Submitted:
08 January 2026
Posted:
09 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology
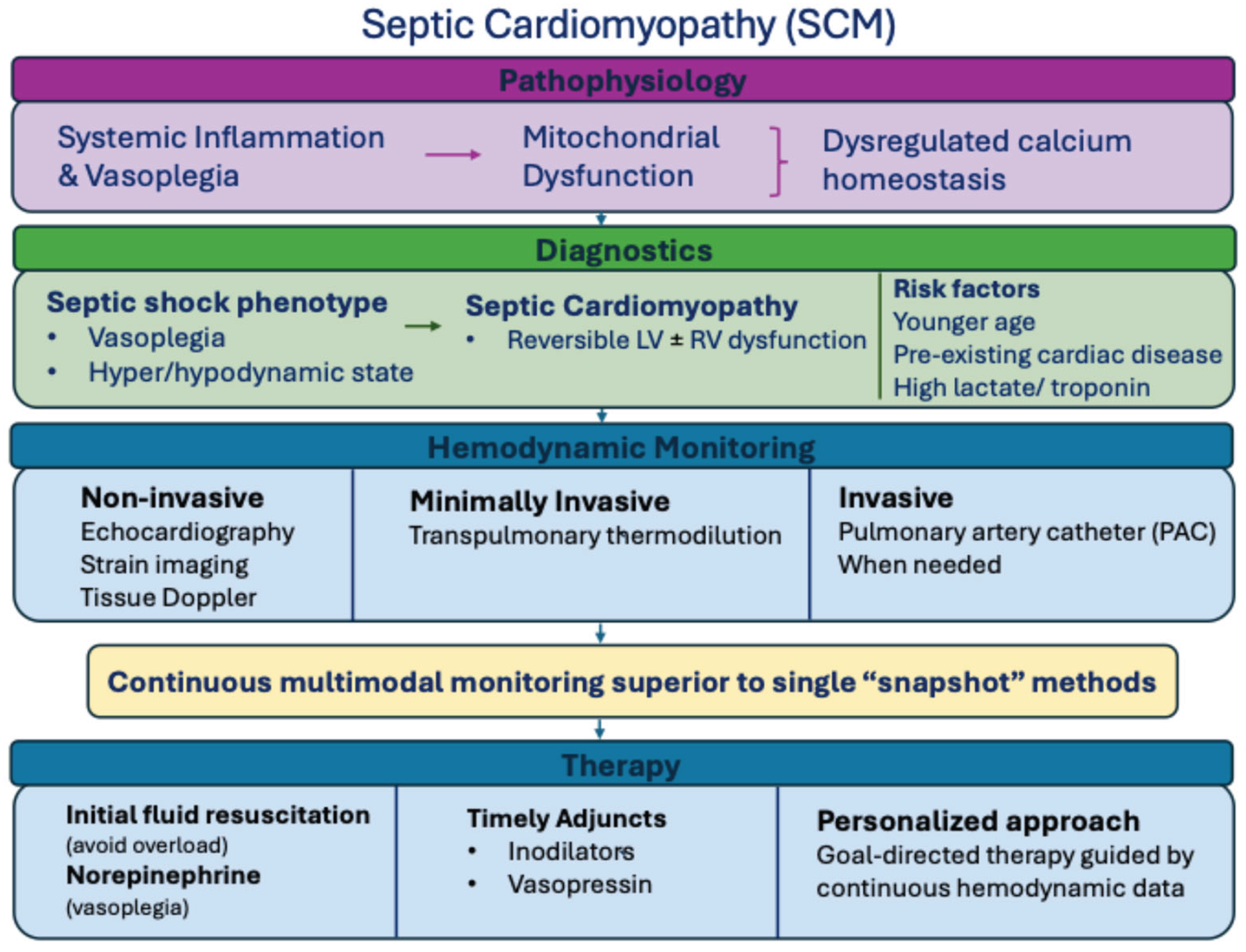
3. Pathophysiological Mechanisms in Adult and Pediatric Sepsis
4. Echocardiographic Findings in Adult and Pediatric Sepsis
5. Basic Hemodynamic Monitoring
6. Advanced Multimodal Hemodynamic Monitoring
7. Current Treatment Concepts Based on Hemodynamic Monitoring
8. Refractory Septic Shock
9. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| CO | Cardiac Output |
| CRT | Capillary refill time |
| CVP | Central venous pressure |
| DO2 | Oxygen Delivery |
| EVLW | Extravascular lung water |
| FAC | Fractional area change |
| FS | Fractional shortening |
| GEDV | Global end-diastolic volume |
| GLS | Global longitudinal strain |
| HR | Heart rate |
| ICU | Intensive Care Unit |
| LVEF | Left ventricular ejection fraction |
| LV | Left ventricle/ventricular |
| MAP | Mean arterial pressure |
| PAC | Pulmonary artery catheter |
| Pv-aCO2 | Venous-to-arterial carbon dioxide difference |
| RV | Right ventricle/ventricular |
| SCM | Sepsis-induced cardiomyopathy |
| STE | Speckle-tracking echocardiography |
| TAPSE | Tricuspid annular plane systolic excursion |
| TDI | Tissue Doppler imaging |
| TPTD | Transpulmonary thermodilution |
| VA-ECMO | Veno-arterial extracorporeal membrane oxygenation |
| VAD | Ventricular assist device |
| VIS | Vasoactive inotrope score |
| SvO2 | Mixed venous oxygen saturation |
| PICCO | Pulse index continuous cardiac output |
References
- Beesley, SJ; Weber, G; Sarge, T; Nikravan, S; Grissom, CK; Lanspa, MJ; et al. Septic Cardiomyopathy. Critical Care Medicine 2018, 46, 625. [Google Scholar] [CrossRef]
- Gupta, S; Sankar, J. Advances in Shock Management and Fluid Resuscitation in Children. Indian J Pediatr 2023, 90, 280–8. [Google Scholar] [CrossRef]
- Lautz, AJ; Zingarelli, B. Age-Dependent Myocardial Dysfunction in Critically Ill Patients: Role of Mitochondrial Dysfunction. Int J Mol Sci 2019, 20, 3523. [Google Scholar] [CrossRef] [PubMed]
- Boissier, F; Aissaoui, N. Septic cardiomyopathy: Diagnosis and management. J Intensive Med 2022, 2, 8–16. [Google Scholar] [CrossRef]
- Chan, JC; Menon, AP; Rotta, AT; Choo, JTL; Hornik, CP; Lee, JH. Use of Speckle-Tracking Echocardiography in Septic Cardiomyopathy in Critically Ill Children: A Narrative Review. Crit Care Explor 2024, 6, e1114. [Google Scholar] [CrossRef] [PubMed]
- Sato, R; Hasegawa, D; Guo, S; Nuqali, AE; Moreno, JEP. Sepsis-induced cardiogenic shock: controversies and evidence gaps in diagnosis and management. J Intensive Care 2025, 13, 1. [Google Scholar] [CrossRef]
- L’Heureux, M; Sternberg, M; Brath, L; Turlington, J; Kashiouris, MG. Sepsis-Induced Cardiomyopathy: a Comprehensive Review. Curr Cardiol Rep 2020, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Sato, R; Kuriyama, A; Takada, T; Nasu, M; Luthe, SK. Prevalence and risk factors of sepsis-induced cardiomyopathy: A retrospective cohort study. Medicine (Baltimore) 2016, 95, e5031. [Google Scholar] [CrossRef]
- Jeong, HS; Lee, TH; Bang, CH; Kim, J-H; Hong, SJ. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: A comparative retrospective study. Medicine (Baltimore) 2018, 97, e0263. [Google Scholar] [CrossRef]
- Lukić, I; Mihić, D; Varžić, SC; Relatić, KS; Zibar, L; Loinjak, D; et al. Septic Cardiomyopathy. Rev Cardiovasc Med 2024, 25, 23. [Google Scholar] [CrossRef]
- Bakker, J; Kattan, E; Annane, D; Castro, R; Cecconi, M; De Backer, D; et al. Current practice and evolving concepts in septic shock resuscitation. Intensive Care Med 2022, 48, 148–63. [Google Scholar] [CrossRef]
- Hernández, G; Ospina-Tascón, GA; Damiani, LP; Estenssoro, E; Dubin, A; Hurtado, J; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–64. [Google Scholar] [CrossRef] [PubMed]
- Guarracino, F; Bertini, P; Pinsky, MR. Heterogeneity of Cardiovascular Response to Standardized Sepsis Resuscitation. Crit Care 2020, 24, 99. [Google Scholar] [CrossRef]
- Raj, S; Killinger, JS; Gonzalez, JA; Lopez, L. Myocardial dysfunction in pediatric septic shock. J Pediatr 2014, 164, 72–77.e2. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J; Carcillo, JA; Choong, K; Cornell, T; Decaen, A; Deymann, A; et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 2009, 37, 666–88. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, LJ; Watson, RS; Sorce, LR; Argent, AC; Menon, K; Hall, MW; et al. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024, 331, 665–74. [Google Scholar] [CrossRef]
- Fernández-Sarmiento, J; Carcillo, JA; Díaz Del Castillo, AME; Barrera, P; Orozco, R; Rodríguez, MA; et al. Venous-arterial CO2 difference in children with sepsis and its correlation with myocardial dysfunction. Qatar Med J 2019, 2019, 18. [Google Scholar] [CrossRef]
- Swami, VS; LA, V; Ghosh, S; Reddy, M. Sepsis-Induced Myocardial Dysfunction in Pediatric Septic Shock: Prevalence, Predictors, and Outcome-A Prospective Observational Study. J Pediatr Intensive Care 2024, 13, 87–94. [Google Scholar] [CrossRef]
- Lee, E-P; Hsia, S-H; Lin, J-J; Chan, O-W; Lee, J; Lin, C-Y; et al. Hemodynamic Analysis of Pediatric Septic Shock and Cardiogenic Shock Using Transpulmonary Thermodilution. Biomed Res Int 2017, 2017, 3613475. [Google Scholar] [CrossRef]
- Liang, Y-W; Zhu, Y-F; Zhang, R; Zhang, M; Ye, X-L; Wei, J-R. Incidence, prognosis, and risk factors of sepsis-induced cardiomyopathy. World J Clin Cases 2021, 9, 9452–68. [Google Scholar] [CrossRef]
- Pulido, JN; Afessa, B; Masaki, M; Yuasa, T; Gillespie, S; Herasevich, V; et al. Clinical Spectrum, Frequency, and Significance of Myocardial Dysfunction in Severe Sepsis and Septic Shock. Mayo Clinic Proceedings 2012, 87, 620–8. [Google Scholar] [CrossRef]
- Yu, Y-Y; Wang, R; Chen, G-Q; Gui, Y-F; Ma, J; Ma, J-H; et al. Mechanisms and Targeted Therapeutic Strategies in Sepsis-Induced Myocardial Dysfunction: The Role of NLRP3 Inflammasome-Mediated Inflammation. J Inflamm Res 2025, 18, 8875–97. [Google Scholar] [CrossRef] [PubMed]
- Beesley, SJ; Weber, G; Sarge, T; Nikravan, S; Grissom, CK; Lanspa, MJ; et al. Septic Cardiomyopathy. Crit Care Med 2018, 46, 625–34. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, D; Ishisaka, Y; Maeda, T; Prasitlumkum, N; Nishida, K; Dugar, S; et al. Prevalence and Prognosis of Sepsis-Induced Cardiomyopathy: A Systematic Review and Meta-Analysis. J Intensive Care Med 2023, 38, 797–808. [Google Scholar] [CrossRef]
- Lu, N-F; Niu, H-X; Liu, A-Q; Chen, Y-L; Liu, H-N; Zhao, P-H; et al. Types of Septic Cardiomyopathy: Prognosis and Influencing Factors - A Clinical Study. Risk Manag Healthc Policy 2024, 17, 1015–25. [Google Scholar] [CrossRef]
- Jain, A; Sankar, J; Anubhuti, A; Yadav, DK; Sankar, MJ. Prevalence and Outcome of Sepsis-induced Myocardial Dysfunction in Children with “Sepsis” “With” and ’Without Shock’-A Prospective Observational Study. J Trop Pediatr 2018, 64, 501–9. [Google Scholar] [CrossRef]
- Ceneviva, G; Paschall, JA; Maffei, F; Carcillo, JA. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics 1998, 102, e19. [Google Scholar] [CrossRef]
- Carbone, F; Liberale, L; Preda, A; Schindler, TH; Montecucco, F. Septic Cardiomyopathy: From Pathophysiology to the Clinical Setting. Cells 2022, 11, 2833. [Google Scholar] [CrossRef] [PubMed]
- Zanotti-Cavazzoni, SL; Hollenberg, SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care 2009, 15, 392–7. [Google Scholar] [CrossRef]
- Stanzani, G; Duchen, MR; Singer, M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochim Biophys Acta Mol Basis Dis 2019, 1865, 759–73. [Google Scholar] [CrossRef]
- Fujimura, K; Karasawa, T; Komada, T; Yamada, N; Mizushina, Y; Baatarjav, C; et al. NLRP3 inflammasome-driven IL-1β and IL-18 contribute to lipopolysaccharide-induced septic cardiomyopathy. J Mol Cell Cardiol 2023, 180, 58–68. [Google Scholar] [CrossRef]
- Zheng, Y; Lin, J; Wan, G; Gu, X; Ma, J. Macrophage Notch1 drives septic cardiac dysfunction by impairing mitophagy and promoting NLRP3 activation. Biology Direct 2025, 20, 65. [Google Scholar] [CrossRef]
- Muehlberg, F; Blaszczyk, E; Will, K; Wilczek, S; Brederlau, J; Schulz-Menger, J. Characterization of critically ill patients with septic shock and sepsis-associated cardiomyopathy using cardiovascular MRI. ESC Heart Fail 2022, 9, 2147–56. [Google Scholar] [CrossRef]
- Hollenberg, SM; Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol 2021, 18, 424–34. [Google Scholar] [CrossRef]
- Werdan, K; Oelke, A; Hettwer, S; Nuding, S; Bubel, S; Hoke, R; et al. Septic cardiomyopathy: hemodynamic quantification, occurrence, and prognostic implications. Clin Res Cardiol 2011, 100, 661–8. [Google Scholar] [CrossRef]
- Ranjit, S; Aram, G; Kissoon, N; Ali, MK; Natraj, R; Shresti, S; et al. Multimodal monitoring for hemodynamic categorization and management of pediatric septic shock: a pilot observational study*. Pediatr Crit Care Med 2014, 15, e17-26. [Google Scholar] [CrossRef] [PubMed]
- Lanspa, MJ; Cirulis, MM; Wiley, BM; Olsen, TD; Wilson, EL; Beesley, SJ; et al. Right Ventricular Dysfunction in Early Sepsis and Septic Shock. Chest 2021, 159, 1055–63. [Google Scholar] [CrossRef]
- De Backer, D; Cecconi, M; Chew, MS; Hajjar, L; Monnet, X; Ospina-Tascón, GA; et al. A plea for personalization of the hemodynamic management of septic shock. Crit Care 2022, 26, 372. [Google Scholar] [CrossRef]
- Furian, T; Aguiar, C; Prado, K; Ribeiro, RVP; Becker, L; Martinelli, N; et al. Ventricular dysfunction and dilation in severe sepsis and septic shock: relation to endothelial function and mortality. J Crit Care 2012, 27, 319.e9–15. [Google Scholar] [CrossRef] [PubMed]
- Boissier, F; Razazi, K; Seemann, A; Bedet, A; Thille, AW; de Prost, N; et al. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med 2017, 43, 633–42. [Google Scholar] [CrossRef] [PubMed]
- Williams, FZ; Sachdeva, R; Travers, CD; Walson, KH; Hebbar, KB. Characterization of Myocardial Dysfunction in Fluid- and Catecholamine-Refractory Pediatric Septic Shock and Its Clinical Significance. J Intensive Care Med 2019, 34, 17–25. [Google Scholar] [CrossRef]
- Sankar, J; Das, RR; Jain, A; Dewangan, S; Khilnani, P; Yadav, D; et al. Prevalence and outcome of diastolic dysfunction in children with fluid refractory septic shock--a prospective observational study. Pediatr Crit Care Med 2014, 15, e370-378. [Google Scholar] [CrossRef]
- Kakihana, Y; Ito, T; Nakahara, M; Yamaguchi, K; Yasuda, T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care 2016, 4, 22. [Google Scholar] [CrossRef]
- Merx, MW; Weber, C. Sepsis and the Heart. Circulation 2007, 116, 793–802. [Google Scholar] [CrossRef]
- Porter, TR; Shillcutt, SK; Adams, MS; Desjardins, G; Glas, KE; Olson, JJ; et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr 2015, 28, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Jentzer, JC; Tabi, M; Wiley, BM; Singam, NSV; Anavekar, NS. Echocardiographic Correlates of Mortality Among Cardiac Intensive Care Unit Patients With Cardiogenic Shock. Shock 2022, 57, 336–43. [Google Scholar] [CrossRef]
- Fenton, KE; Sable, CA; Bell, MJ; Patel, KM; Berger, JT. Increases in serum levels of troponin I are associated with cardiac dysfunction and disease severity in pediatric patients with septic shock. Pediatr Crit Care Med 2004, 5, 533–8. [Google Scholar] [CrossRef] [PubMed]
- Deep, A; Goonasekera, CDA; Wang, Y; Brierley, J. Evolution of haemodynamics and outcome of fluid-refractory septic shock in children. Intensive Care Med 2013, 39, 1602–9. [Google Scholar] [CrossRef]
- Abdalaziz, FA; Algebaly, HAF; Ismail, RI; El-Sherbini, SA; Behairy, A. The use of bedside echocardiography for measuring cardiac index and systemic vascular resistance in pediatric patients with septic shock. Rev Bras Ter Intensiva 2018, 30, 460–70. [Google Scholar] [CrossRef]
- Orde, SR; Pulido, JN; Masaki, M; Gillespie, S; Spoon, JN; Kane, GC; et al. Outcome prediction in sepsis: speckle tracking echocardiography based assessment of myocardial function. Crit Care 2014, 18, R149. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, MR; Cecconi, M; Chew, MS; De Backer, D; Douglas, I; Edwards, M; et al. Effective hemodynamic monitoring. Crit Care 2022, 26, 294. [Google Scholar] [CrossRef]
- Giraud, R; Siegenthaler, N; Merlani, P; Bendjelid, K. Reproducibility of transpulmonary thermodilution cardiac output measurements in clinical practice: a systematic review. J Clin Monit Comput 2017, 31, 43–51. [Google Scholar] [CrossRef]
- Pernbro, F; Wåhlander, H; Romlin, B. Haemodynamic monitoring after paediatric cardiac surgery using echocardiography and PiCCO. Cardiol Young 2024, 34, 2636–40. [Google Scholar] [CrossRef]
- Zhang, Y; Wang, Y; Shi, J; Hua, Z; Xu, J. Cardiac output measurements via echocardiography versus thermodilution: A systematic review and meta-analysis. PLoS One 2019, 14, e0222105. [Google Scholar] [CrossRef]
- Wetterslev, M; Møller-Sørensen, H; Johansen, RR; Perner, A. Systematic review of cardiac output measurements by echocardiography vs. thermodilution: the techniques are not interchangeable. Intensive Care Med 2016, 42, 1223–33. [Google Scholar] [CrossRef] [PubMed]
- Lemson, J; van Die, LE; Hemelaar, AEA; van der Hoeven, JG. Extravascular lung water index measurement in critically ill children does not correlate with a chest x-ray score of pulmonary edema. Crit Care 2010, 14, R105. [Google Scholar] [CrossRef]
- Grindheim, G; Eidet, J; Bentsen, G. Transpulmonary thermodilution (PiCCO) measurements in children without cardiopulmonary dysfunction: large interindividual variation and conflicting reference values. Paediatr Anaesth 2016, 26, 418–24. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X; Persichini, R; Ktari, M; Jozwiak, M; Richard, C; Teboul, J-L. Precision of the transpulmonary thermodilution measurements. Crit Care 2011, 15, R204. [Google Scholar] [CrossRef]
- Slagt, C; de Leeuw, MA; Beute, J; Rijnsburger, E; Hoeksema, M; Mulder, JWR; et al. Cardiac output measured by uncalibrated arterial pressure waveform analysis by recently released software version 3.02 versus thermodilution in septic shock. J Clin Monit Comput 2013, 27, 171–7. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.




