Submitted:
09 January 2026
Posted:
09 January 2026
You are already at the latest version
Abstract
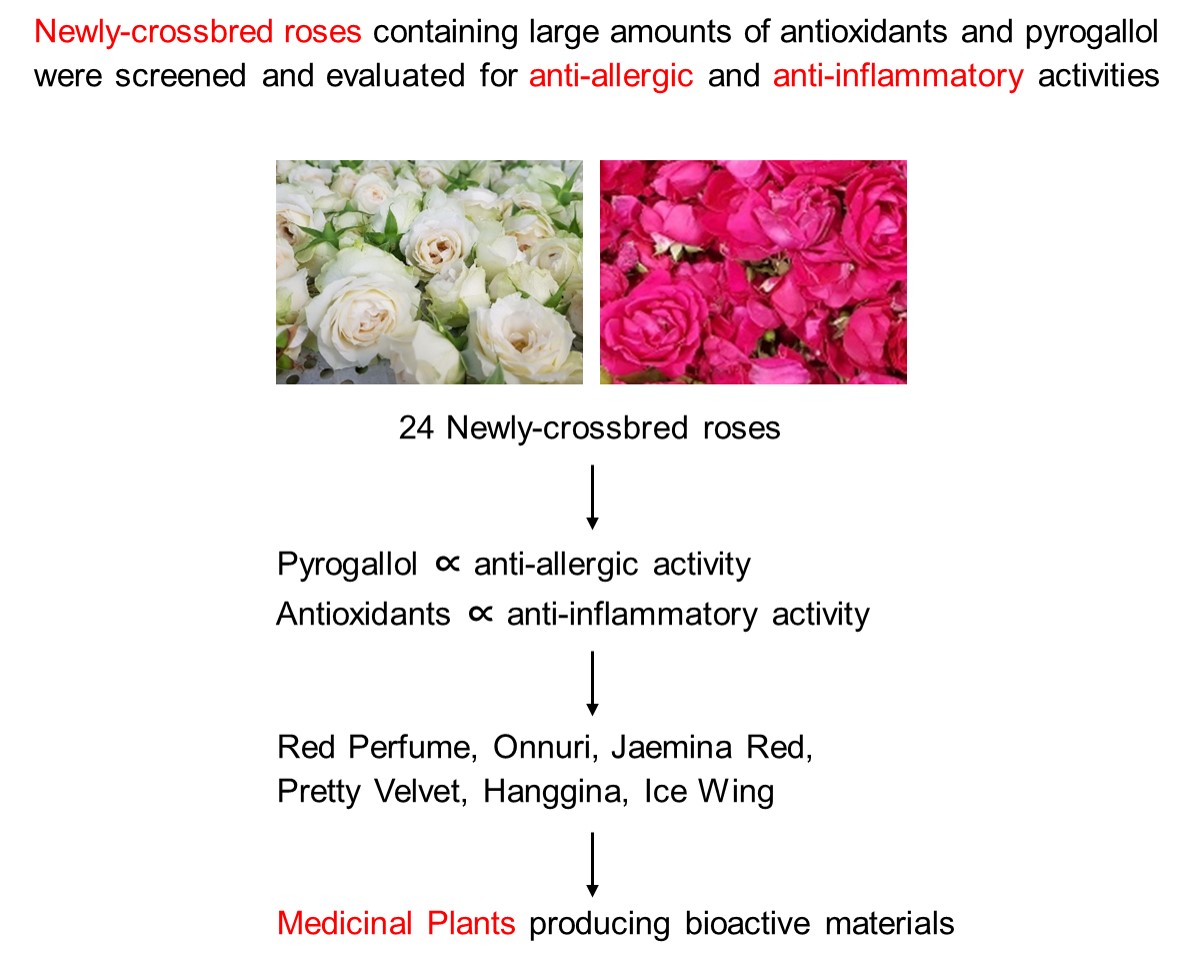
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of Rosebud Extracts
2.2. Analysis of Antioxidative Components
2.2.1. Analysis of Total Polyphenols
2.2.2. Analysis of Flavonoids
2.2.3. Analysis of Tannins
2.2.4. Analysis of Proanthocyanidins
2.2.5. Analysis of Pyrogallol (1,2,3-Benzenetriol)
2.3. Measurement of Mast Cell Degranulation
2.4. Measurement of Macrophage Nitric Oxide (NO) Production
2.5. Measurement of Mouse Allergic Reaction
2.5.1. Animals
2.5.2. Measurement of Systemic Allergic Reaction (Death from Shock)
2.5.3. Measurement of Local Allergic Reaction (Skin Itching)
2.5.4. Measurement of Blood IgE and Histamine
2.5.5. Measurement of Blood Cytokines
2.6. Statistical Analysis
3. Results
3.1. Antioxidant Contents in Rosebud Extracts
3.2. 1,2,3-Benzenetriol Content in Rosebud Extracts
3.3. Inhibition of Mast Cell Degranulation
3.4. Inhibition of Macrophage NO Production
3.5. Inhibition of Systemic Allergic Reaction
3.6. Inhibition of Skin Allergic Reaction
3.7. Inhibition of Blood IgE and Histamine
3.8. Inhibition of Blood Inflammatory Cytokines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Burns-Naas, L.A.; Meade, B.J.; Munson, A.E. Toxic response of the immune system. In The Basic Science of Poisons, 6th ed.; Klaassen, C.D., Ed.; McGraw Hill/Medical: Columbus, OH, USA, 2001; pp. 419–470. [Google Scholar]
- Hossen, M.A.; Fujii, Y.; Ogawa, M.; Takubo, M.; Tsumuro, T.; Kamei, C. Effect of loratadine on mouse models of atopic dermatitis associated pruritus. Int. Immunopharmacol. 2005, 5, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Noh, H.M.; Kweon, S.H.; Jo, E.H.; Jang, H.C.; Kim, H.K.; Park, H.J.; Kim, W.J.; Park, M.C. The study on the environmental factors of atopic dermatitis in oriental-western medicine. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2018, 31, 52–70. [Google Scholar]
- Jeon, J.H.; Kwon, S.C.; Park, D.; Shin, S.; Jang, M.J.; Joo, S.S.; Kang, H.; Kim, S.H.; Oh, J.Y.; Jeong, J.H.; et al. Effects of red and white rose petal extracts and Ganoderma lucidum culture on ovalbumin-induced atopic dermatitis. Lab. Anim. Res. 2008, 24, 347–354. [Google Scholar]
- Li, K. Itch in atopic dermatitis: From pathogenesis to treatment. Allergy Asthma Respir. Dis. 2014, 2, 8–15. [Google Scholar] [CrossRef]
- Trautmann, A.; Anders, D.; Stoevesandt, J. H1-Antihistamine premedication in NSAID-associated urticaria. J. Allergy Clin. Immunol. Pract. 2016, 4, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Korea Ministry of Food and Drug Safety. Regulation on the Evaluation of Functional Cosmetics. 2020. Available online: https://www.law.go.kr.
- Gorji, A. Pharmacological treatment of headache using tranditional Persian medicine. Trends Pharmacol. Sci. 2003, 24, 331–334. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, J.M.; Choi, N.S.; Lee, J.M. The anti-oxidative and anti-microbial ability of ethanol extracts from Rosa hybrida. Korean J. Food Sci. Technol. 2003, 35, 373–378. [Google Scholar]
- Konczak, I.; Zhang, W. Anthocyanins—more than nature’s colours. J. Biomed. Biotechnol. 2004, 2004, 239–240. [Google Scholar] [CrossRef]
- Cho, E.K.; Son, J.-Y.; Kang, K.O. Antioxidant activities of rose, camellia and cockscomb flower extracts. Food Serv. Indust. J. 2015, 11, 21–33. [Google Scholar]
- Vinokur, Y.; Rodov, V.; Reznick, N.; Goldman, G.; Horev, B.; Umiel, N. Rose petal tea as an anti-oxidant rich beverage: Cultivar effects. J. Food Sci. 2006, 71, S42–S47. [Google Scholar] [CrossRef]
- Özkan, G.; Sağdiç, O.; Baydar, N.G.; Baydar, H. Anti-oxidant and anti-bacterial activities of Rosa damascena flower extracts. Food Sci. Technol. Int. 2004, 10, 277–281. [Google Scholar] [CrossRef]
- Wang, C.; Kim, I.J.; Seong, H.R.; Noh, C.H.; Park, S.; Kim, T.M.; Jeong, H.-S.; Kim, K.Y.; Kim, S.T.; Yuk, H.G.; et al. Antioxidative and anti-inflammatory activities of rosebud extracts of newly crossbred roses. Nutrients 2023, 15, 2376. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Kwon, S.C.; Park, D.; Shin, S.; Jeong, J.H.; Hwang, S.Y.; Kim, Y.B.; Joo, S.S. Anti-allergic effects of white rose petal extract and anti-atopic properties of its n-hexane fraction. Arch. Pharm. Res. 2009, 32, 823–830. [Google Scholar] [CrossRef]
- Choi, E.K.; Guo, H.; Choi, J.K.; Jang, S.K.; Shin, K.; Cha, Y.S.; Choi, Y.; Seo, D.W.; Lee, Y.B.; Joo, S.S.; et al. Extraction conditions of white rose petals for the inhibition of enzymes related to skin aging. Lab. Anim. Res. 2015, 31, 148–152. [Google Scholar] [CrossRef]
- Yang, G.; Park, D.; Lee, S.H.; Bae, D.-K.; Yang, Y.-H.; Kyung, J.; Kim, D.; Choi, E.-K.; Hong, J.T.; Jeong, H.-S.; et al. Neuroprotective effects of a butanol fraction of Rosa hybrida petals in an ischemia-reperfusion stroke model. Biomol. Ther. 2013, 21, 454–461. [Google Scholar] [CrossRef]
- Moon, J.; Gwak, H.; Lee, T.-H.; An, E.S.; Kim, Y.B.; Park, D. Antioxidant effects of pyrogallol in neural cells and mice brain. Brain Dig. Learn. 2019, 9, 113–124. [Google Scholar] [CrossRef]
- Nakano, T.; Ikeda, M.; Wakugawa, T.; Kashiwada, Y.; Kaminuma, O.; Kitamura, N.; Yabumoto, M.; Fujino, H.; Kitamura, Y.; Fukui, H.; et al. Identification of pyrogallol from Awa-tea as an anti-allergic compound that suppresses nasal symptoms and IL-9 gene expression. J. Med. Investig. 2020, 67, 289–297. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Ito, T.; Nishida, K.; Wakugawa, T.; Nakano, T.; Tanabe, A.; Watano, T.; Kitamura, N.; Kaminuma, O.; Kimura, K.; et al. Structure-activity relationship studies of pyrogallol as a calcineurin/NFAT signaling suppressor. J. Pharmacol. Sci. 2024, 155, 140–147. [Google Scholar] [CrossRef]
- Yon, J.M.; Kim, Y.B.; Park, D. The ethanol fraction of white rose petal extract abrogates excitotoxicity-induced neuronal damage in vivo and in vitro through inhibition of oxidative stress and proinflammation. Nutrients 2018, 10, 1375. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Duval, B.; Shetty, K. The stimulation of phenolics and antioxidative activity in pea (Pisum sativam) elicited by genetically transformed anise root extract. J. Food Biochem. 2001, 25, 361–377. [Google Scholar] [CrossRef]
- Takahama, U.; Tanaka, M.; Hirota, S. Proanthocyanidins in buckwheat flour can reduce salivary nitrite to nitric oxide in the stomach. Plant Foods Hum. Nutr. 2010, 65, 1–7. [Google Scholar] [CrossRef]
- Ban, H.J.; Park, D.I.; Kang, K.H. Inhibitory effect of Artemisiae asiaticae Herba on degranulation, production of cytokine, and FcεRI expression in RBL-2H3 cells. Korean J. Orient. Physiol. Pathol. 2012, 26, 915–921. [Google Scholar]
- Kim, S.H.; Choi, C.H.; Kim, S.Y.; Eun, J.S.; Shin, T.Y. Anti-allergic effects of Artemisia iwayomogi on mast cell-mediated allergy model. Exp. Biol. 2005, 230, 82–88. [Google Scholar] [CrossRef]
- Inagaki, N.; Igeta, K.; Kim, J.F.; Nagao, M.; Shiraishi, N.; Nakamura, N.; Nagai, H. Involvement of unique mechanisms in the induction of scratching behavior in BALB/c mice by compound 48/80. Eur. J. Pharmacol. 2002, 448, 175–183. [Google Scholar] [CrossRef]
- Joo, S.S.; Park, D.; Shin, S.; Jeon, J.H.; Kim, T.K.; Kim, J.S.; Park, S.K.; Hwang, B.Y.; Lee, D.I.; Kim, Y.B. Anti-allergic effects and mechanisms of the ethanolic extract of Angelica gigas in dinitrofluorobenzene-induced inflammation models. Environ. Toxicol. Pharmacol. 2010, 30, 127–133. [Google Scholar] [CrossRef]
- Shin, S.; Joo, S.S.; Park, D.; Jeon, J.H.; Kim, T.K.; Kim, J.S.; Park, S.K.; Hwang, B.Y.; Kim, Y.B. Ethanol extract of Angelica gigas inhibits croton oil-induced inflammation by suppressing the cyclooxygenase—Prostaglandin pathway. J. Vet. Sci. 2010, 11, 43–50. [Google Scholar] [CrossRef]
- Hwang, M.R.; Kim, H.E.; Park, D.K.; Heu, Y.C.; Lee, H.J.; Kang, N.J. Induction of oxidative stress and activation of antioxidant enzymes by infection of powdery mildew in cucurbita plants. J. Agric. Life Sci. 2013, 47, 75–81. [Google Scholar]
- Middleton, E.; Kandaswami, C. Potential health-promoting properties of citrus flavonoids. Food Technol. 1994, 48, 115–119. [Google Scholar]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Charlesworth, E.N.; Beltrani, V.S. Pruritic dermatoses: Overview of etiology and therapy. Am. J. Med. 2002, 113, 25S–33S. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Bieber, T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003, 112, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.Y.; Kim, S.H.; Suk, K.; Ha, J.H.; Kim, I.; Lee, M.G.; Jun, C.D.; Kim, S.Y.; Lim, J.P.; Eun, J.S.; et al. Anti-allergic effects of Lycopus lucidus on mast cell-mediated allergy model. Toxicol. Appl. Pharmacol. 2005, 209, 255–262. [Google Scholar] [CrossRef]
- Swierczyniska-Machura, D.; Krakowiak, A.; Palczynski, C. Occupational allergy caused by ornamental plants. Med. Pr. 2006, 57, 359–364. [Google Scholar]
- Church, M.K.; Levi-Schaffer, F. The human mast cell. J. Allergy Clin. Immunol. 1997, 99, 155–160. [Google Scholar] [CrossRef]
- Miyajima, I.; Dombrowicz, D.; Martin, T.R.; Ravetch, J.V.; Kinet, J.P.; Galli, S.J. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J. Clin. Investig. 1997, 99, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Park, S.H. Risk and benefit of steroid therapy. J. Korean Soc. Intern. Med. 2009, 77, 298–303. [Google Scholar]
- Demir, A.U.; Karakaya, G.; Kalyoncu, A.F. Allergy symptoms and IgE immune response to rose: An occupational and an environmental disease. Allergy 2002, 57, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Jeon, J.H.; Kwon, S.C.; Shin, S.; Jang, J.Y.; Jeong, J.H.; Lee, H.S.; Kim, D.I.; Kim, Y.B.; Joo, S.S. Antioxidative activities of white rose flower extract and pharmaceutical advantages of its hexane fraction via free radical scavenging effects. Biochem. Cell Biol. 2009, 87, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, H.S.; Kim, S.T.; Park, D.; Hong, J.T.; Kim, Y.B.; Joo, S.S. Anti-inflammatory effects of hexane fraction from white rose flower extracts via inhibition of inflammatory repertoires. Biomol. Ther. 2011, 19, 331–335. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




