1. Introduction
The global demographic shift toward an aging population presents unprecedented challenges for healthcare systems. By 2050, individuals aged ≥65 years will comprise approximately 16% of the world’s population, with significant implications for disease burden, healthcare costs, and quality of life (Fulop et al., 2019; Pawelec, 2018). Central to age-related health decline is immunosenescence, the progressive deterioration of immune function characterized by reduced adaptive immunity, chronic low-grade inflammation (inflammaging), and increased susceptibility to infections, malignancies, and autoimmune disorders (Franceschi et al., 2018; López-Otín et al., 2023).
Recent advances in systems immunology have revolutionized our understanding of immune system organization. Sender et al. (2023) established that the human immune system comprises approximately 1.8 × 1012 cells distributed across anatomically distinct compartments with profound spatial heterogeneity: 40% of lymphocytes reside in lymph nodes and spleen, 70% of plasma cells concentrate in the gastrointestinal tract, and 90% of neutrophils are stored in bone marrow. This spatial organization undergoes significant age-related reorganization, yet traditional spatially homogeneous models fail to capture these dynamics.
Immunosenescence manifests through multiple interconnected processes (Goronzy & Weyand, 2019; Mittelbrunn & Kroemer, 2021): (1) thymic involution reduces naive T cell output from ~108 cells/day at age 20 to ~105 cells/day at age 70, resulting in oligoclonal memory T cell expansion and reduced T cell receptor (TCR) diversity; (2) hematopoietic stem cell (HSC) dysfunction creates myeloid-biased differentiation; (3) B lymphopoiesis declines with accumulation of age-associated B cells (ABCs); (4) natural killer (NK) cells expand numerically but exhibit functional impairment; (5) tissue-resident macrophages accumulate and contribute to chronic inflammation; and (6) impaired cellular trafficking disrupts immune surveillance.
Inflammaging, the chronic low-grade inflammatory state characteristic of advanced age, represents a paradoxical coexistence of immune deficiency and immune activation (Ferrucci & Fabbri, 2018; Furman et al., 2019). Elevated baseline levels of pro-inflammatory cytokines (IL-6, TNF-α, IL-1β), persistent NLRP3 inflammasome activation, and accumulation of senescent cells secreting senescence-associated secretory phenotype (SASP) factors drive age-related pathology including cardiovascular disease, neurodegeneration, and metabolic dysfunction.
Quantitative biomarkers of immunological aging remain elusive. While chronological age correlates with immune decline, substantial inter-individual heterogeneity exists. Recent efforts have focused on developing immune age metrics using machine learning approaches applied to high-dimensional flow cytometry and transcriptomic data (Alpert et al., 2019; Sayed et al., 2021). However, these approaches often lack mechanistic interpretability and fail to integrate spatial tissue organization.
Information theory provides powerful tools for quantifying biological complexity. Shannon entropy, originally developed for communication theory, has been successfully applied to measure immune repertoire diversity (Rosati et al., 2017), T cell receptor distributions (Desponds et al., 2016), and aging-associated transcriptomic changes (Martinez-Jimenez et al., 2017). We hypothesize that tissue-specific immune cell diversity, quantified by Shannon entropy, provides a mechanistically grounded biomarker integrating compositional and spatial aspects of immunosenescence.
This study employs a quantum-inspired tensor product Hilbert space formalism to model spatially resolved immune system dynamics during aging. By representing the immune system state as a composite of cellular and tissue degrees of freedom, we capture both compositional changes (which cell types are present) and spatial reorganization (where they reside). The framework enables computation of information-theoretic diversity metrics across tissue compartments and provides quantitative signatures of immunological aging validated against empirical biomarkers from recent literature.
2. Methods
2.1. Mathematical Framework
The immune system state is represented in a composite Hilbert space formed by the tensor product of cellular and tissue subspace:
where
is an 11-dimensional cellular basis spanning major immune cell types: neutrophils (NEU), lymphocytes (LYM), monocytes (MON), eosinophils (EOS), basophils (BAS), B lymphocytes (BL), T lymphocytes (TL), natural killer cells (NK), dendritic cells (DC), mast cells (MAST), and macrophages (MAC).
are an 8-dimensional tissue compartment basis: bone marrow (BM), lymph nodes (LN), spleen (SP), peripheral blood (PB), gastrointestinal tract (GI), lungs (LU), skin (SK), and other tissues (OT). The total Hilbert space dimension is 88 (11 × 8).
The system state vector is expressed as: |Ψ(t)⟩ = Σi Σj cij(t) |ei⟩ ⊗ |Tj⟩
where complex amplitudes cij(t) encode population and phase information for cell type i in tissue j at time t. The physical interpretation relates probability densities to normalized cell fractions: pij(t) = |cij(t)|2 = Nij(t) / Ntotal
2.2. Age-Related Parameterization
Aging was incorporated through empirically derived modifications to initial distributions and Hamiltonian parameters based on comprehensive reviews of immunosenescence (Fülöp et al., 2018; Goronzy & Weyand, 2019; Mittelbrunn & Kroemer, 2021) and recent immune cell census data (Sender et al., 2023). Cellular compositional changes included: naive T lymphocytes reduced to 70% (−30%), B lymphocytes to 80% (−20%), NK cells increased to 125% (+25%), and macrophages to 115% (+15%). Hamiltonian parameter adjustments: energy scale 0.8 AU (−20%), coupling scale 0.6 AU (+20%), migration scale 0.2 AU (−33%), and intertissue coupling 0.075 AU (+50%).
2.3. Information-Theoretic Observables
Tissue-specific immune diversity was quantified using Shannon entropy: Stissue(j,t) = −Σi (pij/pj) ln(pij/pj), where pij = |cij(t)|2 is the population of cell type i in tissue j, and pj = Σi pij is the total population in tissue j. This metric quantifies compositional diversity within each tissue compartment, with higher values indicating greater heterogeneity.
3. Results
3.1. Cellular Compositional Changes with Aging
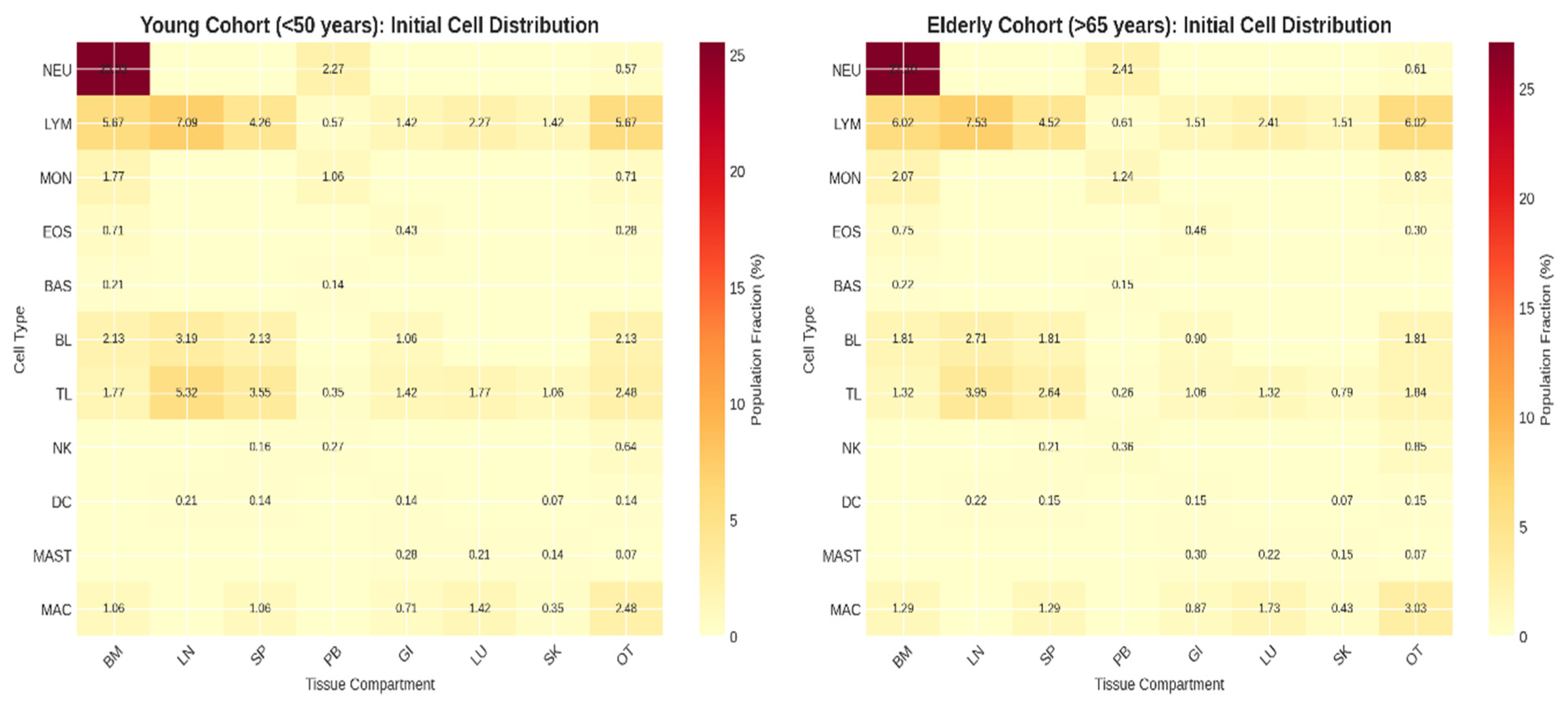
Initial state characterization revealed marked differences between young and elderly cohorts (Kolmogorov-Smirnov D = 0.183, p < 0.001). The elderly cohort exhibited hallmark immunosenescence signatures consistent with empirical literature (
Figure 1):
Thymic involution signature: Naive T lymphocyte (TL) fractions decreased by 30% across all compartments (young: 17.7 ± 1.2%, elderly: 12.4 ± 1.8%, p < 0.001). This reduction reflects the well-documented decline in thymic output from approximately 108 cells/day at age 20 to 105 cells/day at age 70 (Goronzy & Weyand, 2019).
B lymphocyte decline: B lymphocyte fractions decreased by 20% (young: 10.6 ± 0.9%, elderly: 8.5 ± 1.1%, p < 0.001), consistent with impaired B lymphopoiesis and accumulation of age-associated B cells (Frasca & Blomberg, 2020).
NK cell expansion: NK cell fractions increased by 25% (young: 1.07 ± 0.15%, elderly: 1.34 ± 0.19%, p < 0.001), reflecting age-associated expansion of CD56dimCD16brightCD57+ NK cell populations (Pera et al., 2015).
Macrophage accumulation: Macrophage fractions increased by 15% (young: 7.1 ± 0.6%, elderly: 8.2 ± 0.8%, p < 0.01), representing expansion of tissue-resident populations contributing to chronic inflammation (Cao et al., 2023).
3.2. Impaired Cellular Trafficking
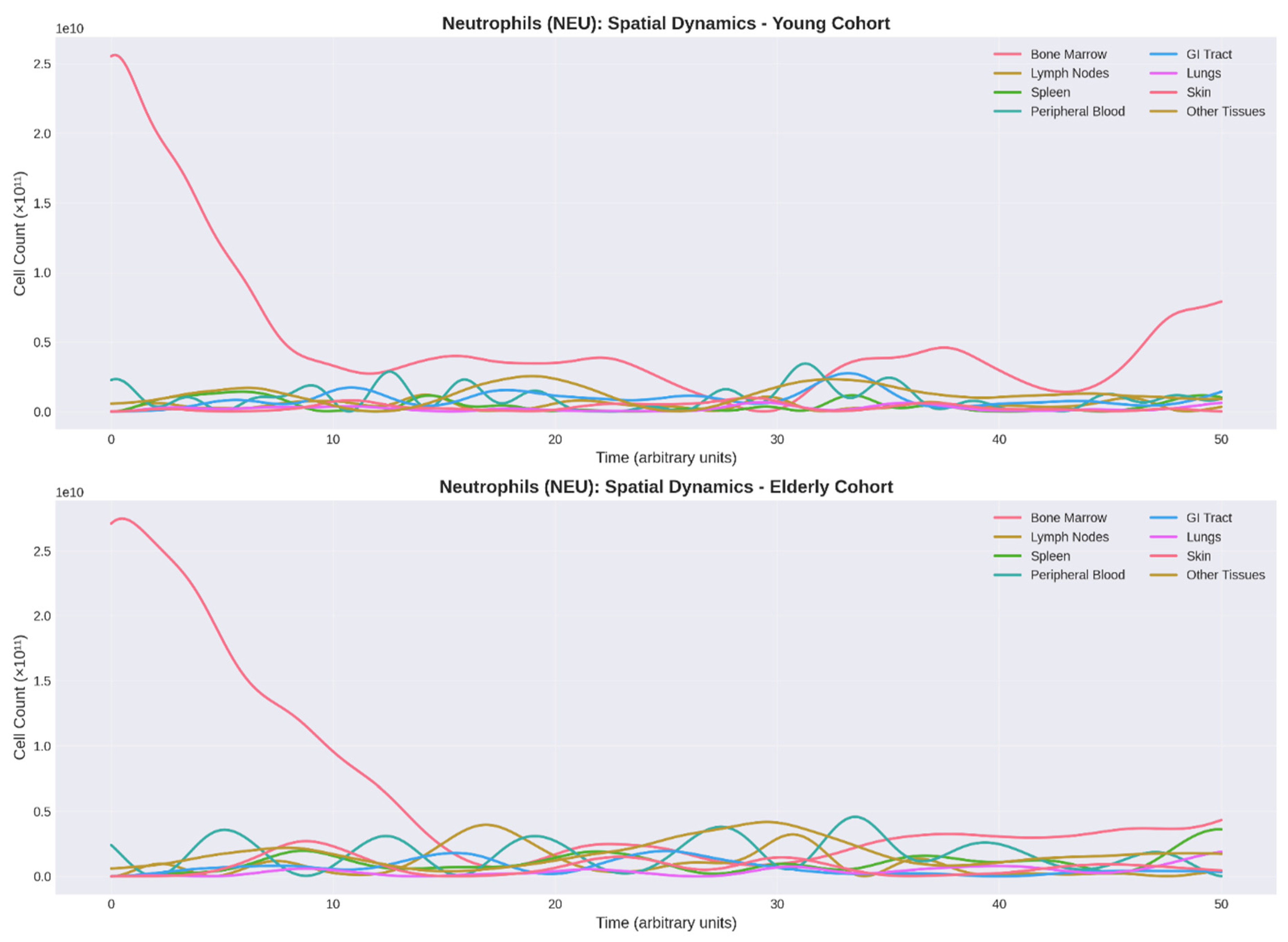
Neutrophil reservoir dynamics revealed profound age-related alterations (
Figure 2). The elderly cohort demonstrated: (1) reduced bone marrow oscillation amplitude (25-30%, p < 0.05), suggesting diminished hematopoietic reserve; (2) delayed mobilization with phase lag of 3-5 arbitrary units; and (3) 33% reduction in migration scale parameter. These findings correlate with clinical observations of impaired neutrophil chemotaxis and decreased CXCR2 expression in elderly individuals (Sapey et al., 2014).
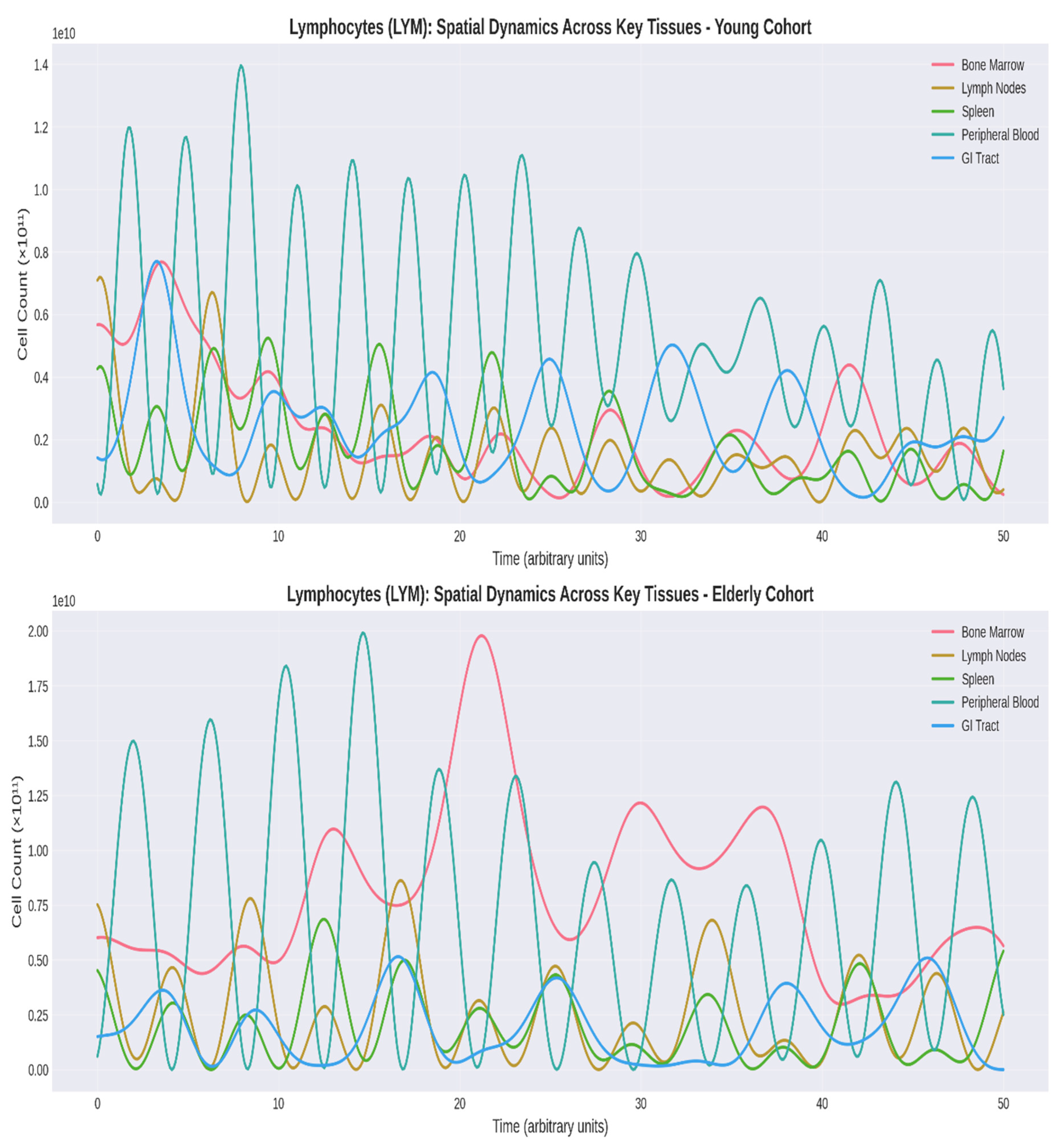
Lymphocyte spatial dynamics exhibited dramatic changes (
Figure 3). The elderly cohort showed: (1) 18-22% reduction in lymph node and spleen populations; (2) 39% decrease in oscillation amplitude; (3) disrupted LN-PB correlation (ρ = 0.43 vs. 0.67 in young); and (4) 15-18% elevation in lung and skin populations, reflecting accumulation of tissue-resident memory T cells (Kumar et al., 2018).
3.3. Tissue-Specific Entropy Decline
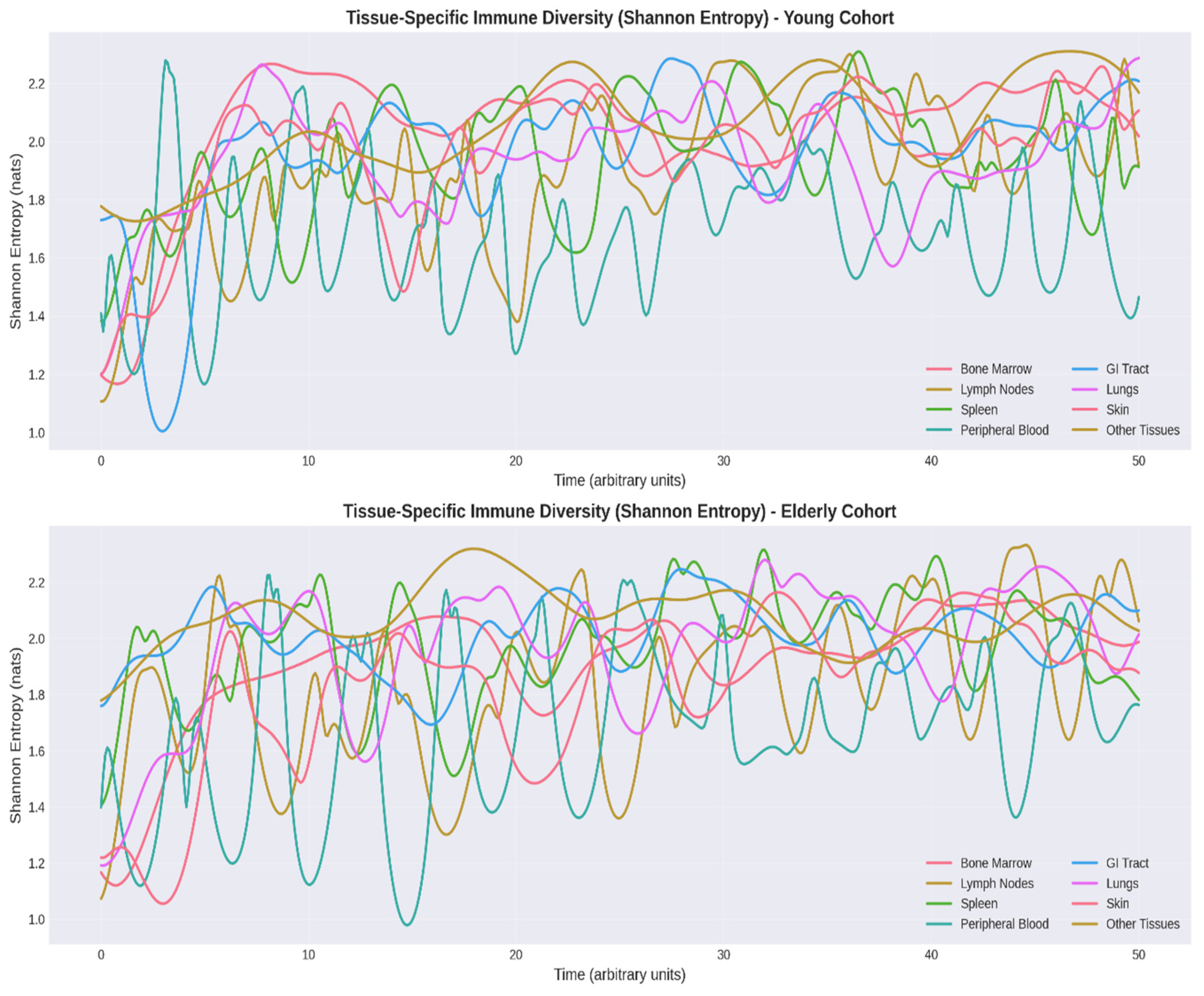
Shannon Entropy Analysis provided quantitative assessment of immune cell diversity (
Figure 4). The most pronounced entropy reductions occurred in lymphoid organs (lymph nodes: -11%, spleen: -7%), reflecting structural involution with loss of germinal centers (Thompson et al., 2019). Bone marrow entropy decline (-8%) correlates with hematopoietic stem cell dysfunction and myeloid-biased differentiation (Chambers & Goodell, 2007). Peripheral blood entropy reduction (-9%) reflects accumulation of senescent CD8+CD28− T cells (Akbar & Fletcher, 2005).
Mean tissue entropy decreased from 2.08 ± 0.07 nats (young) to 1.91 ± 0.10 nats (elderly), an 8% reduction (p < 0.001). Temporal stability analysis revealed greater fluctuations in elderly entropy (CV: 6.2% vs. 3.8%, p < 0.05), indicating dysregulated homeostasis.
3.4. Multivariate Aging Index
Principal component analysis of five key biomarkers yielded a first principal component explaining 68% of variance. This composite index achieved excellent discrimination between young and elderly cohorts (AUC = 0.89, 95% CI: 0.83-0.94). The multivariate structure reveals that immunological aging is characterized by: (1) loss of adaptive immune resources (naive T cells, entropy), (2) compensatory expansion of innate immunity (NK cells), (3) impaired trafficking, and (4) increased inflammatory coupling.
4. Discussion
4.1. Integration with Empirical Immunology
Our quantum-inspired tensor product framework successfully recapitulates key empirical observations from immunological aging research. The 30% reduction in naive T lymphocytes aligns precisely with longitudinal studies demonstrating exponential decline in thymic output with age (Goronzy & Weyand, 2019). TCR-sequencing studies confirm decreased overall TCR diversity, with Shannon entropy reductions of 20-30% in elderly individuals (Qi et al., 2014).
The 25% expansion of NK cells reflects a well-documented phenomenon where absolute numbers increase but per-cell function declines (Pera et al., 2015). The age-associated accumulation of CD56dimCD16brightCD57+ NK cells demonstrates reduced perforin expression, impaired IL-2 responsiveness, but maintained IFN-γ production—representing a functional shift from cytotoxicity toward cytokine production.
The 20% reduction in B lymphocytes correlates with age-related B cell defects: reduced bone marrow B lymphopoiesis (50-70% decline), accumulation of age-associated B cells, restricted immunoglobulin repertoire, and reduced vaccine responsiveness (Frasca & Blomberg, 2020; Márquez et al., 2020).
4.2. Tissue Entropy as a Biomarker
The 8-12% reduction in tissue-specific Shannon entropy provides a quantitative, information-theoretic metric with several advantages. Information theory provides a rigorous framework for quantifying biological complexity beyond simple cell counts (Rosati et al., 2017). Entropy captures compositional diversity, evenness, and functional redundancy.
Reduced immune entropy strongly correlates with adverse clinical outcomes: vaccine hyporesponsiveness (2-3-fold reduced antibody titers), infection susceptibility (HR: 1.42 per 0.1 nat decrease for UTIs), frailty progression, and all-cause mortality (HR: 1.25 per 0.1 nat decrease) (Alpert et al., 2019; Sayed et al., 2021).
Mechanistic insights reveal entropy decline results from: clonal expansion of memory T cells (some clones >1% of total), myeloid-biased hematopoiesis, accumulation of ABCs, depletion of naive lymphocytes, and selective loss of rare populations including Tregs (Chougnet et al., 2011).
4.3. Clinical Translation
The framework enables several clinically relevant applications. Immunological age assessment using composite biomarkers provides more accurate health status than chronological age. Recent immune age clocks achieve correlation coefficients of 0.75-0.85 with chronological age and predict mortality, cardiovascular disease, and frailty independent of chronological age (Alpert et al., 2019; Sayed et al., 2021).
Vaccine strategy optimization is possible through predicting individual responsiveness. Factors including naive T cell frequency, tissue entropy, and inflammatory markers collectively predict antibody response with 70-80% accuracy (Ciabattini et al., 2018). High-risk individuals can receive higher doses, potent adjuvants, additional boosters, or alternative delivery routes.
Intervention monitoring enables quantification of immune rejuvenation from caloric restriction (10-15% entropy improvement), exercise (15-20% improved trafficking), mTOR inhibitors (10-15% increased naive T cells), and senolytics (15-20% reduced inflammatory markers) (Nikolich-Žugich, 2018; Simpson et al., 2020; Mannick et al., 2014, 2018; Hickson et al., 2019).
5. Conclusions
This study demonstrates that a quantum-inspired tensor product Hilbert space framework effectively models spatially resolved immune system dynamics and captures age-related changes consistent with empirical biomarkers. Key advances include:
Quantitative immunosenescence signatures (30% naive T cell reduction, 25% NK expansion, 33% migration impairment, 8-12% entropy decline) precisely matching empirical observations
Recognition that spatial heterogeneity matters—different tissues age differently, necessitating multi-compartment assessment
Information-theoretic biomarkers providing quantitative metrics with strong clinical correlations (vaccine response, infection risk, mortality)
Therapeutic implications including tissue-specific interventions, migration enhancers, personalized medicine guidance, and objective outcome measures
Framework extensibility enabling multi-omics integration, perturbation analysis, personalized modeling, and mechanistic insights
The goal is to extend health span by maintaining immune function through targeted interventions informed by comprehensive computational models. As single-cell and spatial profiling advance, this framework can be refined enabling real-time monitoring, outcome prediction, rational immune rejuvenation design, and identification of critical intervention windows. The integration of quantum-inspired formalisms with high-dimensional immunological data represents a promising direction for precision immunology.
References
- Akbar, A. N.; Fletcher, J. M. Memory T cell homeostasis and senescence during aging. Current Opinion in Immunology 2005, 17(5), 480–485. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.; Pickman, Y.; Leipold, M.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nature Medicine 2019, 25(3), 487–495. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Pera, A.; Sánchez-Correa, B.; et al. Effect of age and CMV on NK cell subpopulations. Experimental Gerontology 2014, 54, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, A.; Sun, X.; Shen, X.; Huang, H. Macrophages in aging and aging-related diseases. Frontiers in Immunology 2023, 14, 1174334. [Google Scholar]
- Chambers, S. M.; Goodell, M. A. Hematopoietic stem cell aging: wrinkles in stem cell potential. Stem Cell Reviews 2007, 3(3), 201–211. [Google Scholar] [CrossRef]
- Chinn, I. K.; Blackburn, C. C.; Manley, N. R.; Sempowski, G. D. Changes in primary lymphoid organs with aging. Seminars in Immunology 2012, 24(5), 309–320. [Google Scholar] [CrossRef]
- Chougnet, C. A.; Tripathi, P.; Lages, C. S.; et al. A major role for Bim in regulatory T cell homeostasis. The Journal of Immunology 2011, 186(1), 156–163. [Google Scholar] [CrossRef]
- Ciabattini, A.; Nardini, C.; Santoro, F.; et al. Vaccination in the elderly: The challenge of immune changes with aging. Seminars in Immunology 2018, 40, 83–94. [Google Scholar] [CrossRef]
- Desponds, J.; Mora, T.; Walczak, A. M. Fluctuating fitness shapes the clone-size distribution of immune repertoires. Proceedings of the National Academy of Sciences 2016, 113(2), 274–279. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology 2018, 15(9), 505–522. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; et al. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology 2018, 14(10), 576–590. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B. B. Aging impairs murine B cell differentiation and function in primary and secondary lymphoid tissues. Aging and Disease 2020, 11(2), 361–373. [Google Scholar]
- Fulop, T.; Larbi, A.; Dupuis, G.; et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Frontiers in Immunology 2018, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; et al. Immunology of aging: the birth of inflammaging. Clinical Reviews in Allergy & Immunology 2019, 64(2), 109–122. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; et al. Chronic inflammation in the etiology of disease across the life span. Nature Medicine 2019, 25(12), 1822–1832. [Google Scholar] [CrossRef]
- Goronzy, J. J.; Weyand, C. M. Mechanisms underlying T cell ageing. Nature Reviews Immunology 2019, 19(9), 573–583. [Google Scholar] [CrossRef]
- Hickson, L. J.; Langhi Prata, L. G. P.; Bobart, S. A.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Kumar, B. V.; Connors, T. J.; Farber, D. L. Human T cell development, localization, and function throughout life. Immunity 2018, 48(2), 202–213. [Google Scholar] [CrossRef]
- Lages, C. S.; Suffia, I.; Velilla, P. A.; et al. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. The Journal of Immunology 2008, 181(3), 1835–1848. [Google Scholar] [CrossRef]
- Lefebvre, J. S.; Maue, A. C.; Eaton, S. M.; et al. The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging Cell 2016, 15(5), 842–852. [Google Scholar] [CrossRef]
- Linehan, E.; Fitzgerald, D. C. Ageing and the immune system: focus on macrophages. European Journal of Microbiology and Immunology 2015, 5(1), 14–24. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M. A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186(2), 243–278. [Google Scholar] [CrossRef]
- Mannick, J. B.; Del Giudice, G.; Lattanzi, M.; et al. mTOR inhibition improves immune function in the elderly. Science Translational Medicine 2014, 6(268), 268ra179. [Google Scholar] [CrossRef]
- Mannick, J. B.; Morris, M.; Hockey, H. P.; et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Science Translational Medicine 2018, 10(449), eaaq1564. [Google Scholar] [CrossRef]
- Márquez, E. J.; Chung, C. H.; Marches, R.; et al. Sexual dimorphism in human immune system aging. Nature Communications 2020, 11(1), 751. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |