Cancer. ACS Pharmacology & Translational Science 2023, 6, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Paez-Munoz, J.M.; Gamez, F.; Fernandez-Afonso, Y.; Gallardo, R.; Pernia Leal, M.; Gutierrez, L.; de la Fuente, J.M.; Caro, C.; Garcia-Martin, M.L. Optimization of iron oxide nanoparticles for MRI-guided magnetic hyperthermia tumor therapy: reassessing the role of shape in their magnetocaloric effect. J Mater Chem B 2023, 11, 11110–11120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: a theranostic nanotool against cancer. Drug Discov Today 2015, 20, 1143–1151. [Google Scholar] [CrossRef]
- Xulu, J.H.; Ndongwe, T.; Ezealisiji, K.M.; Tembu, V.J.; Mncwangi, N.P.; Witika, B.A.; Siwe-Noundou, X. The Use of Medicinal Plant-Derived Metallic Nanoparticles in Theranostics. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Caro, C.; Guzzi, C.; Moral-Sanchez, I.; Urbano-Gamez, J.D.; Beltran, A.M.; Garcia-Martin, M.L. Smart Design of ZnFe and ZnFe@Fe Nanoparticles for MRI-Tracked Magnetic Hyperthermia Therapy: Challenging Classical Theories of Nanoparticles Growth and Nanomagnetism. Adv Healthc Mater 2024, 13, e2304044. [Google Scholar] [CrossRef]
- Caro, C.; Paez-Munoz, J.M.; Pernia Leal, M.; Carayol, M.; Feijoo-Cuaresma, M.; Garcia-Martin, M.L. Metabolically-Driven Active Targeting of Magnetic Nanoparticles Functionalized with Glucuronic Acid to Glioblastoma: Application to MRI-Tracked Magnetic Hyperthermia Therapy. Adv Healthc Mater 2025, 14, e2404391. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct Target Ther 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct Target Ther 2024, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Gamez, J.D.; Guzzi, C.; Bernal, M.; Solivera, J.; Martinez-Zubiaurre, I.; Caro, C.; Garcia-Martin, M.L. Tumor versus Tumor Cell Targeting in Metal-Based Nanoparticles for Cancer Theranostics. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T. Smart drug delivery systems: back to the future vs. clinical reality. Int J Pharm 2013, 454, 527–529. [Google Scholar] [CrossRef]
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat Nanotechnol 2019, 14, 1007–1017. [Google Scholar] [CrossRef]
- Prasad, R.; Ghosh, A.; Patel, V.; Peng, B.; Mendes, B.B.; Win, E.H.A.; Delogu, L.G.; Wong, J.Y.; Pischel, K.J.; Bellare, J.R.; et al. Voices of Nanomedicine: Blueprint Guidelines for Collaboration in Addressing Global Unmet Medical Needs. ACS Nano 2025, 19, 2979–2991. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef]
- Liu, J.; Kang, S.G.; Wang, P.; Wang, Y.; Lv, X.; Liu, Y.; Wang, F.; Gu, Z.; Yang, Z.; Weber, J.K.; et al. Molecular mechanism of Gd@C(82)(OH)(22) increasing collagen expression: Implication for encaging tumor. Biomaterials 2018, 152, 24–36. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, J.; Li, W.; Yang, W.; Qin, L.; Pan, Y. Gold nanoparticles enhance cisplatin delivery and potentiate chemotherapy by decompressing colorectal cancer vessels. Int J Nanomedicine 2018, 13, 6207–6221. [Google Scholar] [CrossRef]
- Caro, C.; Pourmadadi, M.; Eshaghi, M.M.; Rahmani, E.; Shojaei, S.; Paiva-Santos, A.C.; Rahdar, A.; Behzadmehr, R.; García-Martín, M.L.; Díez-Pascual, A.M. Nanomaterials loaded with Quercetin as an advanced tool for cancer treatment. Journal of Drug Delivery Science and Technology 2022, 78, 103938. [Google Scholar] [CrossRef]
- Hossen, M.N.; Rao, G.; Dey, A.; Robertson, J.D.; Bhattacharya, R.; Mukherjee, P. Gold Nanoparticle Transforms Activated Cancer-Associated Fibroblasts to Quiescence. ACS Appl Mater Interfaces 2019, 11, 26060–26068. [Google Scholar] [CrossRef]
- Xia, C.; Pan, J.; Wang, J.; Pu, Y.; Zhang, Q.; Hu, S.; Hu, Q.; Wang, Y. Functional blockade of cancer-associated fibroblasts with ultrafine gold nanomaterials causes an unprecedented bystander antitumoral effect. Nanoscale 2020, 12, 19833–19843. [Google Scholar] [CrossRef] [PubMed]
- Grinde, M.T.; Vik, J.; Camilio, K.A.; Martinez-Zubiaurre, I.; Hellevik, T. Ionizing radiation abrogates the pro-tumorigenic capacity of cancer-associated fibroblasts co-implanted in xenografts. Sci Rep 2017, 7, 46714. [Google Scholar] [CrossRef]
- Bromma, K.; Cicon, L.; Beckham, W.; Chithrani, D.B. Gold nanoparticle mediated radiation response among key cell components of the tumour microenvironment for the advancement of cancer nanotechnology. Sci Rep 2020, 10, 12096. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, A.; Di Silvio, D.; Llarena, I.; Murray, R.A.; Marelli, M.; Lay, L.; Polito, L.; Moya, S.E. Influence of surface coating on the intracellular behaviour of gold nanoparticles: a fluorescence correlation spectroscopy study. Nanoscale 2017, 9, 14730–14739. [Google Scholar] [CrossRef]
- Alhussan, A.; Bromma, K.; Bozdogan, E.P.D.; Metcalfe, A.; Karasinska, J.; Beckham, W.; Alexander, A.S.; Renouf, D.J.; Schaeffer, D.F.; Chithrani, D.B. Investigation of Nano-Bio Interactions within a Pancreatic Tumor Microenvironment for the Advancement of Nanomedicine in Cancer Treatment. Curr Oncol 2021, 28, 1962–1979. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials 2016, 1, 16014. [Google Scholar] [CrossRef]
- Pernia Leal, M.; Caro, C.; Garcia-Martin, M.L. Shedding light on zwitterionic magnetic nanoparticles: limitations for in vivo applications. Nanoscale 2017, 9, 8176–8184. [Google Scholar] [CrossRef] [PubMed]
- Alhussan, A.; Jackson, N.; Calisin, R.; Morgan, J.; Beckham, W.; Chithrani, D.B. Utilizing Gold Nanoparticles as Prospective Radiosensitizers in 3D Radioresistant Pancreatic Co-Culture Model. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Kovacs, D.; Igaz, N.; Marton, A.; Ronavari, A.; Belteky, P.; Bodai, L.; Spengler, G.; Tiszlavicz, L.; Razga, Z.; Hegyi, P.; et al. Core-shell nanoparticles suppress metastasis and modify the tumour-supportive activity of cancer-associated fibroblasts. J Nanobiotechnology 2020, 18, 18. [Google Scholar] [CrossRef]
- Christou, E.; Pearson, J.R.; Beltran, A.M.; Fernandez-Afonso, Y.; Gutierrez, L.; de la Fuente, J.M.; Gamez, F.; Garcia-Martin, M.L.; Caro, C. Iron-Gold Nanoflowers: A Promising Tool for Multimodal Imaging and Hyperthermia Therapy. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Boluda, A.; Vaquero, J.; Laurent, G.; Renault, G.; Bazzi, R.; Donnadieu, E.; Roux, S.; Fouassier, L.; Gazeau, F. Photothermal Depletion of Cancer-Associated Fibroblasts Normalizes Tumor Stiffness in Desmoplastic Cholangiocarcinoma. ACS Nano 2020, 14, 5738–5753. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, M.; Li, S.; Zhang, Z.; Sun, H.; Yang, F.; Liang, H. Developing a Novel Anticancer Gold(III) Agent to Integrate Chemotherapy and Immunotherapy. J Med Chem 2021, 64, 6777–6791. [Google Scholar] [CrossRef]
- Mulens-Arias, V.; Nicolas-Boluda, A.; Pinto, A.; Balfourier, A.; Carn, F.; Silva, A.K.A.; Pocard, M.; Gazeau, F. Tumor-Selective Immune-Active Mild Hyperthermia Associated with Chemotherapy in Colon Peritoneal Metastasis by Photoactivation of Fluorouracil-Gold Nanoparticle Complexes. ACS Nano 2021, 15, 3330–3348. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Ruan, S.; Liu, J.; Qin, L.; Yang, C.; Tong, F.; Lei, T.; Shevtsov, M.; Gao, H.; Qin, Y. Furin-instructed aggregated gold nanoparticles for re-educating tumor associated macrophages and overcoming breast cancer chemoresistance. Biomaterials 2021, 275, 120891. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Chang, L.C.; Song, W.H.; Yang, L.X.; Wang, L.C.; Chia, Z.C.; Chin, Y.C.; Shan, Y.S.; Huang, C.C.; Yeh, C.S. Polyaniline-Based Glyco-Condensation on Au Nanoparticles Enhances Immunotherapy in Lung Cancer. ACS Appl Mater Interfaces 2022, 14, 24144–24159. [Google Scholar] [CrossRef]
- Hao, Y.; Duan, F.; Dong, X.; Bi, R.; Wang, Y.; Zhu, S.; Hu, J. Gold Nanoparticle Inhibits the Tumor-Associated Macrophage M2 Polarization by Inhibiting m(6)A Methylation-Dependent ATG5/Autophagy in Prostate Cancer. Anal Cell Pathol (Amst) 2025, 2025, 6648632. [Google Scholar] [CrossRef]
- Sykes, E.A.; Chen, J.; Zheng, G.; Chan, W.C. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano 2014, 8, 5696–5706. [Google Scholar] [CrossRef]
- Nath, A.; Pal, R.; Singh, L.M.; Saikia, H.; Rahaman, H.; Ghosh, S.K.; Mazumder, R.; Sengupta, M. Gold-manganese oxide nanocomposite suppresses hypoxia and augments pro-inflammatory cytokines in tumor associated macrophages. Int Immunopharmacol 2018, 57, 157–164. [Google Scholar] [CrossRef]
- Du, C.; Jiang, J.; Wan, C.; Pan, G.; Kong, F.; Zhai, R.; Hu, C.; Ying, H. AntiPD-L1 antibody conjugated Au-SPIOs nanoplatform for enhancing radiosensitivity and triggering anti-tumor immune response. Sci Rep 2022, 12, 19542. [Google Scholar] [CrossRef]
- Huang, A.; Li, Q.; Shi, X.; Gao, J.; Ma, Y.; Ding, J.; Hua, S.; Zhou, W. An iron-containing nanomedicine for inducing deep tumor penetration and synergistic ferroptosis in enhanced pancreatic cancer therapy. Mater Today Bio 2024, 27, 101132. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef]
- Lee, C.; Kim, G.R.; Yoon, J.; Kim, S.E.; Yoo, J.S.; Piao, Y. In vivo delineation of glioblastoma by targeting tumor-associated macrophages with near-infrared fluorescent silica coated iron oxide nanoparticles in orthotopic xenografts for surgical guidance. Sci Rep 2018, 8, 11122. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Dong, Z.; Fu, Y.; Gong, T.; Deng, L.; Zhang, Z. Hyaluronic acid modified doxorubicin loaded Fe(3)O(4) nanoparticles effectively inhibit breast cancer metastasis. J Mater Chem B 2019, 7, 5861–5872. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, Y.; Zheng, R.; Xu, K.; Yan, J.; Song, P.; Wang, Y.; Rauf, A.; Pan, Y.; Zhang, H. Immunomodulation of Tumor Microenvironment by Arginine-Loaded Iron Oxide Nanoparticles for Gaseous Immunotherapy. ACS Appl Mater Interfaces 2021, 13, 19825–19835. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; He, Y.; Wu, C.; Zhang, M.; Gu, Z.; Zhang, J.; Liu, E.; Xu, Q.; Asrorov, A.M.; Huang, Y. Magnetism-mediated targeting hyperthermia-immunotherapy in “cold” tumor with CSF1R inhibitor. Theranostics 2021, 11, 6860–6872. [Google Scholar] [CrossRef]
- Nascimento, C.; Castro, F.; Domingues, M.; Lage, A.; Alves, E.; de Oliveira, R.; de Melo, C.; Eduardo Calzavara-Silva, C.; Sarmento, B. Reprogramming of tumor-associated macrophages by polyaniline-coated iron oxide nanoparticles applied to treatment of breast cancer. Int J Pharm 2023, 636, 122866. [Google Scholar] [CrossRef]
- Hu, A.; Pu, Y.; Xu, N.; Cai, Z.; Sun, R.; Fu, S.; Jin, R.; Guo, Y.; Ai, H.; Nie, Y.; et al. Controlled intracellular aggregation of magnetic particles improves permeation and retention for magnetic hyperthermia promotion and immune activation. Theranostics 2023, 13, 1454–1469. [Google Scholar] [CrossRef]
- Darya, G.H.; Zare, O.; Karbalaei-Heidari, H.R.; Zeinali, S.; Sheardown, H.; Rastegari, B. Enzyme-responsive mannose-grafted magnetic nanoparticles for breast and liver cancer therapy and tumor-associated macrophage immunomodulation. Expert Opin Drug Deliv 2024, 21, 663–677. [Google Scholar] [CrossRef]
- Arnosa-Prieto, A.; Diaz-Rodriguez, P.; Gonzalez-Gomez, M.A.; Garcia-Acevedo, P.; de Castro-Alves, L.; Pineiro, Y.; Rivas, J. Magnetic-driven Interleukin-4 internalization promotes magnetic nanoparticle morphology and size-dependent macrophage polarization. J Colloid Interface Sci 2024, 655, 286–295. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Guo, Z.; Hu, Z.; Yin, Y.; Duan, S.; Jia, W.; Lu, W.; Hu, J. Fe(3)O(4) Nanoparticles That Modulate the Polarisation of Tumor-Associated Macrophages Synergize with Photothermal Therapy and Immunotherapy (PD-1/PD-L1 Inhibitors) to Enhance Anti-Tumor Therapy. Int J Nanomedicine 2024, 19, 7185–7200. [Google Scholar] [CrossRef] [PubMed]
- Roudi, R.; Pisani, L.; Pisani, F.; Kiru, L.; Daldrup-Link, H.E. Novel Clinically Translatable Iron Oxide Nanoparticle for Monitoring Anti-CD47 Cancer Immunotherapy. Invest Radiol 2024, 59, 391–403. [Google Scholar] [CrossRef]
- Zhan, S.; Cao, Z.; Li, J.; Chen, F.; Lai, X.; Yang, W.; Teng, Y.; Li, Z.; Zhang, W.; Xie, J. Iron Oxide Nanoparticles Induce Macrophage Secretion of ATP and HMGB1 to Enhance Irradiation-Led Immunogenic Cell Death. Bioconjug Chem 2025, 36, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Fu, Y.; Li, C.; Wu, Z.; Cao, W.; Hu, X.; Sun, X.; He, W.; Cao, X.; Ling, D.; et al. Metal-Organic Framework Nanoparticles for Ameliorating Breast Cancer-Associated Osteolysis. Nano Lett 2020, 20, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, Y.; Huang, X.; Shen, Y.; Zou, Q.; Yang, G.; Fu, L.; Liu, Q.; Luo, D. Photosensitive and dual-targeted chromium nanoparticle delivering small interfering RNA YTHDF1 for molecular-targeted immunotherapy in liver cancer. J Nanobiotechnology 2024, 22, 348. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Wang, X.; Xu, X. The axis of tumor-associated macrophages, extracellular matrix proteins, and cancer-associated fibroblasts in oncogenesis. Cancer Cell Int 2024, 24, 335. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Khafaga, A.F.; Gaballa, M.M.S.; Karam, R.; Shoulah, S.A.; Shamma, R.N.; Khalifa, N.E.; Farrag, N.E.; Noreldin, A.E. Synergistic therapeutic strategies and engineered nanoparticles for anti-vascular endothelial growth factor therapy in cancer. Life Sci 2024, 341, 122499. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Wang, Q.; Ni, N.; Tee, J.K.; Ariga, K.; Ke, P.C.; Ho, H.K.; Wang, Y.; Leong, D.T. Engineering tumoral vascular leakiness with gold nanoparticles. Nat Commun 2023, 14, 4269. [Google Scholar] [CrossRef]
- Luo, L.; Qin, B.; Jiang, M.; Xie, L.; Luo, Z.; Guo, X.; Zhang, J.; Li, X.; Zhu, C.; Du, Y.; et al. Regulating immune memory and reversing tumor thermotolerance through a step-by-step starving-photothermal therapy. J Nanobiotechnology 2021, 19, 297. [Google Scholar] [CrossRef]
- Xu, X.; Gan, M.; Ge, Y.; Yi, C.; Feng, T.; Liu, M.; Wu, C.; Chen, X.; Zhang, W.; Zhao, L.; et al. Multifaceted glycoadjuvant@AuNPs inhibits tumor metastasis through promoting T cell activation and remodeling tumor microenvironment. J Nanobiotechnology 2021, 19, 376. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Li, L.; Guo, R.; Shi, X.; Cao, X. Effective CpG Delivery Using Zwitterion-Functionalized Dendrimer-Entrapped Gold Nanoparticles to Promote T Cell-Mediated Immunotherapy of Cancer Cells. Biosensors (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Felix-Pina, P.; Franco Molina, M.A.; Garcia Coronado, P.L.; Prado-Garcia, H.; Zarate-Trivino, D.G.; Castro-Valenzuela, B.E.; Moreno-Amador, K.A.; Uscanga Palomeque, A.C.; Rodriguez Padilla, C. beta-D-Glucose-Reduced Silver Nanoparticles Remodel the Tumor Microenvironment in a Murine Model of Triple-Negative Breast Cancer. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Sargsian, A.; Koutsoumpou, X.; Girmatsion, H.; Egil, C.; Buttiens, K.; Luci, C.R.; Soenen, S.J.; Manshian, B.B. Silver nanoparticle induced immunogenic cell death can improve immunotherapy. J Nanobiotechnology 2024, 22, 691. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Xia, Q.; Shang, J.; He, Y.; Li, Z.; Chen, Y.; Gao, F.; Yu, X.; Yuan, Z.; et al. Photothermal Fe(3)O(4) nanoparticles induced immunogenic ferroptosis for synergistic colorectal cancer therapy. J Nanobiotechnology 2024, 22, 630. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, G.; Sang, W.; Li, J.; Zhang, Z.; Li, W.; Yan, J.; Zhao, Q.; Dai, Y. Phenolic immunogenic cell death nanoinducer for sensitizing tumor to PD-1 checkpoint blockade immunotherapy. Biomaterials 2021, 269, 120638. [Google Scholar] [CrossRef]
- Lei, H.; Li, Q.; Li, G.; Wang, T.; Lv, X.; Pei, Z.; Gao, X.; Yang, N.; Gong, F.; Yang, Y.; et al. Manganese molybdate nanodots with dual amplification of STING activation for “cycle” treatment of metalloimmunotherapy. Bioact Mater 2024, 31, 53–62. [Google Scholar] [CrossRef]
- Lu, S.; Mi, Z.; Liu, P.; Ding, J.; Ma, Y.; Yang, J.; Rong, P.; Zhou, W. Repolarizing neutrophils via MnO(2) nanoparticle-activated STING pathway enhances Salmonella-mediated tumor immunotherapy. J Nanobiotechnology 2024, 22, 443. [Google Scholar] [CrossRef]
- Zhao, Q.; Gong, Z.; Li, Z.; Wang, J.; Zhang, J.; Zhao, Z.; Zhang, P.; Zheng, S.; Miron, R.J.; Yuan, Q.; et al. Target Reprogramming Lysosomes of CD8+ T Cells by a Mineralized Metal-Organic Framework for Cancer Immunotherapy. Adv Mater 2021, 33, e2100616. [Google Scholar] [CrossRef]
- Dai, L.; Yao, M.; Fu, Z.; Li, X.; Zheng, X.; Meng, S.; Yuan, Z.; Cai, K.; Yang, H.; Zhao, Y. Multifunctional metal-organic framework-based nanoreactor for starvation/oxidation improved indoleamine 2,3-dioxygenase-blockade tumor immunotherapy. Nat Commun 2022, 13, 2688. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Q.; Xu, R.; Lin, P.; Deng, G.; Xia, X. Combination of ferroptosis and pyroptosis dual induction by triptolide nano-MOFs for immunotherapy of Melanoma. J Nanobiotechnology 2023, 21, 383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Wang, W.J.; Zhang, C.Y.; Ling, Y.Y.; Hong, X.J.; Su, Q.; Li, W.G.; Mao, Z.W.; Cheng, B.; Tan, C.P.; et al. Ru(II)-modified TiO(2) nanoparticles for hypoxia-adaptive photo-immunotherapy of oral squamous cell carcinoma. Biomaterials 2022, 289, 121757. [Google Scholar] [CrossRef] [PubMed]
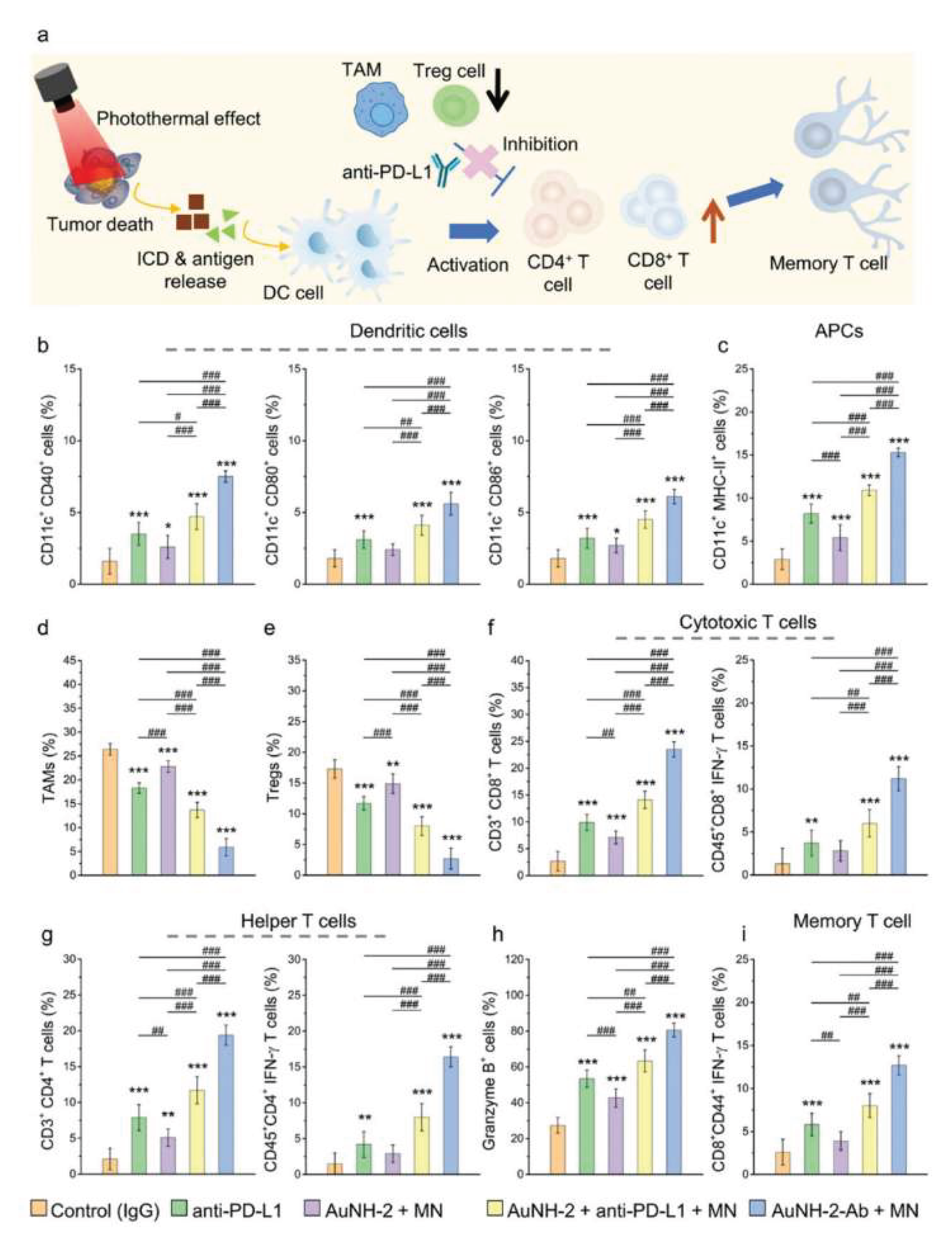
- Cheng, H.W.; Lee, W.; Hsu, F.T.; Lai, Y.H.; Huang, S.R.; Lim, C.S.H.; Lin, Z.K.; Hsu, S.C.; Chiang, C.S.; Jeng, L.B.; et al. Manipulating the Crosstalk between Cancer and Immunosuppressive Cells with Phototherapeutic Gold-Nanohut for Reprogramming Tumor Microenvironment. Adv Sci (Weinh) 2024, 11, e2404347. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Pozo, D. Polysaccharide Colloids as Smart Vehicles in Cancer Therapy. Curr Pharm Des 2015, 21, 4822–4836. [Google Scholar] [CrossRef]
- Li, C.; Clauson, R.; Bugada, L.F.; Ke, F.; He, B.; Yu, Z.; Chen, H.; Jacobovitz, B.; Hu, H.; Chuikov, P.; et al. Antigen-Clustered Nanovaccine Achieves Long-Term Tumor Remission by Promoting B/CD 4 T Cell Crosstalk. ACS Nano 2024, 18, 9584–9604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





